Abstract
The research article describes the effect of an ester group on the in vitro antiproliferative activity in SAR studies of 5-methyl-5H-indolo[2,3-b]quinoline (neocryptolepine) derivatives. The C-2 and/or C-9 ester-substituted neocryptolepines were synthesized starting from indole-3-carboxylates and N-methylanilines, which were bearing an ester group. To these ester-substituted neocryptolepines, various aminoalkylamino substituents were further attached at the C-11, and an in vitro antiproliferative assay was performed by varying the substituents at the C-11 and the position of the ester group in the A and/or D ring of neocryptolepines. Results indicated that the antiproliferative activities of the agents could be improved by introducing an ester substituent at the C-9 position. Among them, the methyl 11-(3-aminopropylamino)-5-methyl-5H-indolo[2,3-b]quinoline-9-carboxylate (8b) was the most potent agent with an IC50 value of 0.044 μM against the human leukemia MV4-11 cell line. The selective cytotoxicity of the agents between the cancer cell lines and normal cell lines were also described. The antiproliferative potency of dimethyl 11-(3-aminopropylamino)-5-methyl-5H-indolo[2,3-b]quinoline-2,9-dicarboxylate (9a) against the human colon cancer cell line HCT116 is 28 times higher than against the normal mice fibroblast cell line BALB/3T3.
Graphical Abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Indoloquinolines isolated from the root of Cryptolepis sanguinolenta, the climbing shrub growing in some African countries, are attracting the constant attention of pharmacologists, since an aqueous macerate or decoction of this root is used in traditional medicine for the treatment of infectious diseases like malaria and various disorders of the body (Alexandra et al., 2000; Cimanga et al., 1996a, b). Among the various isolated products with indoloquinoline structures, cryptolepine i (5-methylindolo[3,2-b]quinoline), and the minor alkaloid neocryptolepine ii (5-methylindolo[2,3-b]quinoline) (Fig. 1) have been intensively studied, which showed broad spectrum of biological activities including antibacterial (Cimanga et al., 1996a, b, 1997, 1998), antifungal (Cimanga et al., 1996a, b, 1997, 1998), and antimalarial (Philippe et al., 1996; Kirby et al., 1995; Wright et al., 1996).
Since cryptolepine i and neocryptolepine ii possess linearly arranged tetracyclic planar structures, they behave as a DNA intercalating agent and inhibit topoisomerase II thus revealing a high level of cytotoxicity (Guittat et al., 2003; Jonckers et al., 2002; Bailly et al., 2000). Consequently, SAR studies on these structures as a lead to search for more active drug candidates have been extensively undertaken (Kumar et al., 2008; Lavrado et al., 2010; Parvatkar et al., 2011).
Our previous research on 5-Me-indolo[2,3-b]quinolines revealed that the 11-amino group is important for their activity, especially the 3-aminopropylamino group, which could increase the activity against MV4-11 about 67 times compared to its 11-chloro precursor (Wang et al., 2012). The antiproliferaive test indicated that the 5-methylated derivatives are usually more cytotoxic than their respective 6-methylated congeners. A synergistic effect of the substituents at the C-2 position was also observed. For example, the C-2-Br derivative was 5 times more active compared to the non-halogenated derivative on 11-(3-aminopropylamino)neocryptolepine, and the 2-chloro-11-(4-methyl-1,4-diazepane-1-yl)neocryptolepine was 65 times more active than its corresponding agent with no substituent at C-2.
On the other hand, a recent publication describing effect of substituents on the anticancer activity indicated that the introduction of an ester group to the core structures affects the activity of other plant-derived anticancer agents such taxol (Ojima et al., 1997), camptothecin (Yang et al., 2002), bryostatin (Wender and Hinkle, 2000), and 5-fluorouracil (Xiong et al., 2009). Such an improvement in the anticancer activity is believed to be due to increasing both the lipophilicity and bioavailability of the drug by introducing the ester group into the core structure. Encouraged by these significant information, we examined the introduction of an ester group to the core and checked its influence on the antiproliferative activity. In this study, we report the further investigation of the anticancer potential of the neocryptolepine core. An ester group was introduced at the C-2 position of the A-ring and/or the C-9 position of the D-ring to establish or extend the structure-activity relationship (SAR) study for these regions.
Synthesis and biological evaluation
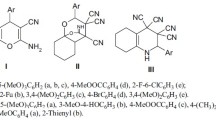
The preparations of the ester substituted 11-chloro-5-methyl-5H-indolo[2,3-b]quinolines 6 were carried out according to the method we previously described (Wang et al., 2012). Since the dimethyl 1H-indole-3,5-dicarboxylate (2b), a key starting compound, was not commercially available, it was synthesized by installation of an ester group at the C-3 position to the methyl 1H-indole-5-carboxylate 1 using the reaction with trichloroacetyl chloride in the presence of pyridine, followed by successive alkaline treatment with KOH at reflux in MeOH and MeI in DMF (Linton and Kozlowski, 2008). The obtained dimethyl 1H-indole-3,5-dicarboxylate (2b) was combined with N-methylaniline (3b) via chlorination with NCS in the presence of 1,4-dimethylpiperazine, giving 2-(N-methylanilino)indole-3,5-dicarboxylate (4b) in 73 % yield. Heating of 4b at 250 °C in diphenyl ether induced intra-molecular acylation at the C-2 of aniline, forming the tetracyclic indolo[2,3-b]quinolinone 5. The reaction of 5 with POCl3 afforded the 11-chloro-indolo[2,3-b]quinoline 6 as a result of dehydrative chlorination. The amination of 6 via an SNAr reaction smoothly proceeded by heating with amines in THF to give various 11-aminoindolo[2,3-b]quinolines 7–9 as depicted in Scheme 1.
Preparation of ester substituted 11-amino-neocryptolepines 7–9. Reagents and conditions: (i) a. Pyridine, trichloroacetyl chloride, THF. b. KOH, MeOH, reflux. (ii) a. N-chlorosuccinimide, 1,4-dimethylpiperazine. b. trichloroacetic acid. (iii) diphenyl ether, reflux. (iv) POCl3, toluene, reflux. (v) appropriate amines
We introduced an ester group to the indole motif at the C-9 position, and varied the C-11 position with different amines (8a–8g). The results of the anti-proliferative evaluation against the human leukemia cell line (MV4-11) are summarized in Table 1. We found that in some cases, their activities were improved compared to those compounds without substituents at C-9. For example, 8a, 8b, 8d, and 8e were shown to be more active compared to compounds 10a, 10b, 10d, and 10e which lack a substitutent at the C-2 or C-9 position. Their IC50 values ranged from 0.044 to 0.811 μM, and the best result was achieved with a 3-aminopropylamino group substituted at C-11 (8b, IC50 = 0.044 μM). Compared with the agent 7, which contained an ester group at the C-2 position on the quinoline subunit, the agent 8b was twice more efficient.

Furthermore, we have synthesized and evaluated agents 9a–9e, bearing the same aminopropylamino group R2 while varying the ester group at R1 or R2 for the SAR study, and a synergistic effect were detected (Table 2). In general, an ester substituent at the C-9 position can efficiently favor the anticancer activity of the agent while the ester substituent at the C-2 position is not as efficient. Comparing compounds 9b and 9d for instance, the compound 9b was twice more active than 9d, the same trend as observed for the agents 7 and 8b. Compared with 8b, the introduction of a substituent at the C-2 position, compounds 9a, 9b, and 9c for instance, has made the compound less active.
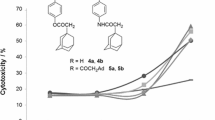
The solubility of an agent in an aqueous medium can significantly influence its further application, despite its high anticancer activity. In order to improve the water solubility of the agents, we tried to hydrolyze the 9-ester substituted neocryptolepines into a 9-carboxylic acid analog by treating the methyl 11-amino-5H-indolo[2,3-b]quinoline-9-carboxylate 8 with NaOH(aq) in MeOH. The mixture was refluxed overnight to afford the 9-carboxylic acid substituted indolo[2,3-b]quinoline 11 in good yield, as shown in Scheme 2. The evaluation results of their anti-proliferative activity are summarized in Table 3.
Unfortunately, the anti-proliferative activity of the hydrolyzed derivatives 11 turned out that though the solubility of the agents in an aqueous medium was improved, their anti-proliferative activities dramatically decreased compared to their precursors 8a–8d.
