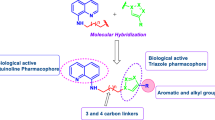
Abstract
A series of novel hybrid 4-aminoquinoline-1,3,5-triazine derivatives were developed and subsequently tested against representative Gram-positive and Gram-negative microorganisms for determination of their antibacterial activity. Screening results indicate that, title molecule exhibit moderate to potent activity in comparison to standard. These hybrid derivatives were synthesized through a facile synthetic routes and structure of reaction intermediates as well as target molecules were recognised with the aid of various spectroscopic techniques viz., FTIR, NMR, mass and elemental analysis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The emergence of multi-drug resistance (MDR) pathogens in last decades significantly jeopardises the global health-care system in both poor and developed countries (Alanis, 2005; Shapiro et al., 2011). Whereas, non-judicious use of these drugs has contributed largely among the other factors that brought up the mutation in microbial genome (Martinez and Baquero, 2000). This has put a selective pressure and necessitates the discovery of novel antimicrobial agents that acted via a novel pathway (Davies and Davies, 2010).
The 1,3,5-triazine scaffold has provided the basis for the design of biologically significant molecules with diverse therapeutic profile, e.g. as antifungal (Singh et al., 2012a, 2013a), anticancer (Corbett et al., 1982), antimalarial (Bhat et al., 2011, 2012a, 2013), antiviral (Lozano et al., 2011) and antibacterial activity (Gahtori et al., 2012). In continuation of our project (Junejo et al., 2011), till now, we had reported numerous antibacterial hybrid conjugates of 1,3,5-triazine with thiazole (Singh et al., 2011), piperazine (Ghosh et al., 2012), 4-aminoquinoline (Bhat et al., 2012b) and 1,3,4-thiadiazole (Dubey et al., 2012), thiazolidine-4-one (Kumar et al., 2013) as potent antimicrobial compounds. In our recent communication, inhibition of bacterial translation was identified as mechanism of action for these conjugates (Singh et al., 2012b). Concerning our endeavour on discovery of antibacterial agents (Singh et al., 2013b) and prompted by these results, herein, we disclosed the facile synthesis and antibacterial activity of hybrid conjugates derived from 4-aminoquinoline and 1,3,5-triazine.
Results and discussion
Chemistry
The synthesis of title hybrid 4-aminoquinoline 1,3,5-triazine derivatives 9 (a–i) were accomplished in five steps. The first step corresponds to the synthesis 7-chloro-4-isothiocyanatoquinoline (3) was achieved by the substitution of potassium thiocynate (2) with 4-chloro of 4,7-dichloroquinoline (1) in the presences of few pieces of tin granules, Scheme 1. Whereas, in second step, synthesis of mono-substituted 1,3,5-triazines viz., 4,6-dichloro-N-(4-nitrophenyl)-1,3,5-triazin-2-amine (6) was accomplished by nucleophilic substitution of the Cl atom of the 2,4,6-trichloro-1,3,5-triazine (4) with p-nitro aniline (5) in the presence of saturated solution of NaHCO3. The above-obtained compound (6) was subsequently treated with different primary and secondary amines (a–i) as presented in Scheme 2 to afford di-substituted 1,3,5-triazines 7(a–i) via nucleophilic substitution at one of Cl atom in the presence of activating base. The synthesis of tri-substituted 1,3,5-triazine 8(a–i) was accomplished by the nucleophilic substitution of the remained Cl atom of di-substituted 1,3,5-triazine 7(a–i) with piperazine in the presence of saturated solution of NaHCO3. Whereas, title compounds 9(a–i) were synthesized by incorporating tri-substituted 1,3,5-triazine moiety 8(a–i) with 7-chloro-4-isothiocyanatoquinoline pharmacophore (3) and serve as step five as showed in Scheme 3.
The series of title hybrid analogues were synthesized in moderate to excellent yields. Whereas, structure of the intermediate as well as target molecules were ascertained on the basis of spectroscopic analysis. FTIR spectra of compounds 9(a–i) in which aromatic C=N group of 1,3,5-triazine was found in range of 1,679.28–1,548.26 cm−1. Whereas quinoline ring aromatic C=C group appears in range of 1,690–1,640 cm−1, C–N group appear in range of 1,275 cm−1 and many strong absorption bands in range of 894–628 cm−1 which confirm the existence of aromatic ring. The 1H NMR spectrums of quinoline expose a signal in the range of 7.27–8.85 ppm. The shielding for bridged NH was usually observed at 3.69–3.59 ppm which is attributable to tri-substituted 1,3,5-triazine but the chemical shift of –NH2 bridge was found in range of 1.93 ppm in case of 8b. Moreover, all mass spectra and elemental analysis are in agreement of proposed structures.
Antibacterial activity
Antibacterial screening of all the synthesized compounds 9(a–i) (Table 1) revealed moderate to potent activities against the tested gram-positive and gram-negative microorganisms in comparison to Ofloxacin as a standard drug. The term significant, moderate and no activity were considered for the concentration range of 3.125, 6.25–25 and 50–100 μg mL−1, respectively. Compound with morpholine substitution on 1,3,5-triazine 9a exhibit significant activity against Staphlococcos aureus, Bacillus cereus, Proteus vulgaris and moderate activity against the Pseudomonas aeruginosa and Bacillus subtilis. Whereas, on replacement of morpholine to hydrazine in the case of compound 9b, presented moderate activities against B. subtilis, S. aureus, P. aeruginosa, Proteus mirabilis, P. vulgaris and Escherichia coli. Compound 9c, formed on replacement of hydrazine by thiosemicarbazide showed the significant antibacterial activity against B. cereus, equipotent to ofloxacin against P. aeruginosa and moderate against the B. subtilis, P. vulgaris and S. aureus. Reasonable activity was observed in case of compound 9d having o-toluidine group as substituent, towards entire microbial strains. Replacement of o-toluidine to aromatic amine in the case of compound 9e, causes significant shift in the activity against B. cereus and moderately against the B. subtilis, S. aureus, P. vulgaris and P. aeruginosa. Compound 9f, resulted from introduction of semicarbazide in place of aromatic amine disclosed the significant antibacterial activity against B. subtilis and moderate activity against the P. aeruginosa, E.coli, S. aureus and P. vulgaris. Moderate activity was observed in case of compounds 9g and 9h having 1,3-diaminopropane and p-aminophenol group as substituent, towards all gram-positive and gram-negative strains. However, substantial-to-significant activity was observed in the case of analogue having p-toluidine 9i against B. cereus and moderate activity against S. aureus, E. coli, B. subtilis, P. vulgaris and P. mirabilis, respectively.
From the bioactivity profile of the target hybrid derivatives, it was inferred that compounds having aliphatic group at 1,3,5-triazine ring viz., 9b, 9c, 9f and 9g showed prominent activity against B. cereus and B. subtilis and no activity against P. aeruginosa, S. aureus, E. coli, P. vulgaris and P. mirabilis. While, replacement of aliphatic group with aromatic 9a, 9d, 9e, 9h and 9i makes the compound prominent active against B. cereus, P. vulgaris and no activity against B. subtilis, E. coli, S. aureus, P. mirabilis and P. aeruginosa. In addition, it was also corroborated that, replacement of aliphatic by aromatic substituent, was lead to remarkable increase in activity for B. cereus and further decrease was reported for rest of the strains.
Experimental
Chemistry
All commercially available solvents and reagents were of analytical grade and used without further purification. Melting points were determined on a Veego, MPI melting point apparatus and FTIR (2.0 cm−1, flat, smooth, abex) were recorded on Perkin-Elmer RX-I spectrophotometer. 1H NMR spectra were recorded on Bruker Avance II 400 NMR and 13C NMR spectra on Bruker Avance II 100 NMR spectrometer in CDCl3 using TMS as internal standard. Mass spectra were obtained on VG-AUTOSPEC spectrometer equipped with electrospray ionisation (ESI) sources. Elemental analysis was carried out on Vario EL-III CHNOS elemental analyser.
