Abstract
The aim of this study is to investigate chemical composition of acetone extract of the lichen Hypogymnia physodes and antioxidant, -microbial, and -cancer activities of some its major metabolites. Four depsidones (fumarprotocetraric-, 3-hydroxyphysodalic-, physodalic-, and physodic acids), two depsides (atranorin and chloroatranorin), and usnic acid were identified from the lichen H. physodes growing in Serbia using high-performance liquid chromatography with photodiode array detector. Antioxidant activity of isolated metabolites was evaluated by free radical scavenging, superoxide anion radical scavenging, and reducing power. As a result of this study physodic acid was found to have stronger antioxidant activities. The antimicrobial activity was estimated by determination of the minimal inhibitory concentration by the broth microdilution method. All isolated compounds were highly active with minimum inhibitory concentration values ranging from 0.0008 to 1 mg/ml. Anticancer activity was tested against FemX (human melanoma) and LS 174 (human colon carcinoma) cell lines using microculture tetrazolium method. Tested compounds were found have strong anticancer activity toward both cell lines with IC50 values ranging from 12.72 ± 0.35 to 24.63 ± 2.15 μg/ml. The present study shows that isolated lichen compounds demonstrated strong antioxidant, -microbial, and -cancer effects. The results suggest that this lichen can be used as new sources of the natural antimicrobial agents, antioxidants, and -cancer compounds.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lichens are complex symbiotic associations between a fungus (mycobiont) and photobiont which can be either an alga or cyanobacterium (Bates et al., 2011). They have been proven to be the earliest colonizers of terrestrial habitats on the earth with a worldwide distribution from arctic to tropical regions and from the plains to the highest mountains. Their specific, even extreme, range of habitats, slow growth, and long life are the reason for them being able to produce numerous protective secondary metabolites against different physical and biological influences (Mitrović et al., 2011a, b).
Lichens’ secondary metabolites are synthesized mostly from fungal metabolism. They are crystal deposits on the surface of hiphes, which are badly dilutable in water and can usually be isolated from lichens by organic dilutants (Otzurk et al., 1999). More than 100 secondary metabolites of lichen have been detected and isolated (Molnar and Farkaš, 2010). Chemical structures of classes of these compounds are similar and identification is often very difficult.
Lichen substances exert a wide variety of biological actions including antibiotic, -mycotic, -viral, -inflammatory, analgesic, antipyretic, -proliferative, and cytotoxic effects (Kosanić et al., 2012a, b; Manojlović et al., 2010). Due to a relatively recent resurgence in lichen bioactivity, the therapeutic potential of many classes of lichen metabolites in medicine has largely remained unexplored. Thus, the aim of the present work was to present results of high-performance liquid chromatography (HPLC) analysis of the acetone extract of the lichen Hypogymnia physodes and evaluate the antioxidant capacity, antimicrobial and cytotoxic activities of its major secondary metabolites.
Results and discussion
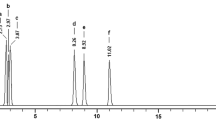
This paper deals with the phytochemical analysis of acetone extract from the species H. physodes, lichen growing in Serbia. For the phytochemical analysis, a HPLC–UV method was used for the identification of phenolic compounds, specially depsides, depsidones, and usnic acid, in this lichen. Comparing the retention times (t R) and UV spectra (200–400 nm) from HPLC–UV with those of authentic substances, it is readily confirmed that the fumarprotocetraric- (t R = 3.12 ± 0.10 min), 3-hydroxyphysodalic- (t R = 3.98 ± 0.1 min), physodalic- (t R = 4.31 ± 0.10 min), physodic- (t R = 5.91 ± 0.10 min), usnic acids (t R = 14.32 ± 0.20 min), atranorin (t R = 16.21 ± 0.10 min), and chloroatranorin (t R = 17.73 ± 0.20 min) are the metabolites present in acetone extract of H. physodes. The chromatograms for standards and H. physodes acetone extract eluted by HPLC are represented in Figs. 1 and 2. Table 1 shows the retention time of the detected lichen substances and their absorbance maxima (nm). Identification of these compounds was achieved by comparison of their retention times values with the standard substances previously isolated from lichens. Compounds identified in the extract mainly belong to depsidones (fumarprotocetraric-, 3-hydroxyphysodalic-, physodalic-, and physodic acids), some of them are depsides (atranorin and chloroatranorin), while usnic acid is well-known lichen metabolite with dibenzofuran structure which exhibits antibiotic activity. The structures of the detected compounds are shown in Fig. 3. After detection of the present compounds in lichens, major lichen metabolites in this species (usnic acid, atranorin, and physodic acid) were isolated by chromatographic column using different solvent systems and used for antioxidant, -microbial, and -cancer investigations.
