Abstract
We here report on the synthesis of new unsymmetrical linked bis-5-arylidene rhodanine derivatives with stereocontrolled Z-configuration. The 6 steps synthesis was achieved and the key steps are the construction of the two 5-arylidene rhodanine moieties using an “one-pot two-steps” method under microwave dielectric heating in a closed reactor. The intermediates 6, 7 and desired unsymmetrical compounds 9 have been also evaluated for their in vitro inhibition of cell proliferation (Huh7 D12, Caco2, MDA-MB 231, HCT116, and NCI-H727 tumoral cell lines). Two of all compounds have shown potent activity against Huh7 D12, Caco2, and MDA-MB 231.
Graphical Abstract

.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The 5-arylidene-2-thioxo-1,3-thiazolidinine-4-ones or 5-arylidene rhodanines and their 2-amino-5-arylidene-5H-thiazol-4-one derivatives belong to five-membered heterocyclic rings (FMHRs) and are considered as “privileged scaffolds” in the medicinal chemistry community because they presented an inherent tendency for a large panel of biological activities (Mentgen et al., 2012). These low-molecular weight inhibitors have been previously studied (Singh et al., 1981; Tomasic and Masic, 2009) and have been subjected to controversies (Lesyk and Zimenkovsky, 2004) due to the high potential of these scaffolds to form intermolecular interactions in diverse receptors. The FMHRs have been known to possesses a wide range of biological properties such as potent and selective inhibitors of the “atypical” dual-specificity phosphatase (DSP) family member-JNK-stimulating phosphatase-1 (JSP-1) (Cutshall et al., 2005), as aldose reductase inhibitor on diabetic peripheral neuropathy (Hotta et al., 2006), as DDX3 inhibitor for HIV replication (Maga et al., 2008).
Our research group is mainly invested in the synthesis of marine alkaloid derivatives as low-molecular weight-inhibitors (Bazureau et al., 2009) of dual specificity, tyrosine phosphorylation-regulated kinases (DYRKs) (Debdab et al., 2011; Tahtouh et al., 2012) and CLKs (cdc2-like kinases) (Aranda et al., 2011). Recently, we have realized a complete structure activity relationship (SAR) study and have identified leucettine L41 (Fig. 1), a synthetic derivative of the marine alkaloid leucettamine B, as first potent inhibitor of DYRK1A (IC50 40 nM) and CLKs, two families of kinase involved in various diseases including Alzheimer’s disease (AD), Down syndrome (Smith et al., 2012), and also cancer (Ionescu et al., 2012; Xiao et al., 2013; Ling et al., 2013). Encouraged by these preliminary results, we continued to explore the design and the synthesis of an expanded series of N,N’-bis-(5-arylidene-4-oxo-3,5-dihydro-4H-imidazol-2-yl)diamines as potential protein kinase inhibitors from appropriate symmetric alkyldiamines as flexible linkers (Coulibaly et al., 2012a). Starting from 1,4-bis(3-aminopropyl)piperazine, this strategy was also extended to the synthetic preparation of symmetric N,N’-disubstituted diamines bearing 5-arylidene-1,3-thiazolidine-4-one moiety and to our surprise one of these compounds has shown nanomolar inhibition potency (IC50 40 nM) towards DYRK1A (Fig. 1) (Coulibaly et al., 2012b). This result prompted us to explore now the use of symmetric 1,2-diamino linker grafted on N-3 position of two different 5-arylidene rhodanine platforms in order to modulate potential biological activities. The goal of the present study was to develop unsymmetrical linked bis-5-arylidene rhodanine derivatives, via “one-pot two steps” reactions under microwave irradiation to build heterocyclic platforms and evaluate biological activities on six representative tumoral cell lines.
Results and discussion
Chemistry
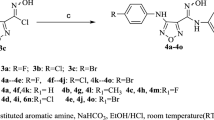
The overall strategy to the target linked bis-5-arylidene rhodanine derivatives is outlined in Scheme 1. Starting from various commercial symmetric diamines 1 as linkers (i.e., ethylenediamine 1a, 1,4-diaminobutane 1b, and 1,4-bis-(3-aminopropyl)piperazine 1c), these diamino building block 1 was subjected to 0.1–0.2 equivalent of di-tert-butyldicarbonate (Boc2O) to afford mainly the mono-Boc-protected amine 2 (Bonger et al., 2009) in 26–56 % yield (Table 1). For the construction of the first 5-arylidene rhodanine moiety grafted on the mono-Boc-protected diamino linker 2, we decided to test a “one-pot two-steps” described protocol (Radi et al., 2010) involving the “Holmberg method” (Holmberg, 1910) (which is based on the reaction of bis(carboxymethyl)-trithiocarbonate 3 with primary amine) and Knoevenagel condensation to afford directly the 5-arylidene rhodanine 6 under microwave irradiation. This choice was guided by the fact that the major benefits of performing reaction under microwave irradiation are higher product yields and significant rate enhancements compared to reactions which run with conventional heating (i.e., in oil bath). A real advantage of commercial laboratory microwave apparatus is their ability to control reaction conditions precisely, by monitoring reaction times and temperature/pressure (de la Hoz and Loupy, 2012; Bazureau and Draye, 2011; Krstenansky and Cotteril, 2000).