7-Chloro-4-isothiocyanatoquinoline (3)
A solution of 4,7-dichloroquinoline (1) (0.01 mol), potassium thiocynate (2) (0.02 mol) and few pieces of tin metal in anhydrous toluene was refluxed at 90–120 °C for 18 h. The completion of reaction was monitored by TLC using ethanol:acetone (1:1) as mobile phase. The reaction mixture was filtered and concentrated under reduced pressure. The resulted residue was dissolved in dichloromethane, washed with brine and dried over Na2SO4. The dried solution was concentrated under reduced pressure to obtain the title compound (3).
Brown crystals, yield: 68 %; m.p.: 197–198 °C; MW: 220.68; R f: 0.48; FTIR (ν max; cm−1 KBr): 1,275 (C–N), 1,630 (C=C), 1,690–1,640 (C=N), 3,000 (C–H), 1,600 (C=C, aromatic ring), 1,470 (C=C, aromatic ring); 1H NMR (400 MHz, CDCl3-d 6, TMS) δ ppm: 8.85 (d, 1H J = 4.97 Hz, quinoline ring), 7.27 (d, 1H J = 4.97 Hz, quinoline ring), 7.76 (d, 1H J = 8.60 Hz, quinoline ring), 8.40 (d, 1H J = 8.60 Hz, quinoline ring), 8.01 (d, 1H J = 1.96 Hz, quinoline ring); 13C NMR (100 MHz, CDCl3); δ ppm: 152.30, 118.30, 138.40, 124.10, 129.80, 128.60, 135.20, 129.10, 137.20; mass: 221.60 (M+H)+; elemental analysis for C10H5ClN2S: calculated: C, 54.43; H, 2.28; N, 12.69. Found: C, 54.41; H, 2.23; N, 12.58.
4,6-Dichloro-N-(4-nitrophenyl)-1,3,5-triazin-2-amine (6)
p-Nitro aniline (5) (0.1 mol) was added into 100 mL of acetone at temperature 0–5 °C. The solution of 2,4,6-trichloro-1,3,5-triazine (4) (0.1 mol) in 25-mL acetone was added to above mixture constantly. The resulting mixture was then stirred for 2 h followed by drop-wise addition of NaHCO3 solution (0.1 mol) taking care that reaction mixture does not become acidic. The completion of reaction was monitored by TLC using benzene:ethyl acetate (9:1) as mobile phase. The product was filtered and washed with cold water and recrystallized with ethanol to afford pure products (6).
Yellow crystals; yield: 83 %; m.p.: 123–124 °C; MW: 286.07; R f: 0.48; FTIR (ν max; cm−1 KBr): 3,289.56 (N–H secondary), 3,056.73 (C–H broad), 1,548.26– 1,446.16 (aromatic C=N); 1H NMR (400 MHz, CDCl3-d 6, TMS) δ ppm: 7.63 (d, 2H J = 8.63 Hz, 2× CH, Ar–H), 7.28(d, 2H J = 8.57 Hz, 2× CH, Ar–H), 3.62 (br, s, 1H, NH); 13C NMR (100 MHz, CDCl3) δ ppm: 118.25, 125.32, 139.26, 144.26, 168.85, 173.56; mass: 287.34 (M+H)+; elemental analysis for C9H5Cl2N5O2: calculated: C, 37.79; H, 1.76; N, 24.48. Found: C, 36.48; H, 1.75; N, 24.86.
General procedure for the synthesis of di-substituted 1,3,5-triazine derivatives 7(a–i)
Various distinguished amines (a–i) (0.1 mol) were added into 100 mL of acetone maintaining temperature 40–45 °C. The solution of mono substituted-1,3,5-triazine (6) (0.1 mol) in 25 mL acetone was added constantly, stirred for 3 h followed by drop-wise addition of NaHCO3 solution (0.1 mol) taking care that reaction mixture does not become acidic. The completion of reaction was monitored by TLC using benzene:ethyl acetate (9:1) as mobile phase. The product was filtered and washed with cold water and recrystallized with ethanol to afford pure products 7(a–i).
4-Chloro-6-morpholino-N-(4-nitrophenyl)-1,3,5-triazin-2-amine (7a)
Light yellow crystals; yield: 76 %; m.p.: 146–148 °C; MW: 336.73; R f: 0.56; FTIR (ν max; cm−1 KBr): 3,288.57 (N–H secondary), 3,055.79 (C–H broad), 1,679.28–1,638.32 (aromatic C=N), 1,348.23–1,023.12 (aromatic C–N), 1,528.24 (NO2), 1,662, 786; 1H NMR (400 MHz, CDCl3-d 6, TMS) δ ppm: 7.28(d, 2H J = 8.57 Hz, 2× CH, Ar–H), 3.58 (br, s, 1H, NH), 3.68 (d, 1H J = 17.31 Hz, 2× CH2, Ar–H) 3.63 (d, 1H J = 18.37 Hz, 2× CH2, Ar–H); 13C NMR (100 MHz, CDCl3) δ ppm: 118.21, 124.22, 138.26, 144.25, 168.81, 173.55, 67.34, 46.67; mass: 337.79 (M+H)+; elemental analysis for C13H13ClN6O3: calculated: C, 46.37; H, 3.89; N, 24.96. Found: C, 46.43; H, 3.97; N, 24.83.
4-Chloro-6-hydrazinyl-N-(4-nitrophenyl)-1,3,5-triazin-2-amine hydrate (7b)
Brown crystals; yield: 86 %; m.p.: 156–158 °C; MW: 299.67; R f: 0.63; FTIR (ν max; cm−1 KBr): 3,315.32 (NH2), 3,288.53 (N–H secondary), 3,043.79 (C–H broad), 1,676.23–1,639.32 (aromatic C=N), 1,668, 1,347.13–1,002.19 (aromatic C–N), 1,525.27 (NO2), 757; 1H NMR (400 MHz, CDCl3-d 6, TMS) δ ppm: 8.12 (d, 2H J = 8.67 Hz, 2× CH, Ar–H), 7.37 (d, 2H, J = 1.95 Hz 2× CH, Ar–H), 3.67 (br, s, 1H, NH), 1.87 (s, 2H, NH2); 13C NMR (100 MHz, CDCl3) δ ppm: 183.24, 172.56, 168.37, 143.24, 137.25, 126.75, 117.28; mass: 301.36 (M+H)+; elemental analysis for C9H10ClN7O3: calculated: C, 36.07; H, 3.36; N, 32.72. Found: C, 35.96; H, 3.28; N, 32.71.
2-(4-Chloro-6-((4-nitrophenyl)amino)-1,3,5-triazin-2-yl)hydrazinecarbothioamide (7c)
Yellow crystals; yield: 69 %; m.p.: 169–170 °C; MW:340.75; R f: 0.68; FTIR (ν max; cm−1 KBr): 3,321.35 (NH2), 3,287.47 (N–H secondary), 3,043.76 (C–H broad), 1,676.53–1,639.36 (aromatic C=N), 1,668, 1,525.27 (NO2), 1,347.13–1,002.19 (aromatic C–N), 1,273.78 (C=S),757; 1H NMR (400 MHz, CDCl3-d 6, TMS) δ ppm: 8.08 (d, 2H J = 8.65 Hz, 2× CH, Ar–H), 7.48 (d, 2H, J = 2.96 Hz, 2× CH, Ar–H), 3.67 (br, s, 1H, NH), 1.87 (s, 1H, NH), 8.77 (s, 1H, NH2); 13C NMR (100 MHz, CDCl3) δ ppm: 184.52, 180.43, 172.20, 165.73, 152.36, 136.32, 128.36, 117.65; mass: 341.76 (M+H)+; elemental analysis for C10H9ClN8O2S: calculated: C, 35.25; H, 2.66; N, 32.88. Found: C, 35.27; H, 2.72; N, 32.86.