HPLC chromatograms acquired at 254 nm of the acetone extract of Hypogymnia physodes. Chromatographic peaks identities are reported in Table 1
The scavenging of 1,1-diphenyl-2-picryl-hydrazyl (DPPH)- and superoxide anion radicals by the tested compounds is shown in Table 2. There was a statistically significant difference between tested samples and control (P < 0.05). Physodic acid showed stronger DPPH- and superoxide anion radicals scavenging activity than usnic acid and atranorin. The inhibition concentration at 50 % inhibition (IC50) values were 69.110 ± 0.35 and 169.65 ± 1.78 μg/ml for physodic acid, 130.73 ± 1.35 and 197.28 ± 1.82 μg/ml for usnic acid, and 131.48 ± 1.93 and 632.01 ± 2.13 μg/ml for atranorin (for DPPH- and superoxide anion radicals scavenging activity, respectively). As shown in Table 3, physodic acid also demonstrated the strongest reducing power.
Various antioxidant activities were compared to ascorbic acid. The results showed that the standard antioxidant had stronger activity than tested compounds.
The antimicrobial activity of the lichen components against the test microorganisms is shown in Table 4. The minimal inhibitory concentration (MIC) for components relative to the tested microorganisms ranged from 0.0008 to 1 mg/ml. Usnic- and physodic acids showed very strong and similar antimicrobial activity, followed by atranorin. Antibacterial activity was stronger than antifungal activity for all compounds.
The antimicrobial activity was compared with the standard antibiotics, streptomycin (for bacteria) and ketoconazole (for fungi). The results showed that standard antibiotics have similar activity as tested compounds as shown in Table 2. In a negative control, dimethyl sulfoxide (DMSO) had no inhibitory effect on the tested organisms.
The cytotoxic activity of the tested compounds against the tested cell lines is shown in Table 5. The tested samples exhibited high cytotoxic activity against the target cells in vitro. The IC50 value for all compounds relative to the tested cells ranged from 12.72 ± 0.35 to 24.63 ± 2.15 μg/ml. The best cytotoxic activity was exhibited by usnic acid, followed by physodic acid and atranorin.
As a shown in the Table 5, positive control cis-diamminedichloroplatinum (cis-DDP) had slightly better cytotoxic activity than the tested components.
Some extracts from H. physodes lichen have been previously investigated for their biological activity. Mitrović et al. (2011a, b) published the results of antioxidant, -microbial, and -proliferative activities of methanol extract of H. physodes. Stojanović et al. (2010) also reported reducing power and radical scavenging activity for this species. Cansaran-Duman et al. (2010) found the antimicrobial effect by various Hypogymnia species.
Since in literature there is no data on biological activity of secondary metabolites from H. physodes lichen, here we report in vitro antioxidant, -microbial, and -cancer activities of compounds usnic acid, atranorin, and physodic acid isolated from this species.
The tested lichen compounds have a strong antioxidant activity against various oxidative systems in vitro. The isolated components belong to phenols, indicating an important role of phenol in the antioxidant activity for lichens. In fact, number of previous studies found that the lichens which were found to have higher content of phenols exert stronger antioxidant activity (Behera et al., 2009; Kosanić et al., 2012a). In most lichens, phenols, including depsides, depsidones, and dibenzofurans, are important antioxidants because of their ability to scavenge free radicals such as singlet oxygen, superoxide, and hydroxyl radicals (Kosanić et al., 2012b). However, some authors believe that the antioxidant activity of lichens may not be necessarily correlated with the content of polyphenolics (Odabasoglu et al., 2004), suggesting that the antioxidant activity of different lichens may also depend on other non-phenol components.