Reagents and reaction conditions: (i) (t-BuO2C)2O, 1,4-dioxane, 25 °C, 24 h. (ii) 3 1 equiv., Et3N 1 equiv., DME, 90 °C, MWI, 10 min. (iii) 5 1 equiv., 110 °C, MWI, 5 min. (iv) HCl 6 M, 1,4-dioxane, 25 °C, 4 h. (v) 3 1 equiv., Et3N 2 equiv., DME, 90 °C, MWI, 10 min. (vi) 5 1 equiv., 110 °C, MWI, 5 min
For the preparation of 4, reaction optimization consisted in varying the reaction time (5-20 min.), the reaction temperature (80–100 °C), the ratio of reagents (ratio 2/3:1–1.3), the use of a polar or no polar solvent (hexane, Et2O, 1,4-dioxane, dimethoxyethane), and the use of closed or open vessel mode and the choice of an organic base (pyridine, i-PrN2Et, Et3N). After all these experiments, the optimal reaction conditions were obtained at 90 °C after 10 min; with a stoichiometric mixture of 2 and 3 solubilized in dimethoxyethane with one equivalent of Et3N. The reaction is conducted in a closed reactor (in this case, the reaction under microwave irradiation is realized in commercial glass tube closed with a snap cap). It is noteworthy that initial attempts to isolate the compound 4 after a time-consuming chromatographic purification were unsuccessful. In this context, we decided to drop the structural identification of 4 in this second step and after the first microwave irradiation period (10 min.), aromatic aldehyde 5 was added directly in the reaction mixture. The resulting mixture was irradiated at 110 °C for 5 min. For this present protocol, we have also examined the chemical reactivity of two commercially available aldehydes 5 (i.e., 4-methoxybenzaldehyde 5a and 3,4-methylenedioxybenzaldehyde 5b). After elimination of the volatiles compounds in vacuo, the desired products 6a–e were isolated after a simple precipitation in MeOH followed by washing with methylene chloride. A set of five designed compounds 6 was prepared in 23–59 % yield (Table 1) and characterized by 1H, 13C NMR, and HRMS. The geometry of the exocyclic double bond (=CH) of these new 5-arylidene rhodanine derivatives 6a–e was performed; the thermodynamically more stable Z-isomers predominated and Z-configuration was assigned as previously reported in literature (Xia et al., 2009). Deprotection of compounds 6 into their corresponding salts 7 was conducted in 1,4-dioxane with a solution of 6 M HCl at room temperature after 4 h of reaction time. The expected hydrochloride salts 7 were synthesized in good yields (65–98 %). Finally, for the installation of the second 5-arylidene-4-oxo-2-thioxo-1,3-thiazolidine-1-yl moiety, we employed again the “one-pot two-steps” protocol under microwave irradiation. To a solution of bis(carboxymethyl)-trithiocarbonate 3 in dimethoxyethane, was added successively two equivalents of triethylamine and appropriate quantity of hydrochloride salt 7. The resulting suspension in a closed reactor was heated at 90 °C under microwave irradiation for 10 min. After this first period of irradiation, aromatic aldehyde 5 (4-methoxybenzaldehyde 5a or benzaldehyde 5c) was added and the mixture was submitted immediately to microwave dielectric heating at 110 °C for 5 min. The desired product 9 was obtained as precipitate after addition of methanol in the solventless crude residue and triturating, followed by washings with cooled hexane, methylene chloride and finally was recrystallized in ethanol to increase the quality of the precipitated product 9. At this stage, the compounds 9a–c were fully analyzed and characterized before entering the biological tests. The structure identification of all these new compounds was based on the 1H and 13C assignments and was performed extensive 1D and 2D NMR spectroscopy. Examination of the 1H NMR spectrum in DMSO-d 6 for 9a showed two separated signals for the two methylene protons (CH=), one appears at δ 7.78 ppm and the second shifted to 7.83 ppm and hence confirmed the unsymmetrical structure of 9a. It is interesting to note that these two methylene protons appear at lower field values than those expected for the E-isomers, which strongly indicates that the two 5-arylidene-4-oxo-2-thioxo-1,3-thiazolidine-1-yl moieties have both the Z-configuration (Guiheneuf et al., 2014). The unsymmetrical structure of 9a was also confirmed in the 13C NMR spectrum because we observed two separated signals for the two methylene (CH=) carbons (δ 133.1 and 133.4 ppm).
Biology
To evaluate the potency of selected compounds 6a–e, 7a–c and 9a–c for their in vitro inhibition of cell proliferation, we used a panel of 6 representative tumoral cell lines, namely Huh7 D12 (differential hepatocellular carcinoma), Caco2 (differentiating colorectal adenocarcinoma), HCT (actively proliferating colorectal carcinoma), PC3 (prostate carcinoma), NCI-H2 (lung carcinoma), MDA-MB 231 (breast carcinoma), and diploid skin fibroblasts as normal cell lines for control. Roscovitine was also used as a positive control and it IC50 values were compared with those obtained for the compounds 6, 7, and 9. Results of the in vitro antiproliferative data activity are reported in Table 2. The most active compound was clearly 6e and exhibited antitumor activities in the Huh7 D12 and Caco2 cell lines with IC50 values lower than 10 μM (Huh7 D12 IC50 8 μM and Caco2 IC50 8 μM). In addition, 7a presented also a selective activity in the MDA MB231 cell line (IC50 7 μM) and did not exhibit the growth of normal fibroblasts (IC50 > 25 μM). On the other hand, in the desired unsymmetrical linked bis-5-arylidene rhodanine derivatives 9a–c, none of these compounds presented a significant activity against the six representative tumoral cell lines.
Conclusion
In summary, the synthesis of unsymmetrical linked bis-5-arylidene rhodanine derivatives was realized in six steps. The key steps of this sequence involved the construction of 5-arylidene-4-oxo-2-thioxo-1,3-thiazolidine-1-yl moieties by “one-pot two-steps” protocol under microwave dielectric heating using primary amine (i.e., intermediates 2 and 7), bis(carboxymethyl)-trithiocarbonate 3 and aromatic aldehydes 5. The intermediates 6, 7 and unsymmetrical compounds 9 have been built with a Z-geometry and their in vitro inhibition of cell proliferation was carried out. None of unsymmetrical compounds showed a significant activity against the six tumoral cell lines, but the intermediates 6e and 7a exhibit activity against Huh7 D12, Caco 2, and MDA MB231 cell lines.
Experimental section
Chemistry
General remarks
Melting points were determined on a Kofler melting point apparatus and were uncorrected. Thin-layer chromatography (TLC) was accomplished on 0.2-mm precoated plates of silica gel 60 F-254 (Merck). Visualization was made with ultraviolet light (254 and 365 nM) or with a fluorescence indicator. 1H NMR spectra were recorded on BRUKER AC 300 P (300 MHz) spectrometer and 13C NMR spectra on BRUKER AC 300 P (75 MHz) spectrometer. Chemical shifts are expressed in parts per million downfield from tetramethylsilane as an internal standard. Data are given in the following order: d value, multiplicity (s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet; and br, broad), number of protons, coupling constants J is given in Hertz. The mass spectra (HRMS) were taken, respectively, on a MS/MS ZABSpec Tof Micromass (EBE TOF geometry) at an ionizing potential of 8 eV and on a VARIAN MAT 311 at an ionizing potential of 70 eV in the Centre Régional de Mesures Physiques de l’Ouest (CRMPO, Rennes). Reactions under microwave irradiations were realized in the Anton Paar Monowave 300® microwave reactor (Anton-Paar France) using borosilicate glass vials of 10 ml equipped with snap caps (at the end of the irradiation, cooling reaction was realized by compressed air). The microwave instrument consists of a continuous focused microwave power output from 0 to 800 W for this Monowave 300® apparatus. All the experiments in the microwave reactor were performed using stirring option. The target temperature was reached with a ramp of 3 min and the chosen microwave power stay constant to hold the mixture at this temperature. The reaction temperature is monitored using calibrated infrared sensor and the reaction time included the ramp period. The microwave irradiation parameters (power and temperature) were monitored by the Monowave software package of the Monowave 300® microwave reactor. Solvents were evaporated with a BUCHI rotary evaporator. All reagents and solvents were purchased from Acros, Sigma-Aldrich Chimie, TCI France and Fluka France and were used without further purification.