6-Chloro-N 2-(4-nitrophenyl)-N 4-(o-tolyl)-1,3,5-triazine-2,4-diamine (7d)
White crystals; yield: 73 %; m.p.: 152–153 °C; MW:356.77; R f: 0.47; FTIR (ν max; cm−1 KBr): 3,285.45 (N–H secondary), 3,045.72 (C–H broad), 1,672.51–1,631.30 (aromatic C=N), 1,667–1,600 (aromatic C=C), 1,528.22 (NO2), 1,346.12–1,016.12 (aromatic C–N), 894, 635; 1H NMR (400 MHz, CDCl3-d 6, TMS) δ ppm: 8.12 (d, 2H J = 8.66 Hz, 2× CH, Ar–H), 7.34 (d, 2H, J = 0.71 Hz, 2× CH, Ar–H), 3.67 (br, s, 1H, NH), 7.12(d, 1H J = 8.64 Hz, 1× CH, Ar–H), 7.03 (d, 1H J = 7.49 Hz, 1× CH, Ar–H), 6.72(d, 1H J = 1.17 Hz, 1× CH, Ar–H), 2.21 (s, 3H, CH3); 13C NMR (100 MHz, CDCl3) δ ppm: 167.32, 164.62, 163.27, 146.23, 141.82, 138.21, 132.82, 129.25, 125.32, 123.73, 122.83, 118.56, 17.63; mass: 357.73 (M+H)+; elemental analysis for C16H13ClN6O2: calculated: C, 53.86; H, 3.67; N, 23.56. Found: C, 54.02; H, 3.64; N, 23.58.
6-Chloro-N 2-(4-nitrophenyl)-N 4-phenyl-1,3,5-triazine-2,4-diamine (7e)
Light brown crystals; yield: 81 %; m.p.: 182–183 °C; MW:342.74; R f: 0.52; FTIR (ν max; cm−1 KBr): 3,285.45 (N–H secondary), 3,045.78 (C–H broad), 1,672.53–1,631.33 (aromatic C=N), 1,671–1,606 (aromatic C=C), 1,524.32 (NO2), 1,345.10–1,016.14 (aromatic C–N), 775, 628; 1H NMR (400 MHz, CDCl3-d 6, TMS) δ ppm: 8.14 (d, 2H J = 8.65 Hz, 2× CH, Ar–H), 7.34 (d, 2H, J = 0.74 Hz, 2× CH, Ar–H), 3.67 (br,s, 1H, NH), 7.53(d, 1H J = 8.29 Hz, 1× CH, Ar–H), 7.48 (d, 1H J = 8.15 Hz, 1× CH, Ar–H), 6.89(d, 1H J = 1.18 Hz, 1× CH, Ar–H); 13C NMR (100 MHz, CDCl3) δ ppm: 167.31, 164.52, 164.23, 143.52, 139.39, 137.25, 129.53, 124.73, 122.86, 119.37, 115.84; mass: 343.76 (M+H)+; elemental analysis for C15H11ClN6O2: calculated: C, 53.86; H, 3.67; N, 23.56. Found: C, 54.02; H, 3.64; N, 23.58.
2-(4-Chloro-6-((4-nitrophenyl)amino)-1,3,5-triazin-2-yl)hydrazinecarboxamide (7f)
Light yellow crystals; yield: 68 %; m.p.: 156–159 °C; MW:324.68; R f: 0.46; FTIR (ν max; cm−1 KBr): 3,321.35 (NH2), 3,287.47 (N–H secondary), 3,043.76 (C–H broad), 1,676.53–1,639.36 (aromatic C=N), 1,668, 1,525.27 (NO2), 1,347.13–1,002.19 (aromatic C–N), 1,753.62 (C=O),758; 1H NMR (400 MHz, CDCl3-d 6, TMS) δ ppm: 8.05 (d, 2H J = 8.67 Hz, 2× CH, Ar–H), 7.65 (d, 2H, J = 0.74 Hz, 2× CH, Ar–H), 3.67 (br,s, 1H, NH), 6.77 (s, 1H, NH), 6.87 (s, 1H, NH2); 13C NMR (100 MHz, CDCl3) δ ppm: 183.35, 171.93, 168.24, 158.42, 147.28, 137.56, 125.35, 118.28; mass: 325.79 (M+H)+; elemental analysis for C10H9ClN8O3: calculated: C, 36.99; H, 2.79; N, 34.51. Found: C, 37.02; H, 2.75; N, 34.57.
N 2-(3-aminopropyl)-6-chloro-N 4-(4-nitrophenyl)-1,3,5-triazine-2,4-diamine (7g)
Yellow white crystals; yield: 75 %; m.p.: 172–173 °C; MW:323.74; R f: 0.54; FTIR (ν max; cm−1 KBr): 3,319.56 (NH2), 3,284.57 (N–H secondary), 3,041.78 (C–H broad), 1,674.45–1,638.12 (aromatic C=N), 1,668, 1,347.13–1,023.19 (aromatic C–N), 1,527.27 (NO2), 759; 1H NMR (400 MHz, CDCl3-d 6, TMS) δ ppm: 8.12 (d, 2H J = 8.66 Hz, 2× CH, Ar–H), 7.37 (d, 2H, J = 2.12 Hz, 2× CH, Ar–H), 3.67 (br,s, 1H, NH), 3.25 (t, 2H J = 6.76 Hz, CH2,), 2.62 (t, 2H, J = 6.85 Hz, CH2), 1.66 (t, 2H, J = 6.76 Hz, CH2), 6.87 (s, 1H, NH2); 13C NMR (100 MHz, CDCl3) δ ppm: 173.25, 171.43, 165.73, 148.12, 136.43, 126.65, 119.28, 38.58, 31.63; mass: 324.83 (M+H)+; elemental analysis for C12H14ClN7O2: calculated: C, 44.52; H, 4.36; N, 30.29. Found: C, 45.03; H, 4.34; N, 30.28.
4-((4-Chloro-6-((4-nitrophenyl)amino)-1,3,5-triazin-2-yl)amino)phenol (7h)
Black crystals; yield: 63 %; m.p.: 184–185 °C; MW:358.74; R f: 0.42; FTIR (ν max; cm−1 KBr): 3,416 (OH stretching), 3,286.51 (N–H secondary), 3,046.58 (C–H broad), 2,800–3,012 (CH2 stretching), 1,672.42–1,637.16 (aromatic C=N), 1,605 (C=C), 1,347.13–1,023.19 (aromatic C–N), 1,527.27 (NO2), 753; 1H NMR (400 MHz, CDCl3-d 6, TMS) δ ppm: 8.14 (d, 2H J = 8.65 Hz, 2× CH, Ar–H), 7.34 (d, 2H, J = 0.73 Hz, 2× CH, Ar–H), 3.67 (br,s, 1H, NH), 7.41 (d, 1H J = 8.73 Hz, Ar–H), 6.82 (d, 1H, J = 2.26 Hz, Ar–H), 5.67 (s, 1H, OH); 13C NMR (100 MHz, CDCl3) δ ppm: 173.25, 169.54, 167.43, 148.52, 143.85, 139.62, 132.21, 125.73, 122.82, 118.27, 114.75; mass: 359.65 (M+H)+; elemental analysis for C15H11ClN6O3: calculated: C, 50.22; H, 3.09; N, 23.43. Found: C, 50.32; H, 3.07; N, 23.46.
6-Chloro-N 2-(4-nitrophenyl)-N 4-(p-tolyl)-1,3,5-triazine-2,4-diamine (7i)
Light yellow crystals; yield: 65 %; m.p.: 158–159 °C; MW:356.77; R f: 0.63; FTIR (ν max; cm−1 KBr): 3,285.45 (N–H secondary), 3,045.72 (C–H broad), 1,672.51–1,631.30 (aromatic C=N), 1,667–1,600 (aromatic C=C), 1,528.22 (NO2), 1,346.12–1,016.12 (aromatic C–N), 894, 635; 1H NMR (400 MHz, CDCl3-d 6, TMS) δ ppm: 8.13 (d, 2H J = 8.64 Hz, 2× CH, Ar–H), 7.32 (d, 2H, J = 0.74 Hz, 2× CH, Ar–H), 3.67 (br,s, 1H, NH), 7.35 (d, 1H J = 8.27 Hz, Ar–H), 6.97 (d, 1H J = 5.49 Hz, Ar–H), 2.21 (s, 3H, CH3); 13C NMR (100 MHz, CDCl3) δ ppm: 172.45, 168.32, 167.36, 152.65, 138.14, 134.53, 130.21, 128.72, 123.87, 118.52, 117.28, 24.37; mass: 357.53 (M+H)+; elemental analysis for C16H13ClN6O2: calculated: C, 53.86; H, 3.67; N, 23.56. Found: C, 54.02; H, 3.64; N, 23.58.