Antioxidant effect of some other lichen compounds was also studied by other researchers. For example, Luo et al. (2009) found antioxidant activity for lecanoric acid from Umbilicaria antarctica. Hidalgo et al. (1994) find antioxidant activity of atranorin isolated from Placopsis sp. and divaricatic acid isolated from Protousnea malaceae. Amo de Paz et al. (2010) explored antioxidant properties of salazinic-, stictic-, and usnic acids from Xanthoparmelia sp.
In our experiments, the tested lichen compounds show very strong antimicrobial activity. This means that lichen components are responsible for the antimicrobial activity of lichens. Differences in antimicrobial activity of different species of lichens are probably a consequence of the presence of different components with antimicrobial activity (Kosanić et al., 2012b). However, it is necessary understand that lichens contain a large number of natural compounds, and their antimicrobial activity is not only a result of the different activities of individual components but may also be the result of their interactions, which can have different effects on the overall activity of lichens.
The intensity of the antimicrobial effect depended on the species of organism tested. The compounds used in this study had a stronger antibacterial than -fungal activity. Also, the tested compounds showed more potent inhibitory effects on Gram-positive bacteria than on -negative. This observation is in accordance with other studies (Yang and Anderson, 1999; Kosanić et al., 2012b; Agrawal and Talele, 2013) focused on the antimicrobial activity which have demonstrated that Gram-positive bacteria are more sensitive to the antimicrobial activity than the Gram-negative bacteria and fungi due to differences in the composition and permeability of the cell wall. The cell wall of Gram-positive bacteria is made of peptidoglycans and teichoic acids, while the cell wall of Gram-negative bacteria is made of peptidoglycans, lipopolysaccharides, and proteins (Heijenoort, 2001; Kosanić et al., 2012a). The cell wall of fungi is poorly permeable and it consists of polysaccharides such as hitchin and glucan (Farkaš, 2003).
Numerous lichen compounds were screened for antimicrobial activity in search of the new antimicrobial agents. Candan et al. (2006) find an antimicrobial activity for gyrophoric acid from Xanthoparmelia pokornyi. Similar results were reported by Turk et al. (2006) for atranorin from Pseudoevernia furfuraceae. Kosanić and Ranković (2011) found out that fumarprotocetraric acid from Cladonia furcata had a strong antimicrobial influence.
In present study, the results clearly demonstrated that isolated compounds from H. physodes induced significant cytotoxic effect on the tested cancer cell lines. Until now, only few researchers proved that lichen compounds have anticancer activity. Einarsdottir et al. (2010) reported significant anticancer effect for (+)-usnic acid from Cladonia arbuscula and (−)-usnic acid from Alectoria ochroleuca. Bogo et al. (2010) explored anticancer properties of lecanoric acid from Parmoterma tinctorum. Burlando et al. (2009) found anticancer activity for several lichen compounds. Some literature data reported that lichen components are responsible for overall anticancer activities of lichens (Bucar et al., 2004; Burlando et al., 2009). However, it is difficult to determine the contribution of individual components for the overall anticancer effect. Often, the activity of lichens may be the result of synergistic or antagonistic effect of several compounds.
Conclusions
In conclusion, it can be stated that tested lichen compounds have a strong antioxidant, -microbial, and -cancer activity in vitro. On the basis of these results, lichens appear to be good natural antioxidant, -microbial, and -cancer agents and also could be of significance in the food industry and to control various human, animal, and plant diseases. Further studies should be done to search new compounds from other lichens that exhibit strong antioxidant, -microbial, and -cancer activity.