Furthermore, the indoloquinoline derivative compounds bearing an ester substituent (7–9) were also evaluated against other cell lines to evaluate the selective anticancer properties (Table 4). Two kinds of cancer cell lines (human lung cancer cell line A549, human colon cancer cell line HCT116) and a kind of normal cell line (normal mice fibroblast cell line BALB/3T3) were chosen for the tests. Most of the tested compounds were cytotoxic against the cancer cell lines, except for the agent 8c with an IC50 value of more than 5 μM. In general, these tested compounds were selectively cytotoxic against the human colon cancer HCT116 cell line, and had a lower cytotoxicity against the A549 or BALB/3T3 cell lines. Their IC50 values against the HCT 116 cell line were generally 4–5 times lower than those against the A549 or BALB/3T3 cell lines. The agents 7, 8a, 8b, 8g, and the 9 series were shown to be more efficient than the wide spectrum anticancer medicine, doxorubicin, against the HCT116 cells. In addition, the agent 9c revealed a better activity against the A549 cells when compared to doxorubicin.
Spectroscopic characterization of neocryptolepine derivative 8b interacting with salmon fish sperm DNA
The DNA binding studies of compounds 8b was performed using UV–vis absorption spectroscopy with salmon fish sperm DNA in phosphate buffer of pH 7.0 at 20 °C. The hypochromic effect was observed in the absorption spectra while the DNA solution was gradually added to the solution of the compound 8b. The results in Fig. 2 showed that the absorption band at 298 nm for the 8b decreased while increasing the DNA concentration. It illustrated that there is a strong interaction between 8b and DNA. Then the binding constant of 8b-DNA was calculated as 2.77 × 105 L mol−1 according to double-reciprocal equation.
Conclusion
To summarize, we have synthesized a series of C-2 and/or C-9 ester substituted 11-amino-5-methyl-5H-indolo[2,3-b]quinolines and evaluated their antiproliferative activity for an SAR study. The results indicated that the activities of the agents could be improved by introducing an ester substituent at the C-9 position. Among them, the methyl 11-(3-aminopropylamino)-5-methyl-5H-indolo[2,3-b]quinoline-9-carboxylate (8b) was the most potent with an IC50 value of 0.044 μM against the human leukemia MV4-11 cell line. While compound 9a showed 28 times more selective cytotoxic activity against the human colon cancer cell line than normal mice fibroblast cell line, BALB/3T3.
Experimental
Chemistry
General
The commercially obtained reagents were directly used without further purification. The 1H NMR and 13C NMR spectra were measured on the Varian INOVA-600 or Varian INOVA-400 spectrometer. High resolution mass spectra were obtained on a Bruker micrOTOF II-SKA spectrometer. Melting points were determined on a J-Science RFS-10 hot stage microscope. The scaffolds 7–8 and were prepared by the method we previously mentioned (Wang et al., 2013).
General procedure for the synthesis of Dimethyl 1H-indole-3,5-dicarboxylate 2b
Pyridine (0.30 mL, 3.54 mmol) was added to a suspension of methyl 1H-indole-5-carboxylate (0.48 g, 2.72 mmol) in anhydrous THF (6 mL) at 0 °C. A solution of trichloroacetyl chloride (0.40 mL, 3.54 mmol) in THF (6 mL) was added dropwise via an addition funnel over 1 h. The reaction mixture was then allowed to warm to room temperature to stir over night. The reaction mixture was quenched in 1 M HCl, dried over Na2SO4, and concentrated under vacuum. The resulting solid was then dissolved in MeOH (54 mL), and KOH(s) was added. The reaction mixture was heated to reflux for 5 h, then stirred at ambient temperature for 1 h, followed by concentration under vacuum. The solid was purified by chromatography (SiO2, 25 % EtOAc/Hexane) in 75 % yield.
Dimethyl 1H-indole-3,5-dicarboxylate 2b
White solids; 1H NMR (400 MHz, DMSO-d 6) δH (ppm) 12.28 (s, 1H), 8.69 (d, J = 1.5 Hz, 1H), 8.23 (s, 1H), 7.83 (dd, J = 8.6, 1.7 Hz, 1H), 7.57 (dd, J = 8.6, 0.5 Hz, 1H), 3.87 (s, 3H), 3.84 (s, 3H), 3.34 (s, 2H). 13C NMR (150 MHz, DMSO-d 6) δC (ppm) 167.0, 164.4, 139.0, 134.3, 125.2, 123.3, 122.7, 112.5, 107.4, 51.9, 50.9.
Methyl 5-bromo-1H-indole-3-carboxylate 2c
White solids; 1H NMR (400 MHz, CDCl3) δH (ppm) 8.68 (s, 1H), 8.33 (d, J = 1.9 Hz, 1H), 7.92 (d, J = 3.0 Hz, 1H), 7.37 (dd, J = 8.6, 1.9 Hz, 1H), 7.29 (dd, J = 8.6, 0.4 Hz, 1H), 3.93 (s, 3H). 13C NMR (100 MHz, CDCl3) δC (ppm) 165.1, 134.6, 131.8, 127.4, 126.3, 124.2, 115.7, 112.9, 108.6, 51.3.
Methyl 2-((4-(methoxycarbonyl)phenyl)(methyl)amino)-1H-indole-3-carboxylate 4a
White solids, Mp: 217–219 °C; 1H NMR (600 MHz, DMSO-d 6): δH (ppm) 12.25 (br s, 1 H), 8.00 (d, J = 7.92 Hz, 1 H), 7.81 (d, J = 8.80 Hz, 2 H), 7.39 (d, J = 7.63 Hz, 1 H), 7.29–7.15 (m, 2 H), 6.73 (d, J = 8.80 Hz, 2 H), 3.78 (s, 3 H), 3.66 (s, 3 H), 3.37 (s, 3 H); 13C NMR (150 MHz, DMSO-d 6) δC (ppm) 166.1, 163.5, 151.7, 145.3, 132.6, 130.7, 125.8, 122.7, 121.5, 121.0, 119.0, 112.9, 111.8, 98.4, 51.6, 50.6, 39.1.
Dimethyl 2-(methyl(phenyl)amino)-1H-indole-3,5-dicarboxylate 4b
White solids, Mp: 168–169 °C; 1H NMR (400 MHz, CDCl3) δH (ppm) 9.09 (s, 1H), 8.80–8.75 (m, 1H), 7.85 (dd, J = 8.5, 1.7 Hz, 1H), 7.25–7.19 (m, 3H), 6.96–6.85 (m, 3H), 3.89 (s, 3H), 3.79 (s, 3H), 3.44 (s, 3H). 13C NMR (100 MHz, CDCl3) δC (ppm) 168.2, 164.3, 149.3, 146.5, 134.9, 129.3, 126.3, 124.0, 123.5(2C), 121.3, 117.1, 110.3, 97.6, 51.9, 51.0, 40.2. ESI-HRMS: m/z Calcd. for C19H17N2O4 [M–H]− 337.1188. Found 337.1166.
Dimethyl 2-((4-(methoxycarbonyl)phenyl)(methyl)amino)-1H-indole-3,5-dicarboxylate 4c
White solids, Mp: 234–236 °C; 1H NMR (400 MHz, CDCl3) δH (ppm) 9.58 (s, 1H), 8.84 (d, J = 1.3 Hz, 1H), 7.96 (dd, J = 8.5, 1.6 Hz, 1H), 7.80–7.73 (m, 2H), 7.38 (d, J = 8.5 Hz, 1H), 6.70–6.63 (m, 2H), 3.93 (s, 3H), 3.82 (d, J = 3.5 Hz, 6H), 3.42 (s, 3H). 13C NMR (100 MHz, CDCl3) δC (ppm) 168.0, 167.3, 163.9, 150.9, 146.7, 135.0, 131.1, 125.7, 124.7, 124.4, 124.0, 120.6, 113.5, 110.9, 100.8, 52.0, 51.8, 51.2, 39.7. ESI-HRMS: m/z Calcd. for C21H20N2O6 [M+Na]+ 419.1219. Found 419.1217.