General procedure for the synthesis of tri-substituted 1,3,5-triazine derivatives 8(a–i)
A solution of di-substituted 1,3,5-triazine compounds 7(a–i) (0.01 mol), piperazine (0.01 mol) and K2CO3 (0.01 mol) in 1,4-dioxane was refluxed for 6–7 h. The completion of reaction was monitored by TLC using benzene:ethyl acetate (9:1) as mobile phase. The reaction mixture was filtered and concentrated under reduced pressure. The resulting residue was purified by ethanol to afford the desired product 8(a–i).
4-Morpholino-N-(4-nitrophenyl)-6-(piperazin-1-yl)-1,3,5-triazin-2-amine (8a)
White crystals; yield: 72 %; m.p.: 216–217 °C; MW: 386.41; R f: 0.58; FTIR (ν max; cm−1 KBr): 3,323.98 (N–H stretching in piperazine), 3,287.37 (N–H secondary), 3,056.78 (C–H broad), 2,813–3,003 (CH2 stretching), 1,282–1,178 (C–N stretching), 1,676.25–1,637.39 (aromatic C=N), 1,616.15 (N–H bending piperazine), 1,528.53 (NO2), 1,605 (C=C), 784; 1H NMR (400 MHz, CDCl3-d 6, TMS) δ ppm: 8.12 (d, 2H J = 8.66 Hz, 2× CH, Ar–H), 7.32 (d, 2H J = 0.71 Hz, 2× CH, Ar–H), 3.62 (br, s, 1H, NH), 3.69 (d, 1H J = 17.80 Hz, 2× CH2, Ar–H) 3.65 (d, 1H J = 15.61 Hz, 2× CH2, Ar–H), 3.12 (d, 4H J = 13.29 Hz, 2× CH2, Ar–H), 2.73 (d,4H J = 3.21 Hz, 2× CH2, Ar–H), 1.94 (s,1H NH); 13C NMR (100 MHz, CDCl3) δ ppm: 183.32, 178.65, 168.86, 147.42, 138.53, 126.21, 119.85, 68.36, 48.73, 46.63, 45.71; mass: 387.63 (M+H)+; elemental analysis for C17H22N8O3: calculated: C, 52.84; H, 5.74; N, 29.00. Found: C, 52.82; H, 5.71; N, 28.85.
4-Hydrazinyl-N-(4-nitrophenyl)-6-(piperazin-1-yl)-1,3,5-triazin-2-amine hydrate (8b)
Light yellow crystals; yield: 59 %; m.p.: 187–188 °C; MW: 349.35; R f: 0.46; FTIR (ν max; cm−1 KBr): 3,323.98 (N–H stretching in piperazine), 3,312.34 (NH2), 3,287.56 (N–H secondary), 3,046.72 (C–H broad), 2,803–3,012 (aromatic CH2) 1,676.23–1,639.32 (aromatic C=N), 1,616.15 (N–H bending piperazine), 1,606 (C=C), 1,349.13-1,008.19 (aromatic C–N), 1,523.27 (NO2), 767; 1H NMR (400 MHz, CDCl3-d 6, TMS) δ ppm: 8.13 (d, 2H J = 8.67 Hz, 2× CH, Ar–H), 7.35 (d, 2H, J = 1.01 Hz 2× CH, Ar–H), 3.65 (br,s 1H, NH), 1.97 (s, 2H, NH2), 3.15 (d, 4H J = 13.25 Hz, 2× CH2, Ar–H), 2.76 (d,4H J = 3.11 Hz, 2× CH2, Ar–H), 1.97 (s,1H NH); 13C NMR (100 MHz, CDCl3) δ ppm: 184.25, 178.54, 171.75, 152.12, 142.65, 128.73, 121.51, 52.48, 47.14; mass: 351.12 (M+H)+; elemental analysis for C13H19N9O3: calculated: C, 44.69; H, 5.48; N, 36.08. Found: C, 44.71; H, 5.49; N, 36.12.
2-(4-((4-Nitrophenyl)amino)-6-(piperazin-1-yl)-1,3,5-triazin-2-yl)hydrazinecarbothioamide (8c)
Brown crystals; yield: 71 %; m.p.: 197–198 °C; MW: 390.42; R f: 0.58; FTIR (ν max; cm−1 KBr):3,328.98 (N–H stretching in piperazine), 3,321.43 (NH2), 3,286.25 (N–H secondary), 3,048.83 (C–H broad), 1,676.25–1,630.48 (aromatic C=N), 1,617.15 (N–H bending piperazine), 1,525.27 (NO2), 1,349.13-1,007.19 (aromatic C–N), 1,274.78 (C=S),753; 1H NMR (400 MHz, CDCl3-d 6, TMS) δ ppm: 8.05 (d, 2H J = 8.67 Hz, 2× CH, Ar–H), 7.94 (d, 2H, J = 0.96 Hz, 2× CH, Ar–H), 3.67 (br,s, 1H, NH), 1.97 (s, 1H, NH), 8.57 (s, 1H, NH2), 3.18 (d, 4H J = 13.29 Hz, 2× CH2, Ar–H), 2.74 (d,4H J = 3.29 Hz, 2× CH2, Ar–H), 1.95 (s,1H NH); 13C NMR (100 MHz, CDCl3) δ ppm: 185.54, 181.32, 178.53, 186.72, 148,75, 139.36, 127.83, 121.59, 49.13, 44.57; mass: 391.56 (M+H)+; elemental analysis for C14H18N10O2S: calculated: C, 43.07; H, 4.65; N, 35.88. Found: C, 43.11; H, 4.67; N, 35.89.
N 2-(4-nitrophenyl)-6-(piperazin-1-yl)-N 4-(o-tolyl)-1,3,5-triazine-2,4-diamine (8d)
Light yellow crystals; yield: 64 %; m.p.: 212–213 °C; MW:406.44; R f : 0.47; FTIR (ν max; cm−1 KBr): 3,328.98 (N–H stretching in piperazine), 3,286.43 (N–H secondary), 3,043.78 (C–H broad), 1,679.56–1,637.35 (aromatic C=N), 1,663–1,602 (aromatic C=C), 1,617.15 (N–H bending piperazine), 1,525.29 (NO2), 1,455.38 (C–C stretching aromatic), 1,349.42–1,018.15 (aromatic C–N), 892, 635; 1H NMR (400 MHz, CDCl3-d 6, TMS) δ ppm: 8.15 (d, 2H J = 8.62 Hz, 2× CH, Ar–H), 7.38 (d, 2H, J = 1.15 Hz, 2× CH, Ar–H), 3.64 (br,s, 1H, NH), 7.14(d, 1H J = 8.42 Hz, 1× CH, Ar–H), 7.01 (d, 1H J = 7.89 Hz, 1× CH, Ar–H), 6.86 (d, 1H J = 1.18 Hz, 1× CH, Ar–H), 2.16 (s, 3H, CH3), 3.21 (d, 4H J = 13.26 Hz, 2× CH2, Ar–H), 2.82 (d,4H J = 3.11 Hz, 2× CH2, Ar–H), 1.98 (s,1H NH); 13C NMR (100 MHz, CDCl3) δ ppm: 184.56, 176.73, 168.63, 152.32, 143.21, 139.92, 134.36, 131.42, 128.57, 125.73, 124.83, 119.25, 52.31, 46.82, 18.64; mass: 407.47 (M+H)+; elemental analysis for C20H22N8O2: calculated: C, 59.10; H, 5.46; N, 27.57. Found: C, 59.13; H, 5.47; N, 27.54.