Experimental
Identification of sample
Lichen samples of H. physodes (L) Nyl. were collected from Kopaonik, Serbia, in September of 2011. The voucher specimen of the lichen (Voucher No. 92) was deposited at the Department of Biology and Ecology, Faculty of Science, University of Kragujevac, Serbia. Determination of the investigated lichens was accomplished using standard methods.
Preparation of the lichen extracts
Finely dry ground thalli from the lichen (100 g) were extracted using acetone in a Soxhlet extractor. The extract was filtered and then concentrated under reduced pressure in a rotary evaporator. The dry extract was stored at −18 °C until it was used for phytochemical screening and process of isolation of secondary metabolites.
HPLC analysis
The extract of the lichen H. physodes was redissolved in 500 μl of acetone and analyzed on an Agilent HPLC instrument 1200 Series with C18 column (C18; 25 cm × 4.6 mm, 10 m) and a UV spectrophotometric detector with methanol–water–phosphoric acid (80:20:0.9, v/v/v) solvent. Phosphoric acid was analytical grade reagent. Methanol was of HPLC grade and was purchased from Merck (Darmstadt, Germany). Deionized water used throughout the experiments was generated by a Milli-Q academic water purification system (Milford, MA, USA). The flow rate was 1.0 ml/min. The sample injection volume was 10 μl. The standards used were obtained from the following sources: 3-hydroxyphysodalic acid (t R = 3.98 ± 0.10), physodalic acid (t R = 4.31 ± 0.10), physodic acid (t R = 5.91 ± 0.10), atranorin (t R = 16.21 ± 0.20), and chloroatranorin (t R = 17.73 ± 0.20) were isolated from lichen P. furfuraceae and usnic acid (t R = 14.32 ± 0.20) from Usnea barbata.
Isolation of lichen metabolites
Isolation of usnic acid
The dried acetone extract of the lichen H. physodes (500 mg) was dissolved in benzene. The precipitate which formed on cooling was collected and HPLC analyzed. HPLC analysis showed that precipitate contains, besides usnic acid, a small amount of atranorin and chloroatranorin. Therefore, precipitate was fractioned on a silica gel column (0.149–0.074 mm; 100–200 mesh) using cyclohexane–ethyl acetate (75:25, v/v). The first eluted compound was usnic acid, which was further recrystallized from chloroform to ethanol, yielding 95 mg pure yellow compound. This compound was further purified by cochromatography and used for structure identification and determination of antioxidant, -microbial, and cytotoxic activities. Usnic acid was identified by its melting point and spectroscopic data (Huneck and Yoshimura, 1996).
Isolation of atranorin
The acetone extract of the lichen H. physodes (100 mg) was fractionated on a silica gel column (0.149–0.074 mm; 100–200 mesh). The column was eluted with methanol–water gradient solvent (6:1, 3:1, and 1:1, v/v) yielding 15 fractions. The last eluted fraction of the lichen extract contains atranorin (21 mg), which was further purified by co- and preparative layer chromatography and used for structure identification and antioxidant, -microbial, and -cancer activities. Atranorin (colorless crystalline substance) was identified by its melting point and spectroscopic data (Huneck and Yoshimura, 1996).
Isolation of physodic acid
The dried acetone extract of the lichen H. physodes (500 mg) was dissolved in benzene. After filtration, the solution was concentrated using an evaporator under reduced pressure. The residue was fractioned on a silica gel column (0.149–0.074 mm; 100–200 mesh). The column was eluted with methanol–chloroform gradient solvent (10:1 and 5:1) yielding seven fractions. The fourth eluted fraction of the lichen extract contains physodic acid (142 mg). This compound was used for structure identification and determination of antioxidant, -microbial, and cytotoxic activities. Physodic acid was identified by its melting point and spectroscopic data (Huneck and Yoshimura, 1996).
Isolated lichen compounds were used for antioxidant, -microbial, and -cancer investigations. The isolated components were dissolved in 5 % DMSO for the experiments. The DMSO was dissolved in sterile distilled water to the desired concentration.