Tert-butyl (2-aminoethyl)carbamate (2a)
In a 250 ml two-necked round-bottomed flask, provided with magnetic stirrer and condenser, commercial 1,2-diaminoethane 1a (14.42 g, 16.03 ml, 0.24 mol.) was solubilized in 1,4-dioxane (69 ml). To this mixture was added dropwise a solution of di-tert-butyldicarbonate (6.5 g, 30 mmol.) in 85 ml of 1,4-dioxane over a period of 3 h at room temperature. After vigorous stirring at 25 °C during 12 h, the volatile compounds of the reaction mixture were removed in vacuo and to the crude reaction mixture was poured 150 ml of deionized water. The mixture was extracted with methylene chloride (5 × 50 ml), organic phases were collected and dried over anhydrous magnesium sulfate. The filtrate was concentrated in a rotary evaporator under reduced pressure and was dried under high vacuum (10−2 Torr) at 25 °C for 10 min. The desired carbamate 2a (1.25 g) was obtained as colorless mobile oil in 26 % yield and was further used without purification. 1H NMR (DMSO-d 6 ) δ: 1.20 (s, 9H, Me3C); 2.60 (t, 2H, J = 5.8 Hz, CH 2); 2.96 (t, 2H, J = 4.3 Hz, CH 2NH); 4.93 (br s, 2H, NH 2 ); 5.71 (br s, 1H, NH). 13C NMR (DMSO-d 6 ) δ: 28.2 (CH3); 41.1 (CH2); 66.8 (CH2NH); 78.7 (Me3 CO); 156.3 (NHCO). HRMS, m/z: 161.1289 found (calculated for C7H17N2O2 [M+H]+ requires 161.1290).
Tert-butyl (4-aminobutyl)carbamate (2b)
In a 250 ml two-necked round-bottomed flask, provided with magnetic stirrer and condenser, commercial 1,4-diaminobutane 1a (14.3 ml, 12.5 g, 0.14 mol.) was solubilized in 1,4-dioxane (69 ml). To this mixture was added dropwise a solution of di-tert-butyldicarbonate (6.5 g, 30 mmol.) in 85 ml of 1,4-dioxane over a period of 3 h at room temperature. After vigorous stirring at 25 °C during 12 h, the volatile compounds of the reaction mixture were eliminated in vacuo and to the crude reaction mixture was poured 150 ml of deionized water. The mixture was extracted with methylene chloride (5 × 50 ml), organic phases were collected and dried over anhydrous magnesium sulfate. The filtrate was concentrated in a rotary evaporator under reduced pressure and was dried under high vacuum (10−2 Torr) at 25 °C for 10 min. The desired carbamate 2b (1.58 g) was obtained as colorless mobile oil in 28 % yield and was further used without purification. 1H NMR (DMSO-d 6 ) δ: 1.34 (s, 9H; Me3CO); 1.40 (m, 4H, CH2); 2.61 (t, 2H, J = 6.7 Hz, CH2); 3.02 (m, 2H, CH2NH); 4.93 (br s, 2H, NH 2 ); 5.71 (br s, 1H, NH). 13C NMR (DMSO-d 6 ) δ: 27.2 (CH2); 28.4 (Me3CO); 29.5 (CH2); 40.2 (CH2); 41.1 (CH2); 78.9 (CO); 156.1 (NHCO). HRMS, m/z: 189.1600 found (calculated for C9H21N2O2 [M+H]+ requires 189.1603).
Tert-butyl {2-[4-(3-aminopropyl)piperazin-1-yl]propyl}carbamate (2c)
In a 250 ml two-necked round-bottomed flask, provided with magnetic stirrer and condenser, commercial 1,4-bis-(3-aminopropyl)piperazine 1c (4.32 ml, 4.21 g, 21 mol.) was solubilized in 1,4-dioxane (34 ml). To this mixture was added a solution of di-tert-butyldicarbonate (812 mg., 37 mmol.) in 16 ml of 1,4-dioxane over a period of 3 h at room temperature. After vigorous stirring at 25 °C during 12 h, the volatile compounds of the reaction mixture were eliminated in vacuo and to the crude reaction mixture was poured 80 ml of deionized water. The mixture was extracted with methylene chloride (5 × 25 ml), organic phases were collected and dried over anhydrous magnesium sulfate. The filtrate was concentrated in a rotary evaporator under reduced pressure and was dried under high vacuum (10−2 Torr) at 25 °C for 10 min. The desired carbamate 2c (3.52 g) was obtained as colorless mobile oil in 56 % yield and was further used without purification. 1H NMR (DMSO-d 6 ) δ: 1.37 (s, 9H, Me3C); 1.60 (m, 4H, CH2); 2.35 (m, 12H, CH2); 2.68 (t, 2H, J = 6.6 Hz, CH2NH2); 3.11 (m, 2H, CH2NH); 4.88 (br s, NH2); 5.42 (br s, 1H, NH). 13C NMR (DMSO-d 6 ) δ: 26.3 (CH2); 28.4 (Me3CO); 29.9 (CH2); 39.8 (CH2NH2); 40.6 (CH2NH); 53.1 (CH2N); 56.5 (NCH2); 78.7 (Me3CO); 156.1 (NHCO). HRMS, m/z: 301.2603 found (calculated for C15H33N4O2 [M+H]+ requires 301.2603).