Dimethyl 2-((4-chlorophenyl)(methyl)amino)-1H-indole-3,5-dicarboxylate 4d
White solids, Mp: 188–189 °C; 1H NMR (600 MHz, CDCl3) δH (ppm) 8.79 (s, 1H), 8.70 (s, 1H), 7.92 (dd, J = 8.5, 1.6 Hz, 1H), 7.26 (d, J = 8.4 Hz, 2H), 7.21–7.16 (m, 2H), 6.82–6.77 (m, 2H), 3.93 (s, 4H), 3.84 (s, 3H), 3.43 (s, 4H). 13C NMR (100 MHz, CDCl3) δC (ppm) 168.01, 164.1, 148.4, 145.4, 134.8, 129.1, 126.1, 125.9, 124.3, 123.8(2C), 117.5, 110.4, 98.8, 52.08, 51.1, 40.1. ESI-HRMS: m/z Calcd. for C19H18ClN2O4 [M+H]+ 373.0960. Found 373.0965.
Dimethyl 2-((4-bromophenyl)(methyl)amino)-1H-indole-3,5-dicarboxylate 4e
White solids, Mp: 193–194 °C; 1H NMR (400 MHz, CDCl3) δH (ppm) 8.85 (s, 1H), 8.79 (s, 1H), 7.91 (dd, J = 8.5, 1.6 Hz, 1H), 7.34–7.26 (m, 3H), 6.75–6.67 (m, 2H), 3.92 (s, 3H), 3.83 (s, 3H), 3.41 (s, 3H). 13C NMR (100 MHz, CDCl3) δC (ppm) 168.0, 164.0, 148.1, 145.9, 134.7, 132.1, 126.1, 124.4, 123.9(2C), 117.7, 113.2, 110.4, 99.1, 52.0, 51.1, 40.0. ESI-HRMS: m/z Calcd. for C19H18BrN2O4 [M+H]+ 417.0450. Found 417.0452.
Methyl 5-bromo-2-((4-(methoxycarbonyl)phenyl)(methyl)amino)-1H-indole-3-carboxylate 4f
White solids, Mp: 217–218 °C; 1H NMR (600 MHz, CDCl3) δH (ppm) 9.08 (s, 1H), 8.29 (d, J = 1.9 Hz, 1H), 7.81–7.76 (m, 2H), 7.37 (dd, J = 8.5, 2.0 Hz, 1H), 7.22 (d, J = 8.6 Hz, 1H), 6.69–6.64 (m, 2H), 3.84 (s, 3H), 3.81 (s, 3H), 3.42 (s, 3H). 13C NMR (150 MHz, CDCl3) δC (ppm) 167.3, 163.8, 151.0, 146.1, 131.1, 130.7, 127.8, 126.4, 124.4, 120.6, 115.7, 113.5, 112.5, 99.7, 51.9, 51.2, 39.7. ESI-HRMS: m/z Calcd. for C19H18BrN2O4 [M+H]+ 417.0450. Found 417.0457.
Methyl 5-bromo-2-((4-chlorophenyl)(methyl)amino)-1H-indole-3-carboxylate 4g
White solids, Mp: 193–194 °C; 1H NMR (600 MHz, CDCl3) δH (ppm) 8.36 (s, 1H), 8.24 (d, J = 1.9 Hz, 1H), 7.31 (dd, J = 8.5, 1.9 Hz, 1H), 7.23–7.16 (m, 2H), 7.11 (d, J = 8.5 Hz, 1H), 6.79–6.75 (m, 2H), 3.82 (s, 3H), 3.41 (s, 3H). 13C NMR (150 MHz, CDCl3) δC (ppm) 164.0, 147.9, 145.5, 130.4, 129.2, 128.2, 125.9(2C), 124.1, 117.5, 115.5, 112.0, 97.9, 51.1, 40.1. ESI-HRMS: m/z Calcd. for C17H15BrClN2O2 [M+H]+ 393.0005. Found 393.0006.
Methyl 5-methyl-11-oxo-6,11-dihydro-5H-indolo[2,3-b]quinoline-2-carboxylate 5a
Pale gray solids, Mp: >300 °C; 1H NMR (600 MHz, DMSO-d 6): δH (ppm) 12.21 (s, 1H), 8.96 (d, J = 2.05 Hz, 1H), 8.28–8.12 (m, 2H), 7.84 (d, J = 8.80 Hz, 1H), 7.48 (d, J = 7.63 Hz, 1H), 7.36–7.17 (m, 2H), 3.98 (s, 3H), 3.91 (s, 3H); 13C NMR (150 MHz, DMSO-d 6): δC (ppm) 171.0, 165.9, 146.9, 142.0, 134.7, 130.9, 127.8, 124.2, 123.7, 123.2, 122.5, 121.5, 120.3, 115.8, 111.0, 102.9, 52.1, 33.7;
Methyl 5-methyl-11-oxo-6,11-dihydro-5H-indolo[2,3-b]quinoline-9-carboxylate 5b
Pale gray solids, Mp:>300 °C; 1H NMR (400 MHz, DMSO-d 6) δH (ppm) 12.45 (s, 1H), 8.86–8.81 (m, 1H), 8.40 (d, J = 7.8 Hz, 1H), 7.94–7.86 (m, 1H), 7.78 (q, J = 9.1 Hz, 2H), 7.55 (d, J = 8.4 Hz, 1H), 7.42 (t, J = 7.1 Hz, 1H), 3.98 (s, 3H), 3.90 (s, 3H). 13C NMR (100 MHz, DMSO-d 6) δC (ppm) 171.6, 166.9, 147.8, 139.2, 137.9, 131.5, 125.8, 124.7, 124.2, 123.8, 122.5, 122.0, 121.6, 115.5, 110.7, 102.1, 51.9, 33.4. ESI-HRMS: m/z Calcd. for C18H13N2O3 [M−H]− 305.0932. Found 305.0867.
Dimethyl 5-methyl-11-oxo-6,11-dihydro-5H-indolo[2,3-b]quinoline-2,9-dicarboxylate 5c
Pale gray solids, Mp:>300 °C; 1H NMR (400 MHz, DMSO-d 6) δH (ppm) 12.53 (s, 1H), 8.91 (d, J = 2.1 Hz, 1H), 8.78 (d, J = 1.3 Hz, 1H), 8.17 (dd, J = 8.9, 2.2 Hz, 1H), 7.89 (dd, J = 8.4, 1.7 Hz, 1H), 7.82 (d, J = 9.0 Hz, 1H), 7.51 (d, J = 8.4 Hz, 1H), 3.95 (s, 3H), 3.91 (d, J = 4.7 Hz, 6H). 13C NMR (150 MHz, DMSO-d 6) δC (ppm) 171.1, 166.9, 165.9, 147.8, 142.0, 137.8, 131.2 127.7, 124.6, 124.1, 123.4, 122.8(2C), 121.8, 116.1, 111.0, 102.7, 52.2, 52.0, 33.8. ESI-HRMS: m/z Calcd. for C20H17N2O5 [M+H]+ 365.1137. Found 365.1140.
Methyl 2-chloro-5-methyl-11-oxo-6,11-dihydro-5H-indolo[2,3-b]quinoline-9-carboxylate 5d
Pale gray solids, Mp: >300 °C; 1H NMR (600 MHz, DMSO-d 6) δH (ppm) 12.54 (s, 1H), 8.81 (s, 1H), 8.29 (s, 1H), 7.92 (d, J = 8.3 Hz, 1H), 7.86 (d, J = 8.5 Hz, 1H), 7.80 (d, J = 9.0 Hz, 1H), 7.56 (d, J = 8.2 Hz, 1H), 3.98 (s, 3H), 3.90 (s, 3H).13C NMR (150 MHz, DMSO-d 6) δC (ppm) 170.3, 166.8, 147.9, 138.0(2C), 131.2, 126.9, 125.9, 124.6(2C), 123.6, 122.7, 121.8, 118.1, 111.0, 102.5, 51.9, 33.8. ESI-HRMS: m/z Calcd. for C18H14ClN2O3 [M+H]+ 341.0693. Found 341.0679.