Antioxidant activity
Scavenging DPPH radicals
The free radical scavenging activity of isolated compounds was measured by DPPH. The method used was similar to the method previously used by some authors (Ibanez et al., 2003; Dorman et al., 2004) but was modified in its details. Two milliliters of methanol solution of DPPH radical in the concentration of 0.05 mg/ml and 1 ml of test samples (1000, 500, 250, 125, and 62.5 μg/ml) were placed in cuvettes. The mixture was shaken vigorously and allowed to stand at room temperature for 30 min. The absorbance was then measured at 517 nm in spectrophotometer (“Jenway” UK). Ascorbic acid was used as positive control. The DPPH radical concentration was calculated using the following equation:
where A 0 is the absorbance of the negative control and A 1 is the absorbance of the reaction mixture or the standard.
The IC50 was the parameter used to compare the radical scavenging activity.
Reducing power
The reducing power of isolated compounds was determined according to the method of Oyaizu (1986). One milliliter of test samples (1000, 500, 250, 125, and 62.5 μg/ml) were mixed with 2.5 ml of phosphate buffer (2.5 ml, 0.2 M, pH 6.6) and potassium ferricyanide (2.5 ml, 1 %). The mixtures were incubated at 50 °C for 20 min. Trichloroacetic acid (10 %, 2.5 ml) was then added to the mixture, which was centrifuged. Finally, the upper layer was mixed with distilled water (2.5 ml) and ferric chloride (0.5 ml, 0.1 %). The absorbance of the solution was measured at 700 nm in a spectrophotometer (“Jenway” UK). A higher absorbance of the reaction mixture indicated that the reducing power was increased. Ascorbic acid was used as a positive control.
Superoxide anion radical scavenging activity
The superoxide anion radical scavenging activity of isolated compounds was detected according to the method of Nishimiki et al. (1972). Briefly, 0.1 ml of test samples (1000, 500, 250, 125, and 62.5 μg/ml) was mixed with 1 ml nitroblue tetrazolium solution (156 μM in 0.1 M phosphate buffer, pH 7.4) and 1 ml nicotinamide adenine dinucleotide solution (468 μM in 0.1 M phosphate buffer, pH 7.4). The reaction was started by adding 100 μl of phenazine methosulfate solution (60 μM in 0.1 M phosphate buffer, pH 7.4). The mixture was incubated at room temperature for 5 min, and the absorbance was measured at 560 nm in spectrophotometer (“Jenway” UK) against blank samples. Decreased absorbance indicated increased superoxide anion radical scavenging activity. Ascorbic acid was used as a positive control. The percentage inhibition of superoxide anion generation was calculated using the following formula:
where A 0 is the absorbance of the negative control and A 1 is the absorbance of the reaction mixture or the standards.
The IC50 was the parameter used to compare the radical scavenging activity.
Antimicrobial activity
Microorganisms and media
The following bacteria were used as test organisms in this study: Bacillus mycoides (ATCC 6462), Bacillus subtilis (ATCC 6633), Staphylococcus aureus (ATCC 25923), Escherichia coli (ATCC 25922), and Klebsiella pneumoniae (ATCC 13883). All of the bacteria used were obtained from the American Type Culture Collection (ATCC). The fungi used as test organisms were: Aspergillus flavus (ATCC 9170), Aspergillus fumigatus (DBFS 310), Candida albicans (ATCC 10231), Penicillium purpurescens (DBFS 418), and Penicillium verrucosum (DBFS 262). They were from the ATCC and the mycological collection maintained by the Mycological Laboratory within the Department of Biology of Kragujevac University’s Faculty of Science (DBFS). The bacterial cultures were maintained on Müller–Hinton agar substrates (Torlak, Belgrade). The fungal cultures were maintained on potato dextrose (PD) and Sabouraud dextrose (SD) agars (Torlak, Belgrade). All of the cultures were stored at 4 °C and subcultured every 15 days.
The sensitivity of microorganisms to tested samples was tested by determining the MIC.