Standard procedure for the preparation of 5-arylidene-2-thioxo-1,3-thiazolidine-4-one carbamates 6a-e under microwave irradiation
In a 10 ml glass tube was placed successively bis(carboxymethyl)trithiocarbonate 3 (0.2 g, 0.88 mmol., 1 equiv.), dimethoxyethane (1 ml), triethylamine (119 μl, 89 mg., 0.88 mmol., 1 equiv.), and carbamate 2 (0.88 mmol., 1 equiv.). The glass tube was sealed with a snap cap and placed in the Monowave 300® Anton Paar microwave cavity (P = 800 Watt). The reaction mixture was irradiated at 90 °C for 10 min. under vigorous magnetic stirring. After microwave dielectric heating, the crude reaction mixture was allowed to cool down at room temperature, aldehyde 5 (0.88 mol, 1 equiv.) was added to the cooled reaction mixture which was immediately submitted to microwave irradiation at 110 °C for 5 min. After cooling, the volatile compounds of the reaction mixture were eliminated in a rotary evaporator under reduced pressure. To the crude reaction mixture was added 2 ml of MeOH and after triturating, the insoluble product 6 was collected by filtration on a Büchner funnel (porosity N°4), washed with cooled methylene chloride (2 × 0.5 ml) and was dried under high vacuum (10−2 Torr) at 25 °C for 1 h. The desired compound 6 was further used without purification.
(5Z)-Tert-butyl [2-(5-(4-methoxybenzylidene)-4-oxo-2-thioxo-1,3-thiazolidin-3-yl)ethyl]carbamate (6a)
Compound 6a was prepared in 23 % yield (80 mg) as yellow powder from tert-butyl (2-aminoethyl)carbamate 2a (9 mg., 0.88 mmol.) and 4-methoxybenzaldehyde 5a (120 mg., 0.88 mmol.) according to the standard procedure. Mp = 134–136 °C. 1H NMR (DMSO-d 6 ) δ: 1.31 (s, 9H, Me3C); 3.25 (q, 2H, J = 5.1 Hz, CH2NH); 3.83 (s, 3H, OCH3); 4.10 (t, 2H, J = 5.3 Hz, CH2); 6.94 (br t, 1H, J = 6.1 Hz, NHCH2); 7.10 (d, 2H, J = 8,8 Hz, H-3′, Ar); 7.60 (d, 2H, J = 8,8 Hz, H-2′, Ar); 7,70 (s, 1H, CH=). 13C NMR (DMSO-d 6 ) δ: 28.1 (CMe3); 36.9 (CH2NH); 44.5 (CH2N); 55.5 (OCH3); 77.7 (OCMe3); 115.2 (C-3′, Ar); 119.4 (C-1′, Ar); 125.6 (C=); 132.5 (CH=); 132.7 (C-2′, Ar); 155.7 (NHCO); 161.4 (C-4′, Ar); 167.3 (C=O); 193.7 (C=S). HRMS, m/z: 417.0921 found (calculated for C18H22N2O4S2Na [M+Na]+ requires 417.0918).
(5Z)-Tert-butyl [2-(5-(1,3-benzodioxol-5ylmethylidene)-4-oxo-2-thioxo-1,3-thiazolidin-3-yl)ethyl]carbamate (6b)
Compound 6b was prepared in 59 % yield (212 mg) as yellow powder from tert-butyl (2-aminoethyl)carbamate 2a (9 mg., 0.88 mmol.) and 3,4-methylendioxybenzaldehyde 5b (132 mg., 0.88 mmol.) according to the standard procedure. Mp = 154–156 °C. 1H NMR (DMSO-d 6 ) δ: 1.32 (s, 9H, Me3C); 3.25 (q, 2H, J = 5.1 Hz, 2H, CH2NH); 4.10 (t, 2H, J = 5,3 Hz, 2H, CH2N); 6.15 (s, 2H, OCH 2 O); 6.93 (t, 1H, J = 6.1 Hz, 1H, NH); 7.10–7.20 (m, 3H, H-2′, H-3′, H-5′, Ar); 7.70 (s, 1H, CH=). 13C NMR (DMSO-d 6 ) δ: 28.1 (Me3C); 36.9 (CH2NH); 44.5 (CH2N); 77.7 (Me3C); 102.1 (OCH2O); 109.3 (C-2′, Ar); 109.5 (CH=); 120.1 (C=); 126.7 (C-5′, Ar); 127.3 (C-1′, Ar); 132.5 (C-6′, Ar); 148.3 (C-4′, Ar); 149.7 (C-3′, Ar); 155.7 (NHCO); 167.2 (C=O); 193.6 (C=S). HRMS, m/z: 431.0709 found (calculated for C18H20N2O5S2Na [M+Na]+ requires 431.0711).
(5Z)-Tert-butyl [2-(5-(4-methoxybenzylidene)-4-oxo-2-thioxo-1,3-thiazolidin-3-yl)butyl]carbamate (6c)
Compound 6c was prepared in 44 % yield (163 mg) as yellow powder from tert-butyl (2-aminobutyl)carbamate 2b (166 mg., 0.88 mmol.) and 4-methoxybenzaldehyde 5a (120 mg., 0.88 mmol.) according to the standard procedure. Mp = 142–146 °C. 1H NMR (DMSO-d 6 ) δ: 1.35 (s, 9H, Me3C); 1.61 (quint, 2H, J = 7.4 Hz, CH2); 2.91 (q, 2H, J = 6.2 Hz, CH2NH); 3.83 (s, 3H, OCH 3 ); 4.00 (t, 2H, J = 7 Hz, CH2N); 6.80 (br s, 1H, NH); 7.12 (d, 2H, J = 9.1 Hz, H-3′, Ar); 7.60 (d, 2H, J = 8.9 Hz, H-2′, Ar); 7.70 (s, 1H, CH=). 13C NMR (DMSO-d 6 ) δ: 23.9 (CH2); 26.7 (CH2); 28.1 (Me3C); 40.3 (CH2N); 55.6 (OCH3); 77.4 (Me3C); 115.2 (C-3′, Ar); 119.0 (C=); 125.5 (C-1′, Ar); 132.9 (C-2′, Ar); 133.2 (CH=); 155.5 (NHCO); 161.5 (C-4′, Ar); 167.0 (C=O); 193.3 (C=S). HRMS, m/z: 445.1234 found (calculated for C20H26N2O4S2Na [M+Na]+ requires 445.1232).