Methyl 2-bromo-5-methyl-11-oxo-6,11-dihydro-5H-indolo[2,3-b]quinoline-9-carboxylate 5e
Pale gray solids, Mp: >300 °C; 1H NMR (600 MHz, DMSO-d 6) δH (ppm) 12.57 (s, 1H), 8.82 (d, J = 1.3 Hz, 1H), 8.44 (d, J = 2.4 Hz, 1H), 7.96–7.87 (m, 2H), 7.81 (d, J = 9.1 Hz, 1H), 7.57 (d, J = 8.4 Hz, 1H), 3.99 (s, 3H), 3.90 (s, 3H). 13C NMR (150 MHz, DMSO-d 6) δC (ppm) 170.2, 166.9, 147.9, 138.3, 138.1, 133.9, 127.8, 126.3, 124.6, 123.6, 122.7, 121.8, 118.3, 114.8, 111.0, 102.5, 51.9, 33.7. ESI-HRMS: m/z Calcd. for C18H13BrN2O3 [M+Na]+ 407.0007. Found 407.0009.
Methyl 9-bromo-5-methyl-11-oxo-6,11-dihydro-5H-indolo[2,3-b]quinoline-2-carboxylate 5f
Pale gray solids, Mp: >300 °C; 1H NMR (600 MHz, DMSO-d 6) δH (ppm) 12.34 (s, 1H), 8.88 (d, J = 2.1 Hz, 1H), 8.22 (s, 1H), 8.15 (dd, J = 8.8, 2.1 Hz, 1H), 7.79 (d, J = 9.0 Hz, 1H), 7.38 (d, J = 1.7 Hz, 2H), 3.92 (s, 3H), 3.90 (s, 3H). 13C NMR (150 MHz, DMSO-d 6) δC (ppm) 171.1, 165.8, 147.2, 142.0, 133.6, 131.2, 127.7, 125.5(2C), 123.9, 122.7, 122.2, 115.9, 113.7, 113.0, 102.0, 52.2, 33.7. ESI-HRMS: m/z Calcd. for C18H13BrN2O3 [M+Na]+ 407.0007. Found 407.0007.
9-bromo-2-chloro-5-methyl-5H-indolo[2,3-b]quinolin-11(6H)-one 5g
Pale gray solids, Mp: >300 °C; 1H NMR (600 MHz, DMSO-d 6) δH (ppm) 12.27 (s, 1H), 8.22 (dd, J = 5.2, 1.8 Hz, 2H), 7.77 (d, J = 9.1 Hz, 1H), 7.73 (dd, J = 9.0, 2.6 Hz, 1H), 7.44–7.30 (m, 2H), 3.91 (s, 3H). 13C NMR (150 MHz, DMSO-d 6) δC (ppm) 170.2, 147.3, 137.8, 133.7, 131.0, 126.7, 125.6(2C), 125.4, 124.6, 122.1, 117.8, 113.5, 112.9, 101.7, 33.6. ESI-HRMS: m/z Calcd. for C16H10BrClN2O [M+Na]+ 382.9563. Found 382.9565.
Methyl 11-chloro-5-methyl-5H-indolo[2,3-b]quinoline-2-carboxylate 6a
Orange solids, Mp: 281–283 °C; 1H NMR (600 MHz, CDCl3): δH (ppm) 8.95 (s, 1H), 8.29 (d, J = 8.22 Hz, 2H), 7.74–7.57 (m, 2H), 7.52 (t, J = 7.48 Hz, 1H), 7.22 (t, J = 7.48 Hz, 1H), 4.24 (s, 3H), 4.00 (s, 3H); 13C NMR (150 MHz, CDCl3): δC (ppm) 166.0, 155.1, 154.5, 139.2, 135.7, 131.3, 130.1, 128.2, 125.4, 123.9, 123.8,123.5, 120.9, 118.5, 117.8, 114.3, 52.4, 33.4.
Methyl 11-chloro-5-methyl-5H-indolo[2,3-b]quinoline-9-carboxylate 6b
Orange solids, Mp: 248–249 °C; 1H NMR (400 MHz, CDCl3) δH (ppm) 9.01 (s, 1H), 8.45 (d, J = 8.2 Hz, 1H), 8.20 (d, J = 8.4 Hz, 1H), 7.88 (t, J = 7.7 Hz, 1H), 7.81 (d, J = 8.6 Hz, 1H), 7.70 (d, J = 8.4 Hz, 1H), 7.60 (t, J = 7.6 Hz, 1H), 4.43 (d, J = 7.3 Hz, 3H), 3.98 (s, 3H). 13C NMR (150 MHz, CDCl3) δC (ppm) 167.4, 157.6, 156.1, 137.1, 136.5, 131.5, 130.9, 126.0, 125.4, 123.4, 122.9(2C), 121.5, 119.2, 116.7, 114.4, 51.9, 33.4. ESI-HRMS: m/z Calcd. for C18H14ClN2O2 [M+H]+ 325.0744. Found 325.0797.
Dimethyl 11-chloro-5-methyl-5H-indolo[2,3-b]quinoline-2,9-dicarboxylate 6c
Orange solids, Mp: 278–280 °C; 1H NMR (600 MHz, CDCl3) δH (ppm) 9.14 (d, J = 1.8 Hz, 1H), 9.03 (d, J = 1.5 Hz, 1H), 8.51 (dd, J = 8.9, 1.8 Hz, 1H), 8.24 (dd, J = 8.4, 1.6 Hz, 1H), 7.91 (d, J = 9.0 Hz, 1H), 7.79 (d, J = 8.4 Hz, 1H), 4.55 (s, 3H), 4.06 (s, 3H), 3.99 (s, 3H). 13C NMR (150 MHz, CDCl3) δC (ppm) 167.2, 165.7, 156.4, 155.7, 139.1, 138.0, 132.2, 131.7, 128.6, 125.8, 125.1, 124.4, 123.0, 122.8, 119.2, 117.1, 115.0, 52.7, 52.1, 34.4. ESI-HRMS: m/z Calcd. for C20H16ClN2O4 [M+H]+ 383.0799. Found 383.0799.
Methyl 2,11-dichloro-5-methyl-5H-indolo[2,3-b]quinoline-9-carboxylate 6d
Orange solids, Mp: 283–284 °C; 1H NMR (600 MHz, CDCl3) δH (ppm) 8.90 (d, J = 1.2 Hz, 1H), 8.33 (d, J = 2.3 Hz, 1H), 8.17 (dd, J = 8.4, 1.7 Hz, 1H), 7.73 (dd, J = 9.1, 2.3 Hz, 1H), 7.63 (dd, J = 21.2, 8.7 Hz, 2H), 4.33 (s, 3H), 3.97 (s, 3H). 13C NMR (150 MHz, CDCl3) δC (ppm) 167.3, 157.6, 155.9, 135.9, 135.1, 131.8, 131.6, 129.2, 125.7, 125.3, 124.7, 122.8, 122.3, 120.4, 117.0, 116.2, 52.1, 33.9. ESI-HRMS: m/z Calcd. for C18H13Cl2N2O2 [M+H]+ 359.0354. Found 359.0357.
Methyl 2-bromo-11-chloro-5-methyl-5H-indolo[2,3-b]quinoline-9-carboxylate 6e
Orange solids, Mp: 289–290 °C; 1H NMR (600 MHz, CDCl3) δH (ppm) 9.06 (d, J = 1.5 Hz, 1H), 8.60 (d, J = 2.2 Hz, 1H), 8.27 (dd, J = 8.4, 1.7 Hz, 1H), 7.96 (dd, J = 9.1, 2.2 Hz, 1H), 7.74 (dd, J = 20.0, 8.7 Hz, 2H), 4.46 (s, 3H), 3.99 (s, 3H). 13C NMR (150 MHz, CDCl3) δC (ppm) 167.5, 158.5, 156.5, 135.7, 134.4, 131.7, 130.9, 128.8, 128.5, 125.9, 125.2, 123.3, 122.2, 120.8, 117.3, 116.3, 52.1, 33.6. ESI-HRMS: m/z Calcd. for C18H13BrClN2O2 [M+H]+ 402.9849. Found 402.9848.