Bacterial inoculi were obtained from bacterial cultures incubated for 24 h at 37 °C on Müller–Hinton agar substrate and brought up by dilution according to the 0.5 McFarland standard to approximately 108 CFU/ml. Suspensions of fungal spores were prepared from freshly mature (3- to 7-day-old) cultures that grew at 30 °C on a PD agar substrate. The spores were rinsed with sterile distilled water, used to determine turbidity spectrophotometrically at 530 nm, and were then further diluted to approximately 106 CFU/ml according to the procedure recommended by NCCLS (National Committee for Clinical Laboratory Standards) (1998).
Minimal inhibitory concentration (MIC)
The MIC was determined by the broth microdilution method using 96-well microtiter plates (Sarker et al., 2007). A series of dilutions with concentrations ranging from 4 to 0.00181 mg/ml for isolated compounds was used in the experiment against every microorganism tested. The starting solutions of the test samples were obtained by measuring off a certain quantity of extract and dissolving it in DMSO. Twofold dilutions of the test samples were prepared in a Müller–Hinton broth for bacterial cultures and a SD broth for fungal cultures. The MIC was determined with resazurin. Resazurin is an oxidation–reduction indicator used for the evaluation of microbial growth. It is a blue non-fluorescent dye that becomes pink and fluorescent when reduced to resorufin by oxidoreductases within viable cells. The boundary dilution without any change in color of resazurin was defined as the MIC for the tested microorganism at a given concentration. As a positive control of growth inhibition, streptomycin was used in the case of bacteria and ketoconazole in the case of fungi. A DMSO solution was used as a negative control for the influence of the solvents. All experiments were performed in triplicate.
Cytotoxic activity
Cell lines
The human melanoma FemX and human colon carcinoma LS 174 cell lines were obtained from the ATCC (Manassas, VA, USA). Both cancer cell lines were maintained in the recommended RPMI 1640 medium supplemented with 10 % heat-inactivated (56 °C) fetal bovine serum (FBS), l-glutamine (3 mM), streptomycin (100 mg/ml), penicillin (100 IU/ml), and 25 mM HEPES and was adjusted to pH 7.2 by bicarbonate solution. The cells were grown in a humidified atmosphere of 95 % air and 5 % CO2 at 37 °C.
Treatment of cell lines
A stock solution of isolated compounds, made in DMSO, was dissolved in corresponding medium to the required working concentrations. Neoplastic FemX- (5,000 cells/well) and neoplastic LS 174 cells (7,000 cells/well) were seeded into 96-well microtiter plates, and 24 h later, after cell adherence, five different, double diluted, concentrations of investigated compounds, were added to the wells. Final concentrations applied to target cells were 200, 100, 50, 25, and 12.5 μg/ml, except to the control wells, where only the nutrient medium was added to the cells. Nutrient medium was RPMI 1640 medium, supplemented with l-glutamine (3 mM), streptomycin (100 lg/ml), and penicillin (100 IU/ml), 10 % heat-inactivated (56 °C) FBS and 25 mM HEPES, and was adjusted to pH 7.2 by bicarbonate solution. The cultures were incubated for 72 h.
Determination of cell survival (MTT test)
The effect of isolated compounds on cancer cell survival was determined by the MTT test (microculture tetrazolium test), according to Mosmann (1983) with modification by Ohno and Abe (1991), 72 h after the addition of the compounds, as it was described earlier. Briefly, 20 μl of MTT solution (5 mg/ml PBS) was added to each well. The samples were incubated for further 4 h at 37 °C in 5 % CO2 in a humidified air atmosphere. Then, 100 μl of 10 % SDS was added to extract the insoluble product formazan, resulting from the conversion of the MTT dye by viable cells. The number of viable cells in each well was proportional to the intensity of the light absorbance, which was then read in an ELISA plate reader at 570 nm. Absorbance (A) at 570 nm was measured 24 h later. To get cell survival (%), A of a sample with cells grown in the presence of various concentrations of the investigated test samples was divided with control optical density (the A of control cells grown only in nutrient medium), and multiplied by 100. It was implied that A of the blank was always subtracted from A of the corresponding sample with target cells. The IC50 concentration was defined as the concentration of an agent inhibiting cell survival by 50 %, compared with a vehicle-treated control. As a positive control cis-DDP was used. All of the experiments were done in triplicate.