(5Z)-Tert-butyl [2-(5-(1,3-benzodioxol-5ylmethylidene)-4-oxo-2-thioxo-1,3-thiazolidin-3-yl)butyl]carbamate (6d)
Compound 6d was prepared in 35 % yield (134 mg) as yellow powder from tert-butyl (2-aminobutyl)carbamate 2b (166 mg., 0.88 mmol.) and 3,4-methylenedioxybenzaldehyde 5b (132 mg., 0.88 mmol.) according to the standard procedure. Mp = 226–228 °C. 1H NMR (DMSO-d 6 ) δ: 1.40 (s, 9H, Me3C); 1.44 (m, 2H, CH2); 1.67 (m, 2H, CH2); 2.97 (q, 2H, J = 6.8 Hz, CH2NH); 4.07 (t, 2H, J = 7.2 Hz, CH2N); 6.13 (s, 2H, OCH 2 O); 6.42 (br s, 1H, NH); 7.07–7.20 (m, 3H, H-2′, H-5′, H-6′, Ar); 7.72 (s, 1H, CH=). 13C NMR (DMSO-d 6 ) δ: 23.9 (CH2); 26.8 (CH2); 28.2 (Me3C); 44.0 (CH2NH); 56.0 (CH2N); 77.5 (CMe3); 102.1 (OCH2O); 109.2 (C-2′, Ar); 109.4 (C-5′, Ar); 120.0 (C=); 126.8 (C-6′, C ipso , Ar); 127.3 (C-1′, C ipso , Ar); 133.1 (CH=); 148.4 (C-4′, Ar); 149.8 (C-3′, Ar); 155.5 (NHCO); 166.9 (C=O); 193.1(C=S). HRMS, m/z: 459.1023 found (calculated for C20H24N2O5S2Na [M+Na]+ requires 459.1024).
(5Z)-Tert-butyl [(4-{[5-(1,3-benzodioxol-5-ylmethylidene)-4-oxo-2-thioxo-1,3-thiazolidin-3-yl]propyl}piperazin-1-yl)propyl]carbamate (6e)
Compound 6e was prepared in 36 % yield (174 mg) as yellow powder from tert-butyl {2-[4-(2-aminopropyl)piperazin-1-yl]propyl}carbamate 2c (263 mg., 0.88 mmol.) and 3,4-methylenedioxybenzaldehyde 5b (132 mg., 0.88 mmol.) according to the standard procedure. Mp = 132–134 °C. 1H NMR (DMSO-d 6 ) δ: 1.34 (s, 9H, Me3C); 1.41 (m, 2H, CH2); 1.80 (m, 2H, CH2); 2.10 (t, 4H, J = 7.1 Hz, 4H, CH2N); 2,25 (m, 8H, 4xNCH2); 2.84 (q, 2H, J = 6.1 Hz, CH2NH); 4.08 (t, 2H, J = 6.9 Hz, CH2N); 6.14 (s, 2H, OCH 2 O); 6.73 (s, 1H, NH); 7.10–7.21 (m, 3H, H-2′, H-5′, H-6′, Ar); 7.72 (s, 1H, CH=). 13C NMR (DMSO-d 6 ) δ: 26.5 (CH2); 28.1 (CMe3); 38.3 (CH2); 40.0 (CH2NH); 43.2 (CH2N); 52.8 (CH2N); 52.9 (CH2N); 55.5 (CH2N); 77.3 (CMe3); 102.2 (OCH2O); 109,3 (CH=); 109.5 (C-2′, Ar); 120,0 (C=); 126.8 (C-5′, Ar); 127.3 (C-1′, Ar); 132.9 (C-6′, Ar); 148.3 (C-4′, Ar); 149.8 (C-3′, Ar); 155.5 (NHCO); 167.2 (C=O); 193.0 (C=S). HRMS, m/z: 549.2195 found (calculated for C26H37N4O5S2 [M+H]+ requires 549.2205).
Standard procedure for the preparation of salts 7a–e after deprotection of the 5-arylidene-2-thioxo-1,3-thiazolidine-4-one carbamates 6
In a 50 ml two-necked round-bottomed flask provided with a magnetic stirrer and condenser, carbamate 6 (0.4 mmol., 1 equiv.) was solubilized in 1,4-dioxane (2 ml) at room temperature under vigorous stirring during 10 min. To this homogeneous solution was added dropwise for 30 min; a solution of 6 M HCl (2 ml) in 1,4-dioxane (2 ml). The reaction mixture was stirred during 4 h at 25 °C and was concentrated in a rotary evaporator under reduced pressure for elimination of volatile compounds. To the crystallized crude reaction mixture was added 5 ml of Et2O and after triturating, the insoluble salt 7 was collected by filtration, then washed with 2 × 5 ml of Et2O. The desired salt 7 was dried under high vacuum (10−2 Torr) at 25 °C for 1 h that gave a yellowish powder and was further used without purification.
(5Z)-3-(2-Aminoethyl)-5-(4-methoxybenzylidene)-2-thioxo-1,3-thiazolidin-4-one hydrochloride (7a)
Compound 7a was prepared in 91 % yield (107 mg) as yellow powder from (5Z)-tert-butyl [2-(5-(4-methoxybenzylidene)-4-oxo-2-thioxo-1,3-thiazolidin-3-yl)ethyl]carbamate 6a (158 mg., 0.4 mmol.) according to the standard procedure. Mp = 260–262 °C. 1H NMR (DMSO-d 6 ) δ: 3.13 (t, 2H, J = 6 Hz, CH2); 3.84 (s, 3H, OCH 3 ); 4.20 (t, 2H, J = 6 Hz, CH2); 7.13 (d, 2H, J = 8.9 Hz, H-3′, Ar); 7.63 (d, 2H, J = 8.8 Hz, H-2′, Ar); 7.79 (s, 1H, CH=); 7.94 (br s, 2H, NH2). 13C NMR (DMSO-d 6 ) δ: 36.2 (CH2); 41.7 (CH2); 55.6 (OCH3); 115.2 (C-3′, Ar); 119.4 (C=); 125.5 (C-1′, Ar); 132.8 (C-2′, Ar); 132.9 (CH=); 161.5 (C-4′, Ar); 167.5 (C=O); 193.8 (C=S). HRMS, m/z: 295.0577 found (calculated for C13H15N2O2S2 [M+H]+ requires 295.0575); 278.0316 found (calculated for C13H12NO2S2 [M-NH3+H]+ requires 278.0309).