Methyl 9-bromo-11-chloro-5-methyl-5H-indolo[2,3-b]quinoline-2-carboxylate 6f
Orange solids, Mp: >300 °C; 1H NMR (600 MHz, CDCl3) δH (ppm) 9.14 (d, J = 1.8 Hz, 1H), 8.53 (d, J = 1.9 Hz, 1H), 8.48 (dd, J = 8.9, 1.9 Hz, 1H), 7.84 (d, J = 9.0 Hz, 1H), 7.70–7.60 (m, 2H), 4.43 (s, 3H), 4.04 (d, J = 8.0 Hz, 3H). 13C NMR (150 MHz, CDCl3) δC (ppm) 166.0, 155.4, 153.4, 139.6, 137.9, 137.2, 132.8, 132.0, 128.7, 126.5, 124.7, 124.3, 119.4, 118.7, 114.7, 113.6, 52.6, 33.6. ESI-HRMS: m/z Calcd. for C18H13BrClN2O2 [M+H]+ 402.9849. Found 402.9850.
9-Bromo-2,11-dichloro-5-methyl-5H-indolo[2,3-b]quinoline 6 g
Orange solids, Mp: 288–290 °C; 1H NMR (600 MHz, CDCl3) δH (ppm) 8.45 (d, J = 1.9 Hz, 1H), 8.37 (d, J = 2.3 Hz, 1H), 7.76 (dd, J = 9.1, 2.4 Hz, 1H), 7.66 (d, J = 9.1 Hz, 1H), 7.62 (dd, J = 8.4, 2.0 Hz, 1H), 7.54 (d, J = 8.4 Hz, 1H), 4.30 (s, 3H). 13C NMR (150 MHz, CDCl3) δC (ppm) 155.0, 153.6, 135.6, 135.5, 132.8, 131.8, 128.6, 126.5, 125.4, 125.0, 124.8, 120.08, 119.1, 115.9, 113.1, 33.5. ESI-HRMS: m/z Calcd. for C16H10BrCl2N2 [M+H]+ 378.9404. Found 378.9406.
General procedure for the synthesis of ester substituted 11-amino-5-methyl-5H-indolo[2,3-b]quinolines 7–9
The methyl 11-chloro-5-methyl-5H-indolo[2,3-b]quinoline-2-carboxylate (6) or methyl 11-chloro-5-methyl-5H-indolo[2,3-b]quinoline-9-carboxylate (7) was heated at reflux with a large excess of an appropriate amine in THF for 3–4 h. The reaction was monitored by TLC. Then the mixture was washed with water and extracted with CH2Cl2. The organic phase was dried over MgSO4 and concentrated under vacuum. The crude product was purified by column chromatography using eluent changed from AcOEt to AcOEt-2 N ammonia in MeOH (10: 1) to give the final product.
Methyl 11-(3-aminopropylamino)-5-methyl-5H-indolo[2,3-b]quinoline-2-carboxylate 7
Yellow solids, Mp: 149–150 °C; 1H NMR (400 MHz, DMSO-d 6) δH (ppm) 9.12 (d, J = 1.8 Hz, 1 H), 8.25 (dd, J = 9.0, 1.8 Hz, 1H), 7.99 (d, J = 7.7 Hz, 1H), 7.90 (d, J = 9.1 Hz, 1H), 7.52 (d, J = 7.5 Hz, 1H), 7.29 (t, J = 7.6 Hz, 1H), 7.09 (t, J = 7.0 Hz, 1H), 4.17 (s, 3H), 3.99 (t, J = 6.5 Hz, 2H), 3.93 (s, 3H), 2.67 (t, J = 6.3 Hz, 2H), 1.78–1.74 (m, 2H); 13C NMR (150 MHz, DMSO-d 6) δC (ppm) 165.9, 156.2, 151.9, 148.1, 140.2, 130.2, 126.5, 124.5, 124.3, 122.1, 121.3, 118.5, 116.9, 115.3, 115.1, 103.7, 52.2, 47.4, 39.9, 33.1, 32.5. ESI-HRMS: m/z Calcd. for C21H21N4O2 [M−H]− 361.1670. Found 375.1666.
Methyl 11-(3-hydroxypropylamino)-5-methyl-5H-indolo[2,3-b]quinoline-9-carboxylate 8a
Orange solids, Mp: 223–224 °C; 1H NMR (400 MHz, DMSO-d 6) δH (ppm) 8.60–8.50 (m, 2H), 7.94–7.88 (m, 2H), 7.86–7.81 (m, 1H), 7.52 (d, J = 8.4 Hz, 1H), 7.50–7.43 (m, 2H), 4.57 (t, J = 4.7 Hz, 1H), 4.19 (s, 3H), 3.97 (q, J = 6.5 Hz, 2H), 3.87 (s, 3H), 3.47 (dd, J = 10.8, 5.9 Hz, 2H), 1.90 (p, J = 6.4 Hz, 2H); 13C NMR (150 MHz, DMSO-d 6) δC (ppm) 167.4, 158.3, 155.8, 149.2, 137.4, 131.2, 125.7, 124.0(2C), 123.6, 121.3, 118.4, 116.0, 115.6(d), 103.1, 58.4, 51.6, 45.8, 40.0(overlap with DMSO-d6 peaks), 33.6, 32.5. ESI-HRMS: m/z Calcd. for C21H20N3O3[M−H]− 362.1492. Found 362.1483.
Methyl 11-(3-aminopropylamino)-5-methyl-5H-indolo[2,3-b]quinoline-9-carboxylate 8b
Yellow solids, Mp: 170–171 °C; 1H NMR (600 MHz, DMSO-d 6) δH (ppm) 8.63 (d, J = 1.4 Hz, 1H), 8.52 (d, J = 7.7 Hz, 1H), 7.93–7.87 (m, 2H), 7.83 (t, J = 7.7 Hz, 1H), 7.52 (d, J = 8.4 Hz, 1H), 7.46 (t, J = 7.4 Hz, 1H), 4.19 (s, 3H), 4.00 (t, J = 6.6 Hz, 2H), 3.86 (s, 3H), 2.71 (t, J = 6.3 Hz, 2H), 1.84–1.78 (m, 2H); 13C NMR (150 MHz, DMSO-d 6) δC (ppm) 167.4, 158.3, 155.7, 149.1, 137.5, 131.1, 125.6, 124.1(2C), 123.6, 121.2, 118.3, 116.0, 115.5(2C), 102.6, 51.6, 47.2, 33.1, 32.5. ESI-HRMS: m/z Calcd. for C21H21N4O2[M−H]− 361.1670. Found 361.1639.
Methyl 5-methyl-11-morpholino-5H-indolo[2,3-b]quinoline-9-carboxylate 8c
Orange solids, Mp: 213–215 °C; 1H NMR (400 MHz, CDCl3) δH (ppm) 9.01 (d, J = 1.6 Hz, 1H), 8.50 (d, J = 8.2 Hz, 1H), 8.20 (dd, J = 8.4, 1.7 Hz, 1H), 7.83 (d, J = 4.1 Hz, 2H), 7.74 (d, J = 8.4 Hz, 1H), 7.57–7.47 (m, 1H), 4.41 (s, 3H), 4.18–4.09 (m, 4H), 3.98 (s, 3H), 3.75–3.66 (m, 4H); 13C NMR (100 MHz, CDCl3) δC (ppm) 167.8, 158.4, 156.7, 151.3, 138.1, 131.0, 129.7, 126.5, 126.0, 122.4, 122.0, 121.0(2C), 120.7, 116.5, 115.1, 67.6, 51.9, 51.0, 33.7. ESI-HRMS: m/z Calcd. for C22H22N3O3 [M+H]+ 376.1661. Found 376.1662.
Methyl 5-methyl-11-(4-methyl-1,4-diazepan-1-yl)-5H-indolo[2,3-b]quinoline-9-carboxylate 8d
Orange solids, Mp: 179–180 °C; 1H NMR (400 MHz, CDCl3) δH (ppm) 8.93 (s, 1H), 8.47 (d, J = 8.2 Hz, 1H), 8.26–8.18 (m, 1H), 7.80 (dd, J = 3.7, 1.7 Hz, 2H), 7.71 (d, J = 8.4 Hz, 1H), 7.50 (ddd, J = 8.1, 4.2, 1.7 Hz, 1H), 4.37 (d, J = 0.8 Hz, 3H), 3.97 (d, J = 0.9 Hz, 3H), 3.93–3.87 (m, 2H), 3.78 (t, J = 6.1 Hz, 2H), 3.14 (d, J = 4.5 Hz, 2H), 3.05–2.90 (m, 2H), 2.63 (s, 3H), 2.39–2.23 (m, 2H); 13C NMR (100 MHz, CDCl3) δC (ppm) 168.1, 159.5, 157.8, 152.3, 138.5, 130.7, 129.7, 126.8, 125.7, 122.8, 122.0, 121.5, 121.0, 120.8, 116.8, 115.0, 61.3, 57.9, 52.9, 52.2, 51.9, 47.2, 33.3, 29.2. ESI-HRMS: m/z Calcd. for C24H27N4O2 [M+H]+ 403.2134. Found 403.2135.