Statistical analyses
Statistical analyses were performed with the EXCEL and SPSS software package. To determine the statistical significance of antioxidant activity, Student’s t test was used. All values are expressed as mean ± SD of three parallel measurements.
References
Agrawal KM, Talele GS (2013) Synthesis and antibacterial, antimycobacterial and docking studies of novel N-piperazinyl fluoroquinolones. Med Chem Res 22:818–831
Amo de Paz G, Raggio J, Gomez-Serranillos MP, Palomino OM, Gonzales-Burgos E, Carretero ME, Crespo A (2010) HPLC isolation of antioxidant constituents from Xanthoparmelia spp. J Pharm Biomed Anal 53:165–171
Bates ST, Cropsey GW, Caporaso JG, Knight R, Fierer N (2011) Bacterial communities associated with the lichen symbiosis. Appl Environ Microbiol 77:1309–1314
Behera BC, Verma N, Sonone A, Makhija U (2009) Optimization of culture conditions for lichen Usnea ghattensis G. awasthi to increase biomass and antioxidant metabolite production. Food Technol Biotechnol 47:7–12
Bogo D, de Fatima Cepa Matos M, Honda NK, Pontes EC, Oguma PM, da Silva Santos EC, de Carvalho JE, Nomizo A (2010) In vitro antitumour activity of orsellinates. Z Naturforsch C 65:43–48
Bucar F, Schneider I, Ogmundsdottir H, Ingolfsdottir K (2004) Antiproliferative lichen compounds with inhibitory activity on 12(S)-HETE production in human platelets. Phytomedicine 11:602–606
Burlando B, Ranzato E, Volante A, Appendino G, Pollastro F, Verotta L (2009) Antiproliferative effects on tumour cells and promotion of keratinocyte wound healing by different lichen compounds. Planta Med 75:607–613
Candan M, Yilmaz M, Tay T, Kivanc M, Turk H (2006) Antimicrobial activity of extracts of the lichen Xanthoparmelia pokornyi and its gyrophoric and stenosporic acid constituents. Z Naturforsch C 61:319–323
Cansaran-Duman D, Cetin D, Simsek H, Coplu N (2010) Antimicrobial activities of the lichens Hypogymnia vittata, Hypogymnia physodes and Hypogymnia tubulosa and HPLC analysis of their usnic acid content. Asian J Chem 22:6125–6132
Dorman HJ, Bachmayer O, Kosar M, Hiltunen R (2004) Antioxidant properties of aqueous extracts from selected Lamiaceae species grown in Turkey. J Agric Food Chem 5:762–770
Einarsdottir E, Groeneweq J, Bjornsdottir GG, Harethardottir G, Omarsdottir S, Ingolfsdottir K, Ogmundsdottir HM (2010) Cellular mechanisms of the anticancer effects of the lichen compound usnic acid. Planta Med 76:969–974
Farkaš V (2003) Structure and biosynthesis of fungal cell walls: methodological approaches. Folia Microbiol 48:469–478
Heijenoort J (2001) Formation of the glycan chains in the synthesis of bacterial peptidoglycan. Glycobiology 11:25–36
Hidalgo ME, Fernandez E, Quilhot W, Lissi E (1994) Antioxidant activity of depsides and depsidones. Phytochemistry 37:1585–1587
Huneck S, Yoshimura I (1996) Identification of lichen substances. Springer, Berlin
Ibanez E, Kubatova A, Senorans FJ, Cavero S, Reglero G, Hawthorne SB (2003) Subcritical water extraction of antioxidant compounds from rosemary plants. J Agric Food Chem 51:375–382
Kosanić M, Ranković B (2011) Antioxidant and antimicrobial properties of some lichens and their constituents. J Med Food 14:1624–1630
Kosanić M, Ranković B, Stanojković T (2012a) Antioxidant, antimicrobial and anticancer activity of 3 Umbilicaria species. J Food Sci 77:T20–T25
Kosanić M, Ranković B, Stanojković T (2012b) Antioxidant, antimicrobial, and anticancer activities of three Parmelia species. J Sci Food Agric 92:1909–1916
