Abstract
To supplement hits from a high through put screening, a docking study on butenolides derivatives was performed as COX-II inhibitors. The fourteen ligands were docked inside the ligand-binding domain (LBD) of protein data bank PDB ID: 3HS5 utilizing Maestro version 9. Out of 14 compounds, compounds I and XII were found to embed in the hydrophobic pocket by forming hydrogen bonds with the amino acids HOH902, THR212, and THR206. Nitrogen of the pyrrolone ring of compound XII formed a strong hydrogen bond with THR206 with the distance of 2.221 Å and had showed highest glide score (−9.3) and lowest energy −95.66 kJ/mol. Glide score of Diclofenac and Celecoxib was found to be −10.33 and −11.37, respectively. Some other docked compounds also showed good glide scores comparable to standard anti-inflammatory drug Diclofenac, were III, V, VI, VII, IX, and XIV. Docking results were further validated by calculation of conformational energy, which was higher in case of Diclofenac and Celecoxib, i.e., −33.57 and −43.7 kJ/mol, respectively, in comparison to hypothetically designed compounds. The compounds that had highest glide score, lowest conformational energy are generally considered better and can be used for further drug designing and synthesize in laboratory. The most potent compound was XII having highest glide score and lowest conformational energy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Non-steroidal anti-inflammatory drugs (NSAIDs) are widely used in the treatment of rheumatoid arthritis and inflammatory diseases. However, long-term use of NSAIDs has been associated with gastrointestinal bleeding and nephrotoxicity (Omar et al., 1996). Anti-inflammatory activity of non-steroidal anti-inflammatory drugs (NSAIDs) is mediated by inhibition of Cyclo-oxygenases, which results in decrease production of prostanoids, i.e. prostaglandins, prostacyclins, and thromboxane. This mechanism is believed to account for both therapeutic as well as adverse effects of NSAIDs. Two forms of COXs have been identified—COX-I and COX-II. While COX-I is expressed in most of body tissues, COX-II is present with low or undetectable levels in some tissues (Sandhu, 2003). The beneficial anti-inflammatory and analgesic activities are based on the inhibition of COX-II, but the gastro-intestinal toxicity and other side effects are due to the concurrent inhibition of COX-1 (Gupta, 1999). Agent which inhibits COX-II, while sparing COX-1 represents a new therapeutics goal for development of safer NSAIDs (Hawkey, 1999; Khannaa et al., 1997; Boehm and Smietana, 1996; Li et al., 1995; Manivannan et al., 2004). Pharmacological inhibition of COX can provide relief from pain and inflammation (en.wikipedia.org/wiki/cyclooxygenase). Pharmacological inhibition of PGs not only play a central role in inflammation, but also regulate other critical physiological responses, i.e. blood clotting, ovulation initiation of labour pain, nerve growth, and development (Singh et al., 2010) (Fig. 1).
Methods for predicting activity modes of organic molecules to protein receptors are widely used within drug discovery efforts. Ligand docking is typically achieved by generating a number of orientations (or poses) of a ligand within the active site, scoring of poses and identification of one or more poses that closely approximate the bioactive conformation. The bioactive conformations are determined by X-ray crystallography (Poulsen et al., 2008). Docking algorithms are also used for identifying putative binders from virtual chemical databases and for estimating the binding affinity of protein–ligand complexes (Prasanna et al., 2009).
Butenolides, also known as furanones and pyrrolones, are reported to have anti-inflammatory, analgesic, antiviral, and many other medicinal properties (Husain et al., 2005, Khan et al., 2002). Using this background information and our current interest in the ligand–protein binding, we attempted in-silico study of various hypothetical butenolides as COX-II inhibitors, by computational docking method. The compounds which were found to have high glide score and low energy were synthesized and evaluated for anti-inflammatory and analgesic activities. However, only docking studies are presented in this research paper (Figs. 2, 3).
Materials and methods
Docking is a computational method to determine possible binding modes of a ligand to the active site of a receptor. Docking studies has been performed with a set of hypothetical butenolide derivatives using Maestro 9.0 on COX-II (PDB ID 3HS5). The X-ray structures of which were accessed from the protein data bank (PDB). The basic structure of analogues is shown in Table 1. The COX-II (PDB ID 3HS5) X-ray structures were accessed from the protein data bank (PDB) (Fig. 4).
Ligand preparation
The molecules were built using Maestro 9.0 and converted to 3D structure from the 2D using Lig Prep version 5.5(Maestro version 9). Lig Prep is a robust collection of tools designed to prepare high quality, all—atom 3D structures for large numbers of drug-like molecules, starting with the 2D structures in SD or Maestro format. The resulting structures were saved in Maestro format. The simplest use of Lig Prep produces a single, low energy, 3D structure with correct chiralities for each successfully proposed input structure. While performing this step, chiralities were determined from 3D structure and original states of ionization were retained. Tautomers were generated using Macromodel method discarding current conformers (Fig. 5).