N 2-(4-nitrophenyl)-N 4-phenyl-6-(piperazin-1-yl)-1,3,5-triazine-2,4-diamine (8e)
Brown crystals; yield: 79 %; m.p.: 217–218 °C; MW: 392.41; R f: 0.59; FTIR (ν max; cm−1 KBr): 3,328.98 (N–H stretching in piperazine), 3,285.45 (N–H secondary), 3,045.78 (C–H broad), 1,672.53–1,631.33 (aromatic C=N), 1,617.15 (N–H bending piperazine), 1,590.27 (C=C stretching aromatic) 1,529.38 (NO2), 1,349.15–1,015.19 (aromatic C–N), 779, 625; 1H NMR (400 MHz, CDCl3-d 6, TMS) δ ppm: 8.13 (d, 2H J = 8.67 Hz, 2× CH, Ar–H), 7.32 (d, 2H, J = 0.69 Hz, 2× CH, Ar–H), 3.68 (br,s, 1H, NH), 7.55 (d, 1H J = 8.15 Hz, 1× CH, Ar–H), 7.46 (d, 1H J = 8.13 Hz, 1× CH, Ar–H), 6.91 (d, 1H J = 1.21 Hz, 1× CH, Ar–H), 3.18 (d, 4H J = 13.29 Hz, 2× CH2, Ar–H), 2.91 (d,4H J = 3.16 Hz, 2× CH2, Ar–H), 1.97 (s,1H NH); 13C NMR (100 MHz, CDCl3) δ ppm: 182.35, 173.42, 168.36, 153.63, 142.23, 138.73, 132.46, 128.92, 124.24, 119.53, 52.85, 47.84; mass: 393.49 (M+H)+; elemental analysis for C19H20N8O2: calculated: C, 58.15; H, 5.14; N, 28.55. Found: C, 58.17; H, 5.13; N, 28.45.
2-(4-((4-Nitrophenyl)amino)-6-(piperazin-1-yl)-1,3,5-triazin-2-yl)hydrazinecarboxamide (8f)
Yellow crystals; yield: 64 %; m.p.: 234–235 °C; MW:374.16; R f: 0.62; FTIR (ν max; cm−1 KBr): 3,328.98 (N–H stretching in piperazine), 3,323.38 (NH2), 3,289.42 (N–H secondary), 3,046.72 (C–H broad), 1,672.56–1,634.38 (aromatic C=N), 1,663, 1,617.15 (N–H bending piperazine) 1,527.29 (NO2), 1,337.17–1,008.29 (aromatic C–N), 1,759.66 (C=O),753; 1H NMR (400 MHz, CDCl3-d 6, TMS) δ ppm: 8.08 (d, 2H J = 8.63 Hz, 2× CH, Ar–H), 7.48 (d, 2H, J = 0.78 Hz, 2× CH, Ar–H), 3.65 (br,s, 1H, NH), 6.73 (s, 1H, NH), 6.82 (s, 1H, NH2), 3.21 (d, 4H J = 13.25 Hz, 2× CH2, Ar–H), 2.96 (d,4H J = 3.12 Hz, 2× CH2, Ar–H), 1.93 (s,1H NH); 13C NMR (100 MHz, CDCl3) δ ppm: 185.93, 178.32, 168.34, 158.63, 148.75, 138,72, 128.42, 121.63, 52.36, 45.83; mass: 375.28 (M+H)+; elemental analysis for C14H18N10O3: calculated: C, 44.92; H, 4.85; N, 37.42. Found: C, 44.95; H, 4.82; N, 37.47.
N 2-(3-aminopropyl)-N 4-(4-nitrophenyl)-6-(piperazin-1-yl)-1,3,5-triazine-2,4-diamine (8g)
White crystals; yield: 65 %; m.p.: 241–242 °C; MW: 373.41; R f: 0.43; FTIR (ν max; cm−1 KBr): 3,327.95 (N–H stretching in piperazine), 3,319.56 (NH2), 3,284.57 (N–H secondary), 3,041.78 (C–H broad), 2,964.65 (C–H stretching in aliphatic chain) 1,674.45–1,638.12 (aromatic C=N), 1,668, 1,618.13 (N–H bending piperazine), 1,347.13–1,023.19 (aromatic C–N), 1,527.27 (NO2), 1,455.38 (C–C stretching), 756; 1H NMR (400 MHz, CDCl3-d 6, TMS) δ ppm: 8.16 (d, 2H J = 8.68 Hz, 2× CH, Ar–H), 7.35 (d, 2H, J = 1.03 Hz, 2× CH, Ar–H), 3.68 (br,s, 1H, NH), 3.27 (t, 2H J = 6.78 Hz, CH2,), 2.74 (t, 2H, J = 6.83 Hz, CH2), 1.69 (t, 2H, J = 6.74 Hz, CH2), 6.74 (s, 1H, NH2), 3.19 (d, 4H J = 13.29 Hz, 2× CH2, Ar–H), 2.84 (d,4H J = 3.14 Hz, 2× CH2, Ar–H), 1.96 (s,1H NH); 13C NMR (100 MHz, CDCl3) δ ppm: 184.52, 172.65, 148.72, 142.83, 128.93, 121.53, 53.42, 46.75, 39.45, 32.21; mass: 374.56 (M+H)+; elemental analysis for C16H23N9O2: calculated: C, 51.46; H, 6.21; N, 33.76. Found: C, 51.48; H, 6.20; N, 33.76.
4-((4-((4-Nitrophenyl)amino)-6-(piperazin-1-yl)-1,3,5-triazin-2-yl)amino)phenol (8h)
Black crystals; yield: 72 %; m.p.: 236–238 °C; MW:408.41; R f: 0.59; FTIR (ν max; cm−1 KBr): 3,328.93 (N–H stretching in piperazine), 3,416 (OH stretching), 3,286.51 (N–H secondary), 3,046.58 (C–H broad), 2,800–3,012 (CH2 stretching), 1,672.42–1,637.16 (aromatic C=N), 1,618.13 (N–H bending piperazine), 1,605 (C=C), 1,347.13–1,023.19 (aromatic C–N), 1,527.27 (NO2), 753; 1H NMR (400 MHz, CDCl3-d 6, TMS) δ ppm: 8.13 (d, 2H J = 8.66 Hz, 2× CH, Ar–H), 7.37 (d, 2H, J = 0.68 Hz, 2× CH, Ar–H), 3.68 (br,s, 1H, NH), 7.37 (d, 1H J = 8.74 Hz, Ar–H), 6.87 (d, 1H, J = 3.22 Hz, Ar–H), 5.48 (s, 1H, OH), 3.24 (d, 4H J = 13.24 Hz, 2× CH2, Ar–H), 2.91 (d,4H J = 3.11 Hz, 2× CH2, Ar–H), 1.98 (s,1H NH); 13C NMR (100 MHz, CDCl3) δ ppm: 183.42, 173.23, 167.84, 153.57, 147.32, 139.24, 134.47, 124.63, 123.21, 119.56, 117.82, 52.31, 46.73; mass: 409.45 (M+H)+; elemental analysis for C19H20N8O3: calculated: C, 55.88; H, 4.94; N, 27.44. Found: C, 55.90; H, 4.92; N, 27.43.
N 2-(4-nitrophenyl)-6-(piperazin-1-yl)-N 4-(p-tolyl)-1,3,5-triazine-2,4-diamine (8i)
Yellow crystals; yield: 73 %; m.p.: 218–219 °C; MW:406.44; R f: 0.54; FTIR (ν max; cm−1 KBr): 3,329.97 (N–H stretching in piperazine), 3,283.48 (N–H secondary), 3,045.71 (C–H broad), 1,674.53–1,633.31 (aromatic C=N), 1,668–1,609 (aromatic C=C), 1,613.19 (N–H bending piperazine), 1,527.28 (NO2), 1,456.35 (C–C stretching aromatic), 1,344.41–1,015.12 (aromatic C–N), 897, 632; 1H NMR (400 MHz, CDCl3-d 6, TMS) δ ppm: 8.14 (d, 2H J = 8.66 Hz, 2× CH, Ar–H), 7.34 (d, 2H, J = 0.69 Hz, 2× CH, Ar–H), 3.67 (br,s, 1H, NH), 7.38 (d, 1H J = 8.09 Hz, Ar–H), 7.05 (d, 1H J = 5.42 Hz, Ar–H), 2.25 (s, 3H, CH3), 3.28 (d, 4H J = 13.29 Hz, 2× CH2, Ar–H), 2.87 (d,4H J = 3.08 Hz, 2× CH2, Ar–H), 1.97 (s,1H NH); 13C NMR (100 MHz, CDCl3) δ ppm: 183.42, 172.53, 168.75, 153.47, 142.76, 134.21, 129.72, 126.42, 121.62, 118.82, 52.12, 46.35; mass: 407.47 (M+H)+; elemental analysis for C20H22N8O2: calculated: C, 59.10; H, 5.46; N, 27.57. Found: C, 59.13; H, 5.47; N, 27.54.