(5Z)-3-(2-Aminoethyl)-5-(1,3-benzodixol-5-ylmethylidene)-2-thioxo-1,3-thiazolidin-4-one hydrochloride (7b)
Compound 7b was prepared in 98 % yield (121 mg) as yellow powder from (5Z)-tert-butyl [2-(5-(1,3-benzodixol-5-ylmethylidene)-4-oxo-2-thioxo-1,3-thiazolidin-3-yl)ethyl]carbamate 6b (163 mg., 0.4 mmol.) according to the standard procedure. Mp = 260–262 °C. 1H NMR (DMSO-d 6 ) δ: 3.13 (t, 2H, J = 5,9 Hz, CH2NH2); 4.26 (t, 2H, J = 5.9 Hz, CH2N); 6.15 (s, 2H, OCH2O); 7.11–7.25 (m, 3H, H-2′, H-5′, H-6′, Ar); 7.75 (s, 1H, CH=); 7.87 (br s, 2H, NH 2 ). 13C NMR (DMSO-d 6 ) δ: 36.3 (CH2NH); 41.7 (CH2N); 102.2 (OCH2O); 109.4 (CH=); 109.6 (C-2′, Ar); 120.1 (C=); 126.9 (C-5′, Ar); 127.1 (C-1′, Ar); 133.0 (C-6′, Ar); 148.4 (C-4′, Ar); 149.9 (C-3′, Ar); 167.5 (C=O); 193.7 (C=S). HRMS, m/z: 309.0369 found (calculated for C13H12N2O3S2 [M+H]+ requires 309.0367); 292.0109 found (calculated for C13H10NO3S2 [M-NH3+H]+ requires 292.0102).
(5Z)-3-(4-Aminobutyl)-5-(1,3-benzodixol-5-ylmethylidene)-2-thioxo-1,3-thiazolidin-4-one hydrochloride (7c)
Compound 7c was prepared in 65 % yield (87 mg) from (5Z)-tert-butyl [2-(5-(1,3-benzodixol-5-ylmethylidene)-4-oxo-2-thioxo-1,3-thiazolidin-3-yl)butyl]carbamate 6d (174 mg., 0.4 mmol.) according to the standard procedure. Mp = 259–260 °C. 1H NMR (DMSO-d 6 ) δ: 1.16 (m, 2H, CH2); 1.75 (m, 2H, CH2); 2.86 (m, 2H, CH2NH2); 4.12 (t, 2H, J = 6.7 Hz, CH2N); 6.22 (s, 2H, OCH2O); 7.17–7.28 (m, H-2′, H-5′, H-6′, Ar); 7.82 (s, 1H, CH=). 13C NMR (DMSO-d 6 ) δ: 23.7 (CH2); 38.3 (CH2); 43.6 (CH2NH2); 66.3 (CH2N); 102.2 (OCH2O); 109.4 (C-2); 109.6 (C-5′, Ar); 119.6 (C=); 127.1 (C-6′, Ar); 133.3 (CH=); 148.4 (C-4′, Ar); 149.9 (C-3′, Ar); 167.1 (C=O); 195.3 (C=S). HRMS, m/z: 337.0681 found (calculated for C15H17N2O3S2 [M+H]+ requires 337.0681).
Standard procedure for the preparation of the linked bis-N,N’-(5-arylidene rhodanin-3-yl) derivatives 9 under microwave irradiation
In a 10 ml glass tube was placed successively bis(carboxymethyl)trithiocarbonate 3 (0.13 g, 0.58 mol., 1 equiv.), dimethoxyethane (1 ml), triethylamine (155 μl, 116 mg., 1.16 mmol., 2 equiv.), and hydrochloride salt 7 (0.58 mmol., 1 equiv.). The glass tube was sealed with a snap cap and placed in the Monowave 300® Anton Paar microwave cavity (P = 800 Watt). The reaction mixture was irradiated at 90 °C for 10 min. under vigorous magnetic stirring. After microwave dielectric heating, the crude reaction mixture was allowed to cool down at room temperature, aldehyde 5 (0.58 mol, 1 equiv.) was added to the cooled reaction mixture which was immediately submitted to microwave irradiation at 110 °C for 5 min. After cooling, the volatile compounds of the reaction mixture were eliminated in a rotary evaporator under reduced pressure. To the crude reaction mixture was added 2 ml of MeOH and after triturating, the insoluble product 9 was collected by filtration on a Büchner funnel (porosity N°4), washed successively with cooled hexane (5 × 2 ml), methylene chloride (3 × 1 ml) and was dried under high vacuum (10−2 Torr) at 25 °C for 1 h. The desired compound 9 was purified by recrystallization in EtOH.
(5Z)-5-Benzylidene-3-{2-[(5Z)-5-(4-methoxybenzylidene)-4-oxo-2-thioxo-1,3-thiazolidin-3-yl]ethyl}-2-thioxo-1,3-thiazolidin-4-one (9a)
Compound 9a was prepared in 47 % yield (136 mg) as yellow powder from (5Z)-3-(2-aminoethyl)-5-(4-methoxybenzylidene)-2-thioxo-1,3-thiazolidin-4-one hydrochloride 7a (363 mg., 0.58 mmol.) and benzaldehyde 5c (62 mg., 0.58 mmol.) according to the standard procedure. Mp = 222–224 °C. 1H NMR (DMSO-d 6 ) δ: 3.84 (s, 3H, OCH3); 4.43 (s, 4H, CH2); 7.11 (d, 2H, J = 8.8 Hz, H-3, Ar); 7.53–7.63 (m, 7H, H-2, H-2′, H-3′, H-4′, Ar); 7.78 (s, 1H, CH=); 7.83 (s, 1H, CH=). 13C NMR (DMSO-d 6 ) δ: 40.4 (CH2N); 55.7 (OCH3); 115.1 (C-3, Ar); 118,5 (C=); 121.8 (C-1, C ipso , Ar); 125.4 (C-1′, C ipso , Ar); 129.5 (C-2′, Ar); 130.8 (C-3′, C-4′, Ar); 132.8 (C-2, Ar); 133.1 (CH=); 133.4 (CH=); 161.6 (C-4, C ipso , Ar); 167.2 (C=O); 193.7 (C=S). HRMS, m/z: 521.0097 found (calculated for C23H18N2O3S4Na [M+Na]+ requires 521.0098).