Methyl 11-(4-aminobutylamino)-5-methyl-5H-indolo[2,3-b]quinoline-9-carboxylate 8e
Yellow oil; 1H NMR (600 MHz, DMSO-d 6) δH (ppm) 8.62 (t, J = 6.1 Hz, 1H), 8.53 (s, 1H), 7.92 (dd, J = 8.3, 7.0, 2H), 7.85 (t, J = 7.7 Hz, 1H), 7.53 (d, J = 8.4 Hz, 1H), 7.49 (t, J = 7.5 Hz, 1 H), 4.20 (s, 3H), 3.90 (t, J = 7.1 Hz, 2H), 3.87 (s, 3H), 2.67 (t, J = 7.4 Hz, 2H), 1.85–1.76 (m, 2H), 1.48 (d, J = 6.4 Hz, 2H); 13C NMR (150 MHz, DMSO-d 6) δC (ppm) 167.4, 158.4, 155.9, 149.0, 137.4, 131.2, 125.8, 124.0 (2C), 123.7, 121.4, 118.4, 116.1, 115.7, 115.5, 103.0, 51.7, 47.3, 38.6, 32.5, 27.6, 24.9. ESI-HRMS: m/z Calcd. for C22H25N4O2 [M + H]+ 377.1978. Found 377.1978.
Methyl 11-(2-(dimethylamino)ethylamino)-5-methyl-5H-indolo[2,3-b]quinoline-9-carboxylate 8f
Yellow solids, Mp: 172–173 °C; 1H NMR (400 MHz, DMSO-d 6) δH (ppm) = 8.70 (s, 1H), 8.54 (d, J = 8.3 Hz, 1H), 7.97–7.91 (m, 2H), 7.86 (t, J = 7.8 Hz, 1H), 7.55 (d, J = 8.4 Hz, 1H), 7.48 (t, J = 7.6 Hz, 1H), 7.26 (t, J = 4.5 Hz, 1H), 4.21 (s, 3H), 4.00 (dd, J = 10.8, 5.5 Hz, 2H), 3.87 (s, 3H), 2.65 (t, J = 6.1 Hz, 2H), 2.27 (s, 6H); 13C NMR (150 MHz, CDCl3) δC (ppm) = 168.2, 157.4, 155.6, 149.3, 138.3, 130.5, 127.0, 125.3, 123.8, 122.5, 120.6, 119.7, 116.1, 115.6, 114.8, 106.0, 58.2, 51.5, 45.2, 44.7, 32.6. ESI-HRMS: m/z Calcd. for C22H23N4O2 [M−H]− 375.1826. Found 375.1806.
Methyl 11-(3-(dimethylamino)propylamino)-5-methyl-5H-indolo[2,3-b]quinoline-9-carboxylate 8g
Yellow solids, Mp: 105–106 °C; 1H NMR (600 MHz, CDCl3) δH (ppm) = 8.65 (d, J = 1.4 Hz, 2H), 8.07 (dd, J = 8.4, 1.5 Hz, 1H), 8.00 (d, J = 8.3 Hz, 1H), 7.70 (dd, J = 11.9, 4.4 Hz, 2H), 7.65 (d, J = 8.6 Hz, 1H), 7.35 (t, J = 7.6 Hz, 1H), 4.23 (d, J = 1.2 Hz, 3H), 4.20–4.10 (m, 2H), 3.94 (s, 3H), 2.72–2.64 (m, 2H), 2.39 (s, 6H), 1.95–1.73 (m, 2H); 13C NMR (150 MHz, CDCl3) δC (ppm) = 168.4, 158.3, 155.5, 149.0, 137.5, 130.3, 126.2, 124.1, 123.6, 123.4, 120.7, 119.1, 115.9, 115.8, 114.5, 103.0, 59.5, 51.5, 50.1, 45.4, 32.5, 26.3. ESI-HRMS: m/z Calcd. for C23H25N4O2[M−H]− 389.1983. Found 389.1949.
Dimethyl 11-(3-aminopropylamino)-5-methyl-5H-indolo[2,3-b]quinoline-2,9-dicarboxylate 9a
Yellow solids, Mp: 75–76 °C; 1H NMR (400 MHz, DMSO-d 6) δH (ppm) 9.17 (s, 1H), 8.63 (s, 1H), 8.28 (d, J = 8.1 Hz, 1H), 7.97–7.89 (m, 2H), 7.55 (d, J = 8.4 Hz, 1H), 4.20 (s, 3H), 4.02 (t, J = 6.6 Hz, 2H), 3.94 (s, 3H), 3.87 (s, 3H), 2.75 (t, J = 6.3 Hz, 2H), 1.87–1.82 (m, 2H). 13C NMR (150 MHz, DMSO-d 6) δC (ppm) 167.4, 165.9, 158.2, 155.4, 148.9, 140.2, 130.7, 126.6, 125.7, 124.1, 123.7, 122.0, 119.0, 116.4, 115.8, 115.2, 102.5, 52.3, 51.6(2C), 47.1, 32.8, 32.5. ESI-HRMS: m/z Calcd. for C23H25N4O4 [M+H]+ 421.1876. Found 421.1877.
Methyl 11-(3-aminopropylamino)-2-chloro-5-methyl-5H-indolo[2,3-b]quinoline-9-carboxylate 9b
Yellow solids, Mp: 185–186 °C; 1H NMR (600 MHz, DMSO-d 6) δH (ppm) 8.59 (d, J = 2.0 Hz, 1H), 8.54 (s, 1H), 7.89 (dd, J = 8.4, 1.4 Hz, 1H), 7.84 (d, J = 9.2 Hz, 1H), 7.79 (dd, J = 9.1, 2.1 Hz, 1H), 7.50 (d, J = 8.3 Hz, 1H), 4.12 (s, 3H), 3.93 (t, J = 6.7 Hz, 2H), 3.86 (s, 3H), 2.67 (t, J = 6.4 Hz, 2H), 1.86–1.77 (m, 2H). 13C NMR (150 MHz, DMSO-d 6) δC (ppm) 167.4, 158.1, 155.8, 147.9, 136.1, 130.7, 125.8(2C), 123.9, 123.2, 118.6, 117.5, 116.7, 116.2, 104.6, 103.1, 51.8, 46.8, 39.9(overlap with DMSO-d6 peaks), 33.3, 32.6. ESI-HRMS: m/z Calcd. for C21H22ClN4O2 [M+H]+ 397.1431. Found 397.1432.
Methyl 11-(3-aminopropylamino)-2-bromo-5-methyl-5H-indolo[2,3-b]quinoline-9-carboxylate 9c
Yellow solids, Mp: 109–112 °C; 1H NMR (400 MHz, DMSO-d 6) δH (ppm) 8.76 (s, 1H), 8.57 (s, 1H), 7.97–7.88 (m, 2H), 7.84 (d, J = 9.2 Hz, 1H), 7.52 (d, J = 8.4 Hz, 1H), 4.16 (s, 3H), 3.95 (t, J = 6.7 Hz, 2H), 3.86 (s, 3H), 2.67 (t, J = 6.4 Hz, 2H), 1.82 (t, J = 6.5 Hz, 2H). 13C NMR (150 MHz, DMSO-d 6) δC (ppm) 167.4, 158.1, 155.8, 147.9, 136.4, 133.4, 126.1, 125.9, 123.9(2C), 118.6, 117.7, 117.3, 116.2, 113.5 103.1, 51.7, 46.6, 39.3, 33.1, 32.6, 32.3. ESI-HRMS: m/z Calcd. for C21H22BrN4O2 [M+H]+ 441.0926. Found 441.0927.