General procedure for the synthesis of titled compounds 9(a–i)
A solution of compound (3) (0.01 mol) and desired tri-substituted 1,3,5-triazine compounds 8(a–i) (0.01 mol) in dry acetone was stirred at 40–45 °C for 8–9 h. The completion of reaction was monitored by TLC using ethanol:acetone (1:1) as mobile phase. The reaction mixture was filtered and concentrated under reduced pressure. The resulting residue was dissolved in dichloromethane, washed with brine and dried over anhydrous Na2SO4. The dried solution was concentrated under reduced pressure to obtain the titled compounds 9(a–i).
N-(7-chloroquinolin-4-yl)-4-(4-morpholino-6-((4-nitrophenyl)amino)-1,3,5-triazin-2-yl)piperazine-1-carbothioamide (9a)
Light black crystals; yield: 57 %; m.p: 256–257 °C; MW:607.09; R f: 0.42; FTIR (ν max; cm−1 KBr): 3,397.12 (C–O stretching), 2,933.32(C–H stretching), 2,344.21 (N–H stretching secondary amine), 1,602.13(N=O stretching), 1,325.63–1,489.96 (C=C stretching), 1,219.82 (C–N stretching), 771.13 (Cl), 685.7 (C=S stretching); 1H NMR (400 MHz, CDCl3-d 6, TMS) δ ppm: 8.36 (d, 1H J = 6.60 Hz, quinoline ring), 7.35 (d, 1H J = 6.65 Hz, quinoline ring), 4.07 (m, 4H, 2× CH2, Ar–H), 2.54 (m, 4H, 2× CH2, Ar–H), 6.90 (m,2H C–H, Ar–H), 4.13 (br, s, 1H, NH); 13C NMR (100 MHz, CDCl3); δ ppm:185.53, 181.25, 174.31, 168.93, 152.73, 151.26, 145.44, 137.51, 134.83, 129.44, 125.62, 124.74, 121.68, 119.21, 68.35, 58.72, 52.14, 48.94; mass: 608.18 (M+H)+; elemental analysis for C27H28ClN11O3S: calculated: C, 53.42; H, 4.48; N, 23.07. Found: C, 53.46; H, 4.46; N, 23.09.
N-(7-chloroquinolin-4-yl)-4-(4-hydrazinyl-6-((4-nitrophenyl)amino)-1,3,5-triazin-2-yl)piperazine-1-carbothioamide hydrate (9b)
Light yellow crystals; yield: 73.32 %; m.p.: 230–236 °C; MW: 570.03; R f: 0.27; FTIR (ν max; cm−1 KBr):3,396.21 (O–H stretching), 2,372.23 (N–H stretching secondary amine), 1,592.25 (N=O stretching), 1,432.81 (C=N stretching), 1,324.41–1,488.73 (C=C stretching aromatic), 1,220.62 (C–N stretching), 771.73 (C–Cl stretching), 676.32 (C=S stretching); 1H NMR (400 MHz, CDCl3-d 6, TMS) δ ppm: 8.36 (d, 1H J = 6.60 Hz, quinoline ring), 7.35 (d, 1H J = 6.65 Hz, quinoline ring), 4.07 (m, 4H, 2× CH2, Ar–H), 6.90 (m,2H C–H, Ar–H), 6.70 (s, 1H, NH), 4.13 (br, s, 1H, NH),; 13C NMR (100 MHz, CDCl3); δ ppm: 186.73, 181.35, 176.42, 153.92, 151.52, 151.17, 150.06, 139.13, 135.58, 126.83, 124.80, 124.36, 119.39, 117.83, 40.74, 39.94; mass: 572.13 (M+H)+; elemental analysis for C23H24ClN11O3S: calculated: C, 48.46; H, 4.24; N, 27.03. Found: C, 48.50; H, 4.21; N, 27.13.
4-(4-(2-Carbamothioylhydrazinyl)-6-((4-nitrophenyl)amino)-1,3,5-triazin-2-yl)-N-(7-chloroquinolin-4-yl)piperazine-1-carbothioamide (9c)
Dark yellow crystals; yield: 64.16 %; m.p.: 270–271 °C; MW: 611.10; R f: 0.49; FTIR (ν max; cm−1 KBr): 3,417.21 (N–H stretching of primary amine), 2,373.52 (N–H stretching of secondary amine), 1,595.72 (N–O stretching), 1,429.31 (C=N stretching), 1,330.32–1,485.72 (C=C aromatic), 1,220.12 (C–N stretching), 771.62 (C–Cl stretching), 677.14 (C=S stretching); 1H NMR (400 MHz, CDCl3-d 6, TMS) δ ppm: 8.39 (d, 1H J = 6.62 Hz, quinoline ring), 7.32 (d, 1H J = 6.64 Hz, quinoline ring), 4.09 (m, 4H, 2× CH2), 6.88 (m,2H C–H, Ar–H), 6.78 (s, 2H, NH2), 2.50 (s, 1H NH), 4.19 (br, s, 1H, NH),; 13C NMR (100 MHz, CDCl3); δ ppm: 185.32, 183.12, 179.72, 175.68, 171.65, 152.81, 146.92, 138.96, 135.32, 126.83, 124.80, 121.47, 120.38, 119.39, 58.94, 54.23; mass: 612.12 (M+H)+; elemental analysis for C24H23ClN12O2S2: calculated: C, 47.17; H, 3.79; N, 27.50. Found: C, 47.16; H, 3.76; N, 27.53.
N-(7-chloroquinolin-4-yl)-4-(4-((4-nitrophenyl)amino)-6-(o-tolylamino)-1,3,5-triazin-2-yl)piperazine-1-carbothioamide (9d)
Light yellow crystals; yield: 48.83 %; m.p.: 274–276 °C; MW: 627.12; R f: 0.46; FTIR (ν max; cm−1 KBr): 3,397.83 (N–H stretching), 2,371.13 (N–H stretching in secondary amine), 1,500.63 (N=O stretching), 1,418.14 (C=N stretching), 1,333.82 (C=C stretching aromatic), 1,222.21 (C–N stretching), 771.63 (C–Cl stretching), 662.85 (C=S stretching); 1H NMR (400 MHz, CDCl3-d 6, TMS) δ ppm: 8.81 (d, 1H J = 8.23 Hz, quinoline ring), 7.90 (d, 1H J = 6.78 Hz, quinoline ring), 4.23 (m, 4H, 2× CH2), 6.93 (m,2H C–H, Ar–H), 3.66–3.85 (d, 4H J = 48.6 Hz, Ar–H), 2.24 (s, 1H NH), 2.51 (s, 3H, CH3), 4.19 (br, s, 1H, NH); 13C NMR (100 MHz, CDCl3); δ ppm: 186.83, 178.64, 169.41, 164.62, 154.12, 149.53, 143.55, 142, 137.92, 136.35, 132.32, 131.96, 129.21, 126.83, 126.12, 123.97, 121.63, 119.26, 58.71, 53.37; mass: 628.35 (M+H)+; elemental analysis for C30H27ClN10O2S: calculated: C, 57.46; H, 4.34; N, 22.33. Found: C, 57.49; H, 4.31; N, 22.36.