The conformational space was searched using the Monte Carlo (MCMM) method as implemented in Macromodel version 9.6 (Halgren, 1999). Each search was continued until the global energy minima were carried out using the truncated Newton Conjugate Gradient (TNCG) and the MMFFs force field as implemented in Macromodel. The conformational searches were done for aqueous solution using the generalized born/solvent accessible surface (GB/SA) continuum salvation (Hasel et al., 1988; Still et al., 1990) (Fig. 6).
Protein preparation
The COX-II (PDB ID 3HS5) X-ray structures were accessed from the protein data bank (PDB). The protein structure with polar hydrogen was prepared using the protein preparation wizard in Maestro (Maestro version, 2010). In this step, bond orders were assigned, all hydrogen’s in the structure were added, and bonds to metals were deleted and adjust the formal charge on the metal and the neighboring atoms and deleting waters that were more than the 5 Å specific distance. With generated Het states options, prediction of ionization, and tautomeric states of the het group at pH 7 was achieved. In protein preparation, optimization of hydrogen bond network was carried out by reorienting hydroxyl group, water molecules, and amino acids. The final step in the preparation process was to refine the structure, with the help of restrained minimization. It was initiated in the imperfect minimization with the 0.3 Å RMSD for the minimization OPLS_2001 force field (Figs. 7, 8).
Docking
Glide searches for favorable interactions between one or more ligand molecules and a receptor molecule, usually a protein. The shape and properties of the receptor were represented on a grid by several different sets of field that provide progressively more accurate scoring of the ligand poses. For receptors that adopted more than one conformation on binding, grids were prepared for each conformation, to ensure that possible activities were not missed. Ligand molecule was picked so it could be excluded from the grid generation with Vander waals radius scaling 1.00 and partial charge cutoff of 0.25. The compounds were docked using Glide with standard settings in standard precision (SP) mode. Grids were generated using Glide version 5.0 following the standard procedure recommended by Schrodinger. The docked poses discussed in it were not necessarily the highest scoring, but were selected as the highest scoring pose with a reasonable conformation and binding mode as judged by the modeler (Fig. 9).
Calculation of the conformational energy
The docked conformation was minimized with Macro Model using default restraining force constant of 100 kJ/mol. This allowed the docked conformations to relax (adjust) to the MMFFs force field. Without the relaxation the energy calculated by MMFFs would be meaningless. This relaxation did not change the conformation as RMS between the docked and relaxed structure are <0.1 Å.
Results and discussion
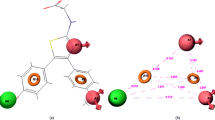
Most important features of docking are the logical interaction of the ligand with the putative-binding site of the enzyme. The molecules built using Maestro 9.0 were converted to 3D structure using Lig Prep version 5.5 (Maestro version 9). Using Lig Prep, a single, low energy, 3D structure with correct chiralities for each successfully proposed input structure was designed. Tautomers were generated using Macromodel method, discarding current conformers, and conformational space. Each search was continued until the global energy minima were carried out. The protein structure with polar hydrogen was prepared and optimization of hydrogen bond network was carried out. The favorable interactions between one or more ligand molecules and a receptor molecule was carried out by preparing grids where ever required, to ensure that possible activities were not missed. The process of evaluating a particular pose was achieved by counting the no. of favorable interactions that might be hydrogen, hydrophobic or electrostatic bonding. Out of fourteen compounds included in this study, two compounds (I and XII) were found to have best results which showed hydrogen bond interaction with amino acids of COX-II protein. The compounds (XIV, IX, VII, VI, V, and III) did not show any hydrogen bond interaction with pocket but good glide score between −9.14 and −8.03 and reasonable conformational energy. The compounds III and XIV did not showed hydrogen bonding but glide score and conformational energy comparable to I and XII, it may be due to some other type of bond interaction, other than hydrogen. From these studies it was observed that binding pocket within the COX-II structure are formed mainly by three amino acid residue HOH902, THR212, and THR206, as shown by most potent compounds I and XII. The results of docking studies by Schrodinger provided very useful information as given in Table 2.
The docking studies revealed useful information with respect to interaction of butenolide derivatives with COX-II receptor. Oxygen of the methoxyl group of compound I showed one hydrogen bond interaction to hydroxy group of amino acid residue HOH902 and same group also formed another hydrogen bond with residue THR212. They are highlighted in yellow colour. H-bond with the HOH902 had a distance of 2.248 and with THR212 had a distance of 1.935. The compound I had Glide score of −6.55.
The nitrogen atom of the quinoline ring of compound XII, formed a strong hydrogen bond with THR206, and showed the distance of 2.221. The compound XII had highest glide score (−9.3) and lowest energy, i.e. −95.66 kJ/mol.
Glide score of standard drug Diclofenac was found to be 10.33, whereas the glide scores of selected compounds III, V, VI, VII, IX, XII, and XIV were found as −9.14, −8.66, −8.03, −8.12, −8.47, 9.39, and 9.03, respectively. These glide scores data indicated that interaction of selected compounds was comparable to standard drug Diclofenac. Moreover, from the literature we found that Glide score of Celecoxib (Bhandari et al., 2009) was found to be −11.37, which was also comparable to hypothetical compounds. The results of glide score were further validated by calculation of conformational energy of Diclofenac (−33.57 kJ/mol) and Celecoxib (−43.7 kJ/mol), which was higher than the hypothetical compounds used in the study. The compounds that had highest glide score and lowest conformational energy, can be used for further drug designing and also provides a way to synthesize new potent compounds in laboratory. The most potent compound of our study was XII, which was having highest glide score and lowest conformational energy.