Methyl 11-(3-aminopropylamino)-9-bromo-5-methyl-5H-indolo[2,3-b]quinoline-2-carboxylate 9d
Yellow solids, Mp: 162–163 °C; 1H NMR (600 MHz, DMSO-d 6) δH (ppm) 9.10 (d, J = 1.6 Hz, 1H), 8.24 (dd, J = 8.9, 1.7 Hz, 1H), 8.10 (d, J = 1.9 Hz, 1H), 7.88 (d, J = 9.0 Hz, 1H), 7.46 (d, J = 8.4 Hz, 1H), 7.39 (dd, J = 8.4, 1.9 Hz, 1H), 4.14 (s, 3H), 3.97 (t, J = 6.6 Hz, 2H), 3.92 (s, 3H), 2.71 (t, J = 6.2 Hz, 2H), 1.83–1.77 (m, 2H). 13C NMR (150 MHz, DMSO-d 6) δC (ppm) 165.8, 156.5, 150.5, 149.0, 140.4, 130.7, 126.7(2C), 126.2, 124.0, 121.5, 118.5, 115.6, 114.9, 110.4, 102.3, 52.2, 47.5, 39.9(overlap with DMSO-d6 peaks), 32.9, 32.6. ESI-HRMS: m/z Calcd. for C21H22BrN4O2 [M+H]+ 441.0926. Found 441.0927.
N-(3-aminopropyl)-9-bromo-2-chloro-5-methyl-5H-indolo[2,3-b]quinolin-11-amine 9e
Yellow solids, Mp: 152–154 °C; 1H NMR (600 MHz, DMSO-d 6) δH (ppm) 8.59 (d, J = 1.8 Hz, 1H), 8.03 (d, J = 1.7 Hz, 1H), 7.86 (d, J = 9.2 Hz, 1H), 7.82 (dd, J = 9.1, 2.0 Hz, 1H), 7.44 (d, J = 8.4 Hz, 1H), 7.39 (dd, J = 8.4, 1.9 Hz, 1H), 4.12 (s, 3H), 3.91 (t, J = 6.6 Hz, 2H), 2.63 (t, J = 6.3 Hz, 2H), 1.77 (p, J = 6.5 Hz, 2H). 13C NMR (150 MHz, DMSO-d 6) δC (ppm) 156.6, 150.9, 148.1, 136.3, 130.7, 126.9, 126.1, 125.2, 124.2, 123.3, 118.3, 117.3, 116.5, 109.9, 102.9, 46.9, 39.5(overlap with DMSO-d6 peaks), 33.3, 32.5. ESI-HRMS: m/z Calcd. for C19H19BrClN4 [M+H]+ 417.0482. Found 417.0480.
General procedure for the synthesis of 11-amino-5-methyl-5H-indolo[2,3-b]quinoline-9-carboxylic acid 11
Methyl 11-amino-5-methyl-5H-indolo[2,3-b]quinoline-9-carboxylate was dissolved in MeOH and refluxed with 20 % NaOH (aq.) over night. The reaction was monitored by TLC. Then the mixture was neutralized with 1 N HCl (aq.) and concentrated to remove the MeOH under vacuum. The crude product was purified by reverse-phase chromatography using eluent changed from H2O to H2O/CH3CN (50:1) to give carboxylic acid.
11-(3-aminopropylamino)-5-methyl-5H-indolo[2,3-b]quinoline-9-carboxylic acid 11a
White solids, Mp: >300 °C; 1H NMR (600 MHz, D2O) δH (ppm) 8.10 (d, J = 8.2 Hz, 1H), 7.90 (t, J = 7.5 Hz, 1H), 7.71 (d, J = 8.4 Hz, 1H), 7.58 (dd, J = 9.9, 4.9 Hz, 2H), 7.53 (s, 1H), 7.17 (dd, J = 7.9, 4.8 Hz, 1H), 3.69–3.57 (m,5H), 2.93–2.83 (m, 2H), 2.06–1.91 (m, 2H). 13C NMR (150 MHz, D2O) δC (ppm) 168.9, 151.6, 146.9, 139.0, 135.8, 133.7, 127.4, 125.1, 123.7, 123.3, 122.4, 118.8, 116.2, 115.4, 111.0, 97.9, 44.8, 36.4, 34.3, 27.9. ESI-HRMS: m/z Calcd. for C20H19N4O2 [M−H]− 347.1513. Found 347.1505.
11-(2-(dimethylamino)ethylamino)-5-methyl-5H-indolo[2,3-b]quinoline-9-carboxylic acid 11b
White solids, Mp: 276–280 °C; 1H NMR (400 MHz, DMSO-d 6) δH (ppm) = 9.03 (d, J = 8.0 Hz, 2H), 8.57 (s, 1H), 8.15 (dd, J = 19.0, 8.6 Hz, 2H), 8.04 (t, J = 7.8 Hz, 1H), 7.82 (d, J = 8.4 Hz, 1H), 7.71 (t, J = 7.7 Hz, 1H), 4.37 (dd, J = 11.4, 5.7 Hz, 2H), 4.30 (s, 3H), 3.55 (t, J = 5.5 Hz, 2H), 2.78 (s, 6H). 13C NMR (150 MHz, DMSO-d 6) δC (ppm) 167.6, 152.5, 149.3, 140.71, 136.7, 133.3, 127.7, 125.2, 124.9, 124.5 (2C), 120.6, 116.9, 112.2, 99.5, 56.0, 43.1, 42.8, 35.7, 34.4. ESI-HRMS: m/z Calcd. for C21H21N4O2 [M−H]− 361.1670. Found 361.1653.
11-(3-(Dimethylamino)propylamino)-5-methyl-5H-indolo[2,3-b]quinoline-9-carboxylic acid 11c
Pale Yellow solids, Mp: 280–282 °C; 1H NMR (400 MHz, DMSO-d 6) δH (ppm) 8.97 (s, 1H), 8.89 (d, J = 8.5 Hz, 1H), 8.52 (s, 1H), 8.12 (dd, J = 16.6, 8.6 Hz, 2H), 8.05–7.98 (m, 1H), 7.79 (d, J = 8.4 Hz, 1H), 7.70 (t, J = 7.7 Hz, 1H), 4.26 (s, 3H), 4.01 (dd, J = 12.9, 6.5 Hz, 2H), 3.15–3.04 (m, 2H), 2.70 (s, 6H), 2.31 (dt, J = 14.0, 7.2 Hz, 2H); 13C NMR (150 MHz, DMSO-d 6) δC (ppm)167.7, 152.4, 149.3, 136.8, 133.2, 127.3, 124.9, 124.5 (2C), 124.2, 120.8, 116.9, 116.7, 112.2, 98.6, 53.7, 45.3, 42.1, 35.6, 25.1. ESI-HRMS: m/z Calcd. for C22H23N4O2 [M−H]− 375.1826. Found 375.1828.
11-(3-Hydroxypropylamino)-5-methyl-5H-indolo[2,3-b]quinoline-9-carboxylic acid 11d
White solids, Mp: >300 °C; 1H NMR (600 MHz, DMSO-d 6) δH (ppm) 8.86–8.72 (m, 2H), 8.59 (s, 1H), 8.10 (d, J = 8.5, 1H), 8.05 (d, J = 8.3, 1H), 7.99 (t, J = 7.6, 1H), 7.76 (d, J = 8.3, 1H), 7.66 (t, J = 7.5, 1H), 4.24 (s, 3H), 4.04 (dd, J = 11.8, 6.0, 2H), 3.46 (t, J = 5.6, 2H), 2.07–1.82 (m, 2H); 13C NMR (150 MHz, DMSO-d 6) δC (ppm) 167.4, 152.4, 148.7, 140.2, 136.7, 132.9, 127.0, 124.8, 124.4, 124.1, 123.9, 120.6, 116.8, 116.5, 111.9, 98.2, 58.0, 46.1, 35.6, 32.8. ESI-HRMS: m/z Calcd. for C20H18N3O3 [M–H]− 348.1354. Found 348.1327.
Antitumor screening test
Cell lines
Established in vitro, human cell line: MV4-11 (leukemia), A549 (lung cancer), HCT116 (colon cancer), and normal mice fibroblast (Balb/3T3) were used. These lines were obtained from American Type Culture Collection (Rockville, Maryland, USA) and were being maintained at the Institute of Immunology and Experimental Therapy, Wroclaw, Poland.