N-(7-chloroquinolin-4-yl)-4-(4-((4-nitrophenyl)amino)-6-(phenylamino)-1,3,5-triazin-2-yl)piperazine-1-carbothioamide (9e)
Brown crystals; yield: 49.23 %; m.p.: 247–248 °C; MW: 613.09; R f: 0.54; FTIR (ν max; cm−1 KBr): 3,020.93 (C–H stretching aromatic), 2,336.21–2,402.35 (N–H stretching secondary amine), 1,500,24 (N=O stretching), 1,417.68 (C=N stretching), 1,330.26–1,493.47 (C=C stretching), 1,217.48 (C–N stretching), 771 (C–Cl stretching), 670.85 (C=S stretching); 1H NMR (400 MHz, CDCl3-d 6, TMS) δ ppm: 8.13 (d, 1H J = 8.76 Hz, quinoline ring), 7.78 (d, 1H J = 6.36 Hz, quinoline ring), 4.12 (m, 4H, 2× CH2), 6.85 (m,2H C–H, Ar–H), 3.78 (m. 4H, 4× CH, Ar–H), 2.50 (s, 1H NH), 4.19 (br, s, 1H, NH),; 13C NMR (100 MHz, CDCl3); δ ppm: 186.85, 181.65, 173.42, 168.96, 158.47, 151.28, 149.36, 146.73, 139.96, 138.21, 136.24, 129.43, 128.94, 126.72, 125.93, 121.67, 119.85, 117.85, 113.38, 57.71, 53.12; mass: 614.17 (M+H)+; elemental analysis for C29H25ClN10O2S: calculated: C, 56.81; H, 4.11; N, 22.85. Found: C, 56.78; H, 4.15; N, 22.87.
2-(4-(4-((7-Chloroquinolin-4-yl)carbamothioyl)piperazin-1-yl)-6-((4-nitrophenyl)amino)-1,3,5-triazin-2-yl)hydrazinecarboxamide (9f)
Dark yellow crystals; yield: 46.91 %; m.p.: 214–215 °C; MW: 595.04; R f: 0.52; FTIR (ν max; cm−1 KBr):3,116.63–3,411.87 (N–H stretching of primary amine), 3,020.21 (C–H stretching), 2,402.23 (N–H stretching secondary amine), 1,782.83 (C=O stretching), 1,488.53 (N=O stretching), 1,437.13 (C=N stretching), 1,322.64–1,363.52 (C=C stretching), 1,217.15 (C–N stretching), 770.37 (C–Cl stretching), 670.49 (C=S stretching); 1H NMR (400 MHz, CDCl3-d 6, TMS) δ ppm: 8.47 (d, 1H J = 6.24 Hz, quinoline ring), 7.61 (d, 1H J = 5.12 Hz, quinoline ring), 4.21 (m, 4H, 2× CH2), 6.82 (m, 2H C–H, Ar–H), 6.54 (s, 2H, NH2), 2.60 (s, 1H NH), 4.32 (br, s, 1H, NH),; 13C NMR (100 MHz, CDCl3); δ ppm: 185.63, 182.36, 176.32, 171.92, 163.41, 154.48, 151.52, 149.32, 146.12, 138.81, 136.94, 129.42, 124.81, 123.42, 121.65, 119.72, 118.54, 57.71, 53.48; mass: 597.07 (M+H)+; elemental analysis for C24H23ClN12O3S: calculated: C, 48.44; H, 3.90; N, 28.25. Found: C, 48.47; H, 3.93; N, 28.24.
4-(4-((3-Aminopropyl)amino)-6-((4-nitrophenyl)amino)-1,3,5-triazin-2-yl)-N-(7-chloroquinolin-4-yl)piperazine-1-carbothioamide (9g)
Dark brown crystals; yield: 88.83 %; m.p.: 196–197 °C; MW: 594.09; R f: 0.24; FTIR (ν max; cm−1 KBr):3,371.43 (N–H stretching primary amine), 3,020.71 (C–H stretching), 2,369.83 (N–H secondary amine), 1,485.47 (N=O stretching), 1,419.52 (C=N stretching), 1,326.12 (C=C stretching), 1,217.57 (C–N stretching), 768.82 (C–Cl stretching), 671.75 (C=S stretching); 1H NMR (400 MHz, CDCl3-d 6, TMS) δ ppm: 8.67 (d, 1H J = 5.87 Hz, quinoline ring), 7.70 (d, 1H J = 5.37 Hz, quinoline ring), 4.78 (m, 4H, 2× CH2), 6.895 (m, 2H C–H, Ar–H), 6.68 (s, 2H, NH2), 3.67 (m, 2H, CH2) 2.50 (s, 1H NH), 4.38 (br, s, 1H, NH),; 13C NMR (100 MHz, CDCl3); δ ppm: 184.53, 178.91, 171.20, 164.52, 154.38, 151.21, 149.62, 148.36, 138.57, 136.29, 131.53, 129.78, 126.85, 123.62, 119.83, 118.38, 58.21, 53.28, 41.81, 32.17; mass: 596.12 (M+H)+; elemental analysis for C26H28ClN11O2S: calculated: C, 52.56; H, 4.75; N, 25.93. Found: C, 52.52; H, 4.77; N, 25.98.
N-(7-chloroquinolin-4-yl)-4-(4-((4-hydroxyphenyl)amino)-6-((4-nitrophenyl)amino)-1,3,5-triazin-2-yl)piperazine-1-carbothioamide (9h)
Brown yellow crystals; yield: 81.84 %; m.p.: 310–311 °C; MW: 629.09; R f: 0.46; FTIR (ν max; cm−1 KBr): 3,439.83 (O–H stretching), 3,020.73 (C–H stretching), 2,335.52–2,375.81 (N–H stretching in secondary amine), 1,625.38 (C=C stretching), 1,488.57 (N=O stretching), 1,414.32 (C=N stretching), 1,218.31 (C–N stretching), 770.83 (C–Cl stretching), 672.35 (C=S stretching); 1H NMR (400 MHz, CDCl3-d 6, TMS) δ ppm: 8.92 (d, 1H J = 7.54 Hz, quinoline ring), 7.45 (d, 1H J = 5.32 Hz, quinoline ring), 4.09 (m, 4H, 2×, Ar–H), 7.53 (m, 4H, 4× CH, Ar–H), 6.75 (m, 4H, 4× CH, Ar–H), 5.40 (s, 1H Ar-OH),4.05 (br, s, 1H, NH); 13C NMR (100 MHz, CDCl3); δ ppm: 185.62, 178.41, 171.28, 167.76, 161.78, 154.53, 149.51, 147.12, 139.96, 134.26, 132.54, 131.29, 128.92, 125.74, 123.15, 121.64, 118.63, 117.28, 115.38, 58.26, 53.42; mass: 630.21 (M+H)+; elemental analysis for C29H25ClN10O3S: calculated: C, 55.37; H, 4.01; N, 22.26. Found: C, 55.38; H, 4.00; N, 22.32.
N-(7-chloroquinolin-4-yl)-4-(4-((4-nitrophenyl)amino)-6-(p-tolylamino)-1,3,5-triazin-2-yl)piperazine-1-carbothioamide (9i)
Brown crysals; yield: 78.82 %; m.p.: 145–148 °C; MW: 627.12; R f: 0.64; FTIR (ν max; cm−1 KBr): 3,376.63–3,490.13 (N–H stretching), 2,934.23–3,078.51 (C–H stretching), 2,343.65 (N–H stretching secondary amine), 1,489.73 (N=O stretching), 1,414.57 (C=N stretching), 1,327.12 (C=C stretching), 1,218.57 (C–N stretching), 769.18(C–Cl stretching), 673.37 (C=S stretching); 1H NMR (400 MHz, CDCl3-d 6, TMS) δ ppm: 8.84 (d, 1H J = 7.42 Hz, quinoline ring), 7.43 (d, 1H J = 5.38 Hz, quinoline ring), 4.09 (m, 4H, 2× CH2, Ar–H), 3.67 (m, 4H, 2× CH2, Ar–H), 7.08 (m, 2H, 2× CH, Ar–H), 7.43 (m, 2H, 2× CH, Ar–H), 2.49 (t, 3H, CH3),4.17 (br, s, 1H, NH); 13C NMR (100 MHz, CDCl3); δ ppm: 186.56, 182.38, 172.94, 168.36, 161.24, 153.58, 148.36, 145.58, 141.27, 138.69, 136.94, 132.26, 131.86, 129.47, 126.64, 123.74, 121.87, 118.26, 58.73, 51.54, 23.47; mass: 628.10 (M+H)+; elemental analysis for C30H27ClN10O2S: calculated: C, 57.46; H, 4.34; N, 22.33. Found: C, 57.49; H, 4.32; N, 22.35.