From the graph shown in Fig. 10, we found that there was a good correlation between glide score and conformational energy. As the conformational energy decreases, glide score increases in the same passion. The highest is glide score, lowest is conformational energy and compound is more stable in binding pocket.
Conclusion
Docking studies have helped us to know about the binding modes of the furanones to elicit their COX-II inhibitory activity. These investigations were found to be very useful during the synthesis of selected compounds, which were more potent and selective COX-II inhibitors. Moreover it was also proved from above discussion that geometry of receptor plays important role in defining drug action.
References
Bhandari SV, Dangre SC, Bothara KG, Patil AA, Sarkate AP, Lokwani DK, Gore ST, Deshmane BJ, Raparti VT, Khachane CV (2009) Design, synthesis and pharmacological screening of novel nitric oxide donars containing 1,5-diaryl pyrazolin-3-one as nontoxic NSAIDs. Eu J Med Chem 44(11):4622–4636
Boehm JC, Smietana JM (1996) 1-substituted-4-aryl-5-pyridinylimidazoles: a new class of cytokine suppressive drugs with low 5-lipoxygenase and cyclooxygenase inhibitory potency. J Med Chem 39:3929–3937
Gupta S (1999) Inhibitory activities of new series of 4,5-diaryl thiadiazoles derivatives on lipopolysaccharides-induced COX-2 expressions. Ind J Pharmacol 31(5):322–332
Halgren TA (1999) MMFF VI. MMFF94s option for energy minimization studies. J Comput Chem 20:720
Hasel WH, Hendrickson TF, Still WC (1988) A rapid approximation to the solvent accessible surface areas of atoms. Tetrahedron Comput Methodol 1(2):103–116
Hawkey CJ (1999) COX-2 inhibitors. Lancet 353:307–314
Husain A, Khan MSY, Hasan SM, Alam MM (2005) 2-Arylidene-4-(4-phenoxy-phenyl)but-3-en-4-olide:synthesis, reaction and biological activity. Eur J Med Chem 40(12):1394–1404
Khan MSY, Husain A, Sharma S (2002) Studies on butenolides: 2-arylidene-4-(substituted aryl) but-3-en-4-olides-synthesis, reactions and biological activity. Ind J Chem 41B(10):2160–2171
Khannaa IS, Weier RM, Collins PW (1997) 1,2-Diarylpyrrole as selective inhibitors of cyclo-oxygenase-2. J Med Chem 40:1619–1633
Li JJ, Anderson GD, Reitz DB (1995) 1,2-Diarylcyclopentenes as selective cyclooxygenase-2 inhibitors and orally active anti-inflammatory agents. J Med Chem 38:4570–4578
Maestro, version 9.0 (2010) Macromodel version 9.6; Glide version 5.5, Schrodinger, New York, NY, 2010: LigPrep, version5.5, Schrodinger, New York, NY
Manivannan F, Prasanna S, Chaturvedi SC (2004) Rationalization of physico-chemical properties of 5,6-diarylthiazolo[3,2-b] 1,2,4-triazoles towards cyclooxygenase-2 (COX-2) inhibition: a QSAR approach. Ind J Biochem Biophys 41:179–183
Omar FA, Mahfouz NM, Rahman MA (1996) Design, synthesis and anti-inflammatory activity of some 1,3,4-oxadiazole derivatives. Eur J Med Chem 31:819–825
Poulsen A, William A, Lee A, Blanchard S, Teo E, Deng W, Tu N, Tan E, Sun E, Goh KL, Ong WC, Ng CP, Goh KC, Bonday ZJ (2008) Structure based design of aurora A and B inhibitors. J Comput Aided Mol Modeling Des 22(12):897–906
Prasanna S, Daga PR, Xie A, Doerksen RJ (2009) Glycogen synthase kinase-3 inhibition by 3-anilino-4-phenylmaleimides: insights from 3D-QSAR and docking. J Comput Aided Mol Modeling Design 23(2):113–127
Sandhu JS (2003) Renal effects of selective cyclooxygenase-2 (COX-2) inhibitors. J Ind Acad Clinical Med 4(1):18–20
Singh AK, Pandey A, Tewari M, Prakash K, Shukla HS, Pandey HP (2010) A discussion on chemoprevention of oral cancer by selective cyclooxygenase-2 (COX-2) inhibitors. Digest J Nanomat Biostr 5(2):285–295
Still WC, Tempczyk A, Hawley RC, Hendrickson T (1990) Semi-analytical treatment of salvation for molecular mechanics and dynamics. J Am Chem Soc 112:6127–6129
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khokra, S.L., Monga, J., Husain, A. et al. Docking studies on butenolide derivatives as Cox-II inhibitors. Med Chem Res 22, 5536–5544 (2013). https://doi.org/10.1007/s00044-013-0511-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-013-0511-x