(5Z)-5-(1,3-Benzodioxol-5-ylmethylidene)-3-{2-[(5Z)-5-benzylidene-4-oxo-2-thioxo-1,3-thiazolidin-3-yl]ethyl}-2-thioxo-1,3-thiazolidin-4-one (9b)
Compound 9b was prepared in 43 % yield (128 mg) from (5Z)-3-(2-aminoethyl)-5-(1,3-benzodixol-5-ylmethylidene)-2-thioxo-1,3-thiazolidin-4-one hydrochloride 7b (200 mg., 0.58 mmol.) and benzaldehyde 5c (62 mg., 0.58 mmol.) according to the standard procedure. Mp = 260–262 °C. 1H NMR (DMSO-d 6 ) δ: 4.43 (t, 4H, J = 3.9 Hz, CH2); 6.15 (s, 2H, OCH2O); 7.10–7.23 (m, 3H, H-2′, H-5′, H-6′, Ar); 7.53 (d, 3H, J = 7.3 Hz, H-3′, H-4′, Ar); 7.66 (d, 2H, J = 5.7 Hz, H-2′, Ar); 7.75 (s, 1H, CH=); 7.83 (s, 1H, CH=). 13C NMR (DMSO-d 6 ) δ: 41.5 (CH2); 102.2 (OCH2O); 109.4 (C-2, Ar); 109.7 (C-5, Ar); 119.1 (C=); 119.4 (C=); 121.8 (C-6, Ar); 121.8 (C-1, C ipso , Ar); 127.2 (C-2′, Ar); 129.5 (C-3′, Ar); 130.8 (C-4′, Ar); 132.7 (CH=); 133.4 (C-1′, C ipso , Ar); 148.4 (C-3, C ipso , Ar); 150,0 (C-4, C ipso , Ar); 167.2 (C=O); 193.6 (C=S). HRMS, m/z: 513.0070 found (calculated for C23H17N2O4S4 [M+H]+ requires 513.0071).
(5Z)-5-(1,3-Benzodioxol-5-ylmethylidene)-3-{2-[(5Z)-5-(4-methoxybenzylidene)-4-oxo-2-thioxo-1,3-thiazolidin-3-yl]ethyl}-2-thioxo-1,3-thiazolidin-4-one (9c)
Compound 9c was prepared in 27 % yield (85 mg) from (5Z)-3-(2-aminoethyl)-5-(1,3-benzodixol-5-ylmethylidene)-2-thioxo-1,3-thiazolidin-4-one hydrochloride 7b (200 mg., 0.58 mmol.) and 4-methoxybenzaldehyde 5a (79 mg., 0.58 mmol.) according to the standard procedure. Mp = 164–166 °C. 1H NMR (DMSO-d 6 ) δ: 3.84 (s, 3H, OCH3); 4.41 (s, 4H, CH2); 6.15 (s, 2H, OCH2O); 7.10 (d, 2H, J = 8.8 Hz, H-2′, Ar); 7.09-7.20 (m, 7H, H-2, H-5, H-6, Ar); 7.60 (d, 2H, J = 8.8 Hz, H-3′, Ar); 7.74 (s, 1H, CH=); 7.78 (s, 1H, CH=). 13C NMR (DMSO-d 6 ) δ: 41.6 (CH2); 55.6 (OCH3); 99.5 (OCH2O); 115.2 (C-3′, C-2, Ar); 118.5 (C=); 119.1 (C-1′, C ipso , Ar); 125.4 (C-1, C ipso , Ar); 127.0 (C-6, Ar); 133.1 (C-2′, C-5, Ar); 133.4 (CH=); 148.4 (C-4, C ipso , Ar); 150.0 (C-3, C ipso , Ar); 161.6 (C-4′, C ipso , Ar); 167.2 (C=O); 213.1 (C=S). HRMS, m/z: 543.0172 found (calculated for C21H19N2O4S4 [M+H]+ requires 543.0177).
Cell culture and survival assays
Skin diploid fibroblastic cells were provided by BIOPREDIC International Company (Rennes, France). Caco2 (Ref ECACC: 86010202), Huh-7D12 (Ref ECACC: 01042712), MDA-MB-231 (Ref ECACC: 92020424), HCT-116 (Ref ECACC: 91091005), PC3 (Ref ECACC: 90112714) and NCI-H727 (Ref ECACC: 94060303) cell lines were obtained from the ECACC collection. Cells were grown according to ECACC recommendations (Nakabayashi et al., 1982). The toxicity test of the compounds on these cells was as follows: 2 × 103 cells for HCT-116 cells or 4 x 103 for the other cells were seeded in 96 multiwell plates in triplicate and left for 24 h for attachment, spreading, and growing. Then, cells were exposed for 48 h to increasing concentrations of the compounds, ranging from 0.1 to 25 μM in a final volume of 120 μl of culture medium. Cells were fixed in cooled solution of acetic acid/ethanol (90:5 %), nuclei were stained with Hoechst 3342 (Sigma) and counted using automated imaging analysis (Cellomics Arrayscan VTI/HCS Reader, Thermo/Scientific). The IC50 were graphically determined.