Luo H, Yamamoto Y, Kim JA, Jung JS, Koh YJ, Hur JS (2009) Lecanoric acid, a secondary lichen substance with antioxidant properties from Umbilicaria antarctica in maritime Antarctica (King George Island). Polar Biol 32:1033–1040
Manojlović N, Vasiljević P, Gritsanapan W, Supabphol R, Manojlović I (2010) Phytochemical and antioxidant studies of Laurera benguelensis growing in Thailand. Biol Res 43:169–176
Mitrović T, Stamenković S, Cvetković V, Nikolić M, Tošić S, Stojičić D (2011a) Lichens as source of versatile bioactive compounds. Biol Nyssana 2:1–6
Mitrović T, Stamenković S, Cvetković V, Tošić S, Stanković M, Radojević I, Stefanović O, Čomić L, Đačić D, Ćurčić M, Marković S (2011b) Antioxidant, antimicrobial and antiproliferative activities of five lichen species. Int J Mol Sci 12:5428–5448
Molnar K, Farkaš E (2010) Current results on biological activities of lichen secondary metabolites: a review. Z Naturforsch C 65:157–173
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
NCCLS (National Committee for Clinical Laboratory Standards), (1998) Reference method for broth dilution antifungal susceptibility testing of conidium-forming filamentous fungi: proposed standard M38-P. NCCLS, Wayne
Nishimiki M, Rao NA, Yagi K (1972) The occurrence of super-oxide anion in the reaction of reduced phenazine methosulfate and molecular oxygen. Biochem Biophys Res Commun 46:849–853
Odabasoglu F, Aslan A, Cakir A, Suleyman H, Karagoz Y, Halici M, Bayir Y (2004) Comparison of antioxidant activity and phenolic content of three lichen species. Phytother Res 18:938–941
Ohno M, Abe T (1991) Rapid colorimetric assay for the quantification of leukemia inhibitory factor (LIF) and interleukin-6 (IL-6). J Immunol Methods 145:199–203
Otzurk S, Guvenc S, Arikan N, Yylmaz O (1999) Effect of usnic acid on mitotic index in root tips of Allium cepa L. Lagascalia 21:47–52
Oyaizu M (1986) Studies on products of browning reaction prepared from glucoseamine. Jpn J Nutr 44:307–314
Sarker SD, Nahar L, Kumarasamy Y (2007) Microtitre plate-based antibacterial assay incorporating resazurin as an indicator of cell growth, and its application in the in vitro antibacterial screening of phytochemicals. Methods 42:321–324
Stojanović G, Stojanović I, Stankov-Jovanović V, Mitić V, Kostić D (2010) Reducing power and radical scavenging activity of four Parmeliaceae species. Cent Eur J Biol 5:808–813
Turk H, Yılmaz M, Tay T, Turk AO, Kivanc M (2006) Antimicrobial activity of extracts of chemical races of the lichen Pseudevernia furfuracea and their physodic acid, chloroatranorin, atranorin, and olivetolic acid constituents. Z Naturforsch C 61:499–507
Yang Y, Anderson EJ (1999) Antimicrobial activity of a porcine myeloperoxidase against plant phatgenic bacteria and fungi. J Appl Microbiol 86:211–220
Acknowledgments
This work was financed in part by the Ministry of Science, Technology, and Development of the Republic of Serbia and was carried out within the framework of Projects No. 173032, 175011, and 172015.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ranković, B., Kosanić, M., Manojlović, N. et al. Chemical composition of Hypogymnia physodes lichen and biological activities of some its major metabolites. Med Chem Res 23, 408–416 (2014). https://doi.org/10.1007/s00044-013-0644-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-013-0644-y