Antibacterial screening
Minimum inhibitory concentration
Entire target compounds were screened for their minimum inhibitory concentration (MIC, μg/mL) against selected gram-positive organisms viz. B. subtilis (NCIM-2,063), B. cereus (NCIM-2,156), S. aureus (NCIM-2079) and gram-negative organism viz. P. aeruginosa (NCIM-2036), E. coli (NCIM-2065), P. mirabilis (NCIM-2241), P. vulgaris (NCIM-2027) by the broth dilution method as recommended by the National Committee for Clinical Laboratory Standards with minor modifications. Ofloxacin was used as standard antibacterial agent. Solutions of the test compounds and reference drug were prepared in dimethyl sulfoxide (DMSO) at concentrations of 100, 50, 25, 12.5, 6.25 and 3.125 μg mL−1. Eight tubes were prepared in duplicate with the second set being used as MIC reference controls (16–24 h visual). After sample preparation, the controls were placed in a 37 °C incubator and read for macroscopic growth (clear or turbid) the next day. Into each tube, 0.8 mL of nutrient broth was pipette (tubes 2–7), tube 1 (negative control) received 1.0 mL of nutrient broth and tube 8 (positive control) received 0.9 mL of nutrient. Tube 1, the negative control, did not contain bacteria or antibiotic. The positive control, tube 8, received 0.9 mL of nutrient broth since it contained bacteria but not antibiotic. The test compound were dissolved in DMSO (100 μg/mL), 0.1 mL of increasing concentration of the prepared test compounds which are serially diluted from tube 2 to tube 7 from highest (100 μg mL−1) to lowest (3.125 μg mL−1) concentration (tube 2–7 containing 100, 50, 25, 12.5, 6.25, 3.125 μg mL−1). After this process, each tube was inoculated with 0.1 mL of the bacterial suspension, concentration of which corresponded to 0.5 McFarland scale (9 × 108 cells/mL) and each bacterium was incubated at 37 °C for 24 h. The final volume in each tube was 1.0 mL. The incubation chamber was kept humid. At the end of the incubation period, MIC values were recorded as the lowest concentration of the substance that gave no visible turbidity, i.e. no growth of inoculated bacteria, (National Committee for Clinical Laboratory Standards, 1982) results were shown in Table 1.
Conclusion
As a concluding remark, we had developed a new series of hybrid 4-aminoquinoline-1,3,5-triazine conjugates as potent antibacterial agents through facile and economical route. In addition, this study suggests the potential utility of this hybrid skeleton to develop newer antibacterial agents. Our studies are in progress towards the development of newer entities of this skeleton and reported subsequently in future.
References
Alanis AJ (2005) Resistance to antibiotics: are we in the post-antibiotic era. Arch Med Res 36:697–705
Bhat HR, Singh UP, Yadav PS, Kumar V, Das A, Chetia D, Prakash A, Mahanta J (2011) Synthesis, characterization and antimalarial activity of hybrid 4-aminoquinoline-1,3,5-triazine derivatives. Arabian J Chem. doi:10.1016/j.arabjc.2011.07.001
Bhat HR, Ghosh SK, Prakash A, Gogoi K, Singh UP (2012a) In vitro antimalarial activity and molecular docking analysis of 4-aminoquinoline-clubbed 1,3,5-triazine derivatives. Lett Appl Microbiol 54:483–486
Bhat HR, Gupta SK, Singh UP (2012b) Discovery of potent, novel antibacterial hybrid conjugates from 4-aminoquinoline and 1,3,5-triazine: design, synthesis and antibacterial evaluation. RSC Adv 2:12690–12695
Bhat HR, Singh UP, Gahtori P, Ghosh SK, Gogoi K, Prakash A, Singh RK (2013) Antimalarial activity and docking studies of novel bi-functional hybrids derived from 4-aminoquinoline and 1,3,5-triazine against wild and mutant malaria parasites as pf-DHFR inhibitor. RSC Adv. doi:10.1039/C2RA21915H
Corbett TH, Leopold WR, Dykes DJ, Roberts BJ, Griswold DP Jr, Schabel FM Jr (1982) Toxicity and anticancer activity of a new triazine antifolate (NSC 127755). Cancer Res 42:1707–1715
Davies J, Davies D (2010) Origins and evolution of antibiotic resistance. Microbiol Mol Biol 74:417–733
Dubey V, Pathak M, Bhat HR, Singh UP (2012) Design, facile synthesis and antibacterial activity of hybrid 1,3,4-thiadiazole-1,3,5-triazine derivatives tethered via S-bridge. Chem Biol Drug Des 80:598–604
Gahtori P, Ghosh SK, Singh B, Singh UP, Bhat HR, Uppal A (2012) Synthesis, SAR and antibacterial activity of hybrid chloro, dichloro-phenylthiazolyl-s-triazines. Saudi Pharm J 20:35–43
Ghosh SK, Saha A, Hazarika B, Singh UP, Bhat HR, Gahtori P (2012) Design, facile synthesis, antibacterial activity and structure-activity relationship of novel di- and tri-substituted 1,3,5-triazines. Lett Drug Des Dis 9:329–335
Junejo JA, Ghosh SK, Shaikh M, Gahtori P, Singh UP (2011) Facile synthesis, antibacterial activity and molecular properties prediction of Some new 1,3-dihydroimidazol-2-thione derivatives. Lett Drug Des Dis 8:763–768
Kumar S, Bhat HR, Kumawat MK, Singh UP (2013) Design and one-pot synthesis of hybrid thiazolidin-4-one-1,3,5-triazines as potent antibacterial agent against human disease causing pathogens. New J Chem. doi:10.1039/C2NJ41028A
Lozano V, Aguado L, Hoorelbeke B, Renders M, Camarasa MJ, Schols D, Balzarini J, San-Félix A, Pérez-Pérez MJ (2011) Targeting HIV entry through interaction with envelope glycoprotein 120 (gp120): synthesis and antiviral evaluation of 1,3,5-triazines with aromatic amino acids. J Med Chem 54:5335–5348
Martinez JL, Baquero F (2000) Mutation frequencies and antibiotic resistance. Antimicrob Agents Chemother 44:1771–1777
National Committee for Clinical Laboratory Standards (1982) Standard methods for dilution antimicrobial susceptibility test for bacteria which grow aerobically. NCCLS, Villanova, p 242
Shapiro RS, Robbins N, Cowen LE (2011) Regulatory circuitry governing fungal development, drug resistance, and disease. Microbiol Mol Biol Rev 75:213–267
Singh UP, Singh RK, Bhat HR, Subhaschandra YP, Kumar V, Kumawat MK, Gahtori P (2011) Synthesis and antibacterial evaluation of a series of novel trisubstituted-s-triazines derivatives. Med Chem Res 20:1603–1610
Singh UP, Bhat HR, Gahtori P (2012a) Antifungal activity, SAR and physicochemical correlation of some thiazole-1,3,5-triazine derivatives. J Mycol Med 22:134–141
Singh UP, Pathak M, Dubey V, Bhat HR, Gahtori P, Singh RK (2012b) Design, synthesis, antibacterial activity and molecular docking studies on novel hybrid 1,3-thiazine-1,3,5-triazine derivatives as potential bacterial translation inhibitor. Chem Biol Drug Des 80:572–583
Singh UP, Bhat HR, Gahtori P, Singh RK (2013) Hybrid thiazole-1,3,5-triazines target cytosolic Leucyl-tRNA synthetase for antifungal action revealed by molecular docking studies. In Silico Pharmacology, In press
Singh UP, Bhat HR, Singh RK (2013b) Ceric ammonium nitrate catalysed expeditious one-pot synthesis of 1,3-thiazine as IspE kinase inhibitor of gram-negative bacteria using polyethylene glycol (PEG-400) as an efficient recyclable reaction medium. C. R. Chime. doi:10.1016/j.crci.2012.11.019
Acknowledgments
Authors are gratified to SAIF, Central Drug Research Institute, Lucknow, India for providing spectral data of compounds synthesized herein and SHIATS for providing basic facilities to carry out the project.
Conflict of interest
Authors declare no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bhat, H.R., Pandey, P.K., Ghosh, S.K. et al. Development of 4-aminoquinoline-1,3,5-triazine conjugates as potent antibacterial agent through facile synthetic route. Med Chem Res 22, 5056–5065 (2013). https://doi.org/10.1007/s00044-013-0521-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-013-0521-8