References
Aranda S, Laguna A, de la Luna S (2011) DYRK family of protein kinases: evolutionary relationships, biochemical properties, and functional roles. FASEB J 25:449–462
Bazureau JP, Draye M (2011) Ultrasound and microwaves: recent advances in organic chemistry, 1st edn. Transworld Research Network, Kerala
Bazureau JP, Carreaux F, Renault S, Meijer L, Lozach O (2009) Imidazolone derivatives, preparation method thereof and biological use of same. Patent WO 2009/05032 A2, 23 April 2009
Bonger KM, Hoogendoorn S, van Koppen CJ, Timmers CM, Overkleeft HS, van der Marel GA (2009) Synthesis and pharmacological evaluation of dimeric follicle-stimulating hormone receptor antagonists. ChemMedChem 4:2098–2102
Coulibaly WK, Paquin L, Bénie A, Bekro YA, Durieu E, Meijer L, Bazureau JP (2012a) Synthesis of N,N’-bis(5-arylidene-4-oxo-3,5-dihydro-4H-imidazol-2-yl)diamines bearing various linkers and biological evaluation as potential inhibitors of kinases. Eur J Med Chem 58:581–590
Coulibaly WK, Paquin L, Bénie A, Bekro YA, Durieux E, Rucheau S, Meijer L, Le Guével R, Corlu A, Bazureau JP (2012b) Synthesis of new N,N’-bis(5-arylidene-4-oxo-4,5-dihydro-thiazolidine-2-yl)piperazine derivatives under microwave irradiation and preliminary biological evaluation. Sci Pharm 80:825–836
Cutshall NS, O’Day C, Prezhdo M (2005) Rhodanine derivatives as inhibitors of JSP-1. Bioorg Med Chem Lett 15:3374–3382
de la Hoz A, Loupy A (2012) Microwaves in organic synthesis, 3rd edn. Wiley-VCH, Weinheim
Debdab M, Carreaux F, Renault S, Soundararajan M, Fedorov O, Filippakopoulos P, Lozach O, Babault L, Tahtouh T, Baratte B, Ogawa Y, Hagiwara M, Eisenreich A, Rauch U, Knapp S, Meijer L, Bazureau JP (2011) Design, synthesis and biological evaluation of leucettines, a class of potent CLK and DYRK kinases inhibitors derived from the marine sponge leucettamine B. Modulation of alternative RNA splicing. J Med Chem 54:4172–4186
Guiheneuf S, Paquin L, Carreaux F, Durieu E, Roisnel T, Meijer L, Bazureau J-P (2014) New 5-ylidene rhodanine derivatives based on the dispacamide A model. Mol Divers 18:375–388
Holmberg B (1910) Estersäuren von schwefelsubstituierter kohlensäure mit aliphatischen alkoholsäuren. J Prakt Chem 81:451–465
Hotta N, Akanuma Y, Kawamori R, Matsuoka K, Oka Y, Shichiri M, Toyata T, Nakoshima M, Yoshimura I, Sakamoto N, Shigeta Y (2006) Long-Term clinical effects of epalrestat, an aldose reductase inhibitor, on diabetic peripheral neuropathy: the 3-year, multicenter, comparative aldose reductase inhibitor-diabetes complications trial. Diabetes Care 29:1538–1541
Ionescu A, Dufrasne F, Gelbcke M, Jabin I, Kiss R, Lamoral-Theys D (2012) DYRK1A kinase inhibitors with emphasis on cancer. Mini Rev Med Chem 12:1315–1329
Krstenansky JL, Cotteril I (2000) Recent advances in microwave-assisted organic syntheses. Curr Opin Drug Discovery Dev 3:454–461
Lesyk RB, Zimenkovsky BS (2004) 4-Thiazolidones: centenarian history, current status and perspectives for modern organic and medicinal chemistry. Curr Org Chem 8:1547–1577
Ling Y, Wang Z-Q, Xiao YA, Zhu C, Shen L, Wang X-M, Hui Y, Wang X-Y (2013) Benzylidene 2-aminoimidazolones derivatives: synthesis and in vitro evaluation of anti-tumor carcinoma activity. Chem Pharm Bull 61:1081–1084
Maga G, Falchi F, Garbelli A, Belfiore A, Witvrow M, Manetti F, Botta M (2008) Pharmacophore modeling and molecular docking led to the discovery of inhibitors of human immunodeficiency virus-1. Replication targeting the human cellular aspartic acid-glutamic acid-alanine-aspartic acid box polypeptide 3. J Med Chem 51:6635–6638
Mentgen T, Steuer C, Klein CD (2012) Privileged scaffolds or promiscuous binders: a comparative study on rhodanines and related heterocycles in medicinal chemistry. J Med Chem 55:743–753
Nakabayashi H, Taketssa K, Miyano K, Yamane T, Sato J (1982) Growth of human hepatoma cell lines with differentiated functions in chemically defined medium. Cancer Res 42:3858–3863
Radi M, Botta L, Casaluce G, Bernardini M, Botta M (2010) Practical one-pot two-step protocol for the microwave-assisted synthesis of highly functionalized rhodanine derivatives. J Comb Chem 10:200–205
Singh SP, Parmar SS, Raman K, Stenberg VI (1981) Chemistry and biological activity of thiazolidinones. Chem Rev 81:175–203
Smith B, Medda F, Gokhale V, Dunckley T, Hulme C (2012) Recent advances in the design, synthesis, and biological evaluation of selective DYRK1A inhibitors: a new avenue for a disease modifying treatment of Alzheimer’s? ACS Chem Neurosci 11:857–872
Tahtouh T, Elkins JM, Filippakopoulos P, Soundararajan M, Burgy G, Durieu E, Cochet C, Schmid RS, Lo DC, Delhommel F, Oberholzer AE, Pearl LH, Carreaux F, Bazureau JP, Knapp S, Meijer L (2012) Selectivity, cocrystal structures, and neuroprotective properties of leucettines, a family of protein kinase inhibitors derived from the marine sponge alkaloid leucettamine B. J Med Chem 55:9312–9330
Tomasic T, Masic LP (2009) Rhodanine as a privileged scaffold in drug discovery. Curr Med Chem 16:1596–1629
Xia Z, Knaak C, Ma J, Beharry ZM, Mc Innes C, Wang W, Kraft AS, Smith CD (2009) Synthesis and evaluation of novel inhibitors of Pim-1 and Pim-2 protein kinases. J Med Chem 52:74–86
Xiao YA, Wang ZQ, Wang XM, Hui Y, Ling Y, Wang XY, He LQ (2013) Synthesis and in vitro biological evaluation of novel 2-aminoimidazolinone derivatives as anti-tumor agents. Chin Chem Lett 24:727–730
Acknowledgments
One of us (K.W.C.) wishes to thank the Agence Universitaire de la Francophonie AUF, contract N°0486″ for a research fellowship. Financial support of this program carried out under the French National Cancer Institute “Cancéropôle Grand Ouest” by contracts PRIR 04-8390 and ACI 04-2254, is gratefully acknowledged.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Coulibaly, W.K., Paquin, L., Bénie, A. et al. Prospective study directed to the synthesis of unsymmetrical linked bis-5-arylidene rhodanine derivatives via “one-pot two steps” reactions under microwave irradiation with their antitumor activity. Med Chem Res 24, 1653–1661 (2015). https://doi.org/10.1007/s00044-014-1186-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-014-1186-7