MV4-11 cells were cultured in the RPMI 1640 supplemented with 2 mM l-glutamine, 1.0 mM sodium pyruvate and 10 % fetal bovine serum (all from Sigma-Aldrich Chemie GmbH, Steinheim, Germany), HCT 116, and A549 cells were cultured in the RPMI 1640 + OptiMEM (50:50) medium (Gibco, Scotland, UK) supplemented with 2 mM l-glutamine and 5 % fetal bovine serum (all from Sigma-Aldrich Chemie GmbH, Steinheim, Germany), BALB/3T3 cells were cultured in Dulbecco medium (IIET) supplemented with 2 mM l-glutamine and 1.0 mM sodium pyruvate, 10 % fetal bovine serum (all from Sigma-Aldrich Chemie GmbH, Steinheim, Germany). All culture medium was supplemented with 100 units/ml penicillin and 100 μg/ml streptomycin (both from Polfa, Tarchomin S.A., Poland). All cell lines were grown at 37 °C with 5 % CO2 humidified atmosphere.
Antiproliferative assay in vitro
Test solutions of the compounds tested (1 mg/ml) were prepared by dissolving the substances in 100 μl of DMSO completed with 900 μl of tissue culture medium. Afterward, the tested compounds were diluted in culture medium to reach the final concentrations of 10, 1, 0.1, 0.01, and 0.001 μg/ml.
Twenty-four hours before the addition of the tested compounds, the cells were plated in 96-well plates (Sarstedt, Germany) at a density of 1 × 104 cells per well. The assay was performed after 72 h of exposure to varying concentrations of the tested agents. The in vitro cytotoxic effect of all agents was examined using the MTT (MV4-11) or SRB (A549, HCT116 and Balb/3T3) assay.
The results were calculated as an IC50 (inhibitory concentration 50)—the dose of tested agent which inhibits proliferation of 50 % of the cancer cell population. IC values were calculated for each experiment separately and mean values ± SD are presented in the Table 1, 2, 3 and 4. Each compound in each concentration was tested in triplicate in a single experiment, which was repeated 3–5 times.
MTT assay
This technique was applied for the cytotoxicity screening against leukemia cells growing in suspension culture. An assay was performed after 72 h exposure to varying concentrations (from 0.001 to 10 μg/ml) of the tested agents. For the last 3–4 h of incubation 20 μl of MTT solution were added to each well (MTT: 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide; stock solution: 5 mg/ml, Sigma-Aldrich, Germany). The mitochondria of viable cells reduce the pale yellow MTT to a navy blue formazan: the more viable cells are present in well, the more MTT will be reduced to formazan. When incubation time was completed, 80 μl of the lysing mixture were added to each well (lysing mixture: 225 ml dimethylformamide, POCh, Gliwice, Poland, 67.5 g sodium dodecyl sulfate, Sigma-Aldrich, Germany, and 275 ml of distilled water). After 24 h, when formazan crystals had been dissolved, the optical densities of the samples were read on an Multiskan RC photometer (Labsystems, Helsinki, Finland) at 570 nm wavelength. Each compound in given concentration was tested in triplicates in each experiment, which was repeated 3–5 times.
SRB assay
This technique was applied for the cytotoxicity screening against cells growing in adherent culture. The details of this technique were described by Skehan (Skehan et al., 1990). The cytotoxicity assay was performed after 72-h exposure of the cultured cells to varying concentrations (from 0.01 to 10 μg/ml) of the tested agents. The cells attached to the plastic were fixed by gently layering cold 50 % TCA (trichloroacetic acid, Aldrich-Chemie, Germany) on the top of the culture medium in each well. The plates were incubated at 4 °C for 1 h and then washed five times with tap water. The background optical density was measured in the wells filled with culture medium, without the cells. The cellular material fixed with TCA was stained with 0.4 % sulforhodamine B (SRB, Sigma, Germany) dissolved in 1 % acetic acid (POCh, Gliwice, Poland) for 30 min. Unbound dye was removed by rinsing (4x) with 1 % acetic acid. The protein-bound dye was extracted with 10 mM unbuffered Tris base (Sigma, Germany) for determination of optical density (at 540 nm) in a computer-interfaced, 96-well microtiter plate reader Multiskan RC photometer (Labsystems, Helsinki, Finland).
References
Alexandra P, Elsa TG, Jonathan S, Dave CW, Peter JH (2000) Antiplasmodial activity of cryptolepis sanguinolenta alkaloids from leaves and roots. Planta Med 66:30–34
Bailly C, Laine W, Baldeyrou B, De Pauw-Gillet MC, Colson P, Houssier C, Cimanga K, Van Miert S, Vlietinck AJ, Pieters L (2000) DNA intercalation, topoisomerase II inhibition and cytotoxic activity of the plant alkaloid neocryptolepine. Anti Canc Drug Des 15:191–201
Cimanga K, De Bruyne T, Lasure A, Van poel B, Pieters L, Claeys M, Vanden Berghe D, Kambu K, Tona L, Vlietinck AJ (1996a) In vitro biological activities of alkaloids from cryptolepis sanguinolenta. Planta Med 62:22–27
Cimanga K, De Bruyne T, Pieters L, Claeys M, Vlietinck A (1996b) New alkaloids from Cryptolepis sanguinolenta. Tetrahedron Lett 37:1703–1706
Cimanga K, De Bruyne T, Pieters L, Vlietinck AJ, Turger CA (1997) In vitro and in vivo antiplasmodial activity of cryptolepine and related alkaloids from cryptolepis sanguinolenta. J Nat Prod 60:688–691
Cimanga K, De Bruyne T, Pieters L, Totte J, Tona L, Kambu K, Vanden Berghe D, Vlietinck AJ (1998) Antibacterial and antifungal activities of neocryptolepine, biscryptolepine, and cryptoquindoline, alkaloids isolated from Cryptolepis sanguinolenta. Phytomedicine 5:209–221
Guittat L, Alberti P, Rosu F, Van Miert S, Thetiot E, Pieters L, Gabelica V, De Pauw E, Ottaviani A, Riou JF, Mergny JL (2003) Interactions of cryptolepine and neocryptolepine with unusual DNA structures. Biochimie 85:535–547
Jonckers THM, Van Miert S, Cimanga K, Bailly C, Colson P, De Pauw-Gillet MC, van den Heuvel H, Claeys M, Lemiere F, Esmans EL, Rozenski J, Quirijnen L, Maes L, Dommisse R, Lemiere GLF, Vlietinck A, Pieters L (2002) Synthesis, cytotoxicity, and antiplasmodial and antitrypanosomal activity of new neocryptolepine derivatives. J Med Chem 45:3497–3508
Kirby GC, Paine A, Warhurst DC, Noamese BK, Phillipson JD (1995) In vitro and in vivo antimalarial activity of cryptolepine, a plant-derived indoloquinoline. Phytother Res 9:359–363
Kumar EVKS, Etukala JR, Ablordeppey SY (2008) Indolo[3,2-b]quinolines: synthesis, biological evaluation and structure activity-relationships. Mini-Rev in Med Chem 8:538–554
Lavrado J, Moreira R, Paulo A (2010) Indoloquinolines as scaffolds for drug discovery. Curr Med Chem 17:2348–2370
Linton EC, Kozlowski MC (2008) Catalytic enantioselective meerwein-eschenmoser claisen rearrangement: asymmetric synthesis of allyl oxindoles. J Am Chem Soc 130:16162–16163
Ojima I, Kuduk SD, Pera P, Veith JM, Bernacki RJ (1997) Synthesis and structure-activity relationships of nonaromatic taxoids: effects of alkyl and alkenyl ester groups on cytotoxicity. J Med Chem 40:279–285
Parvatkar PT, Parameswaran PS, Tilve SG (2011) Isolation, biological activities, and synthesis of indoloquinoline alkaloids: cryptolepine, isocryptolepine, and neocryptolepine. Curr Org Chem 15:1036–1057
Philippe G, Lobo R, Valerie M, Eric D, Joseph S, Francois F, Francois T, Bernard B, Jean-Louis P (1996) Antimalarial activity of cryptolepine and isocryptolepine, alkaloids isolated from Cryptolepis sanguinolenta. Phytother Res 10:317–321
Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR (1990) New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst 82:1107–1112
Wang L, Switalska M, Mei ZW, Lu WJ, Takahara Y, Feng XW, El-Sayed IE, Wietrzyk J, Inokuchi T (2013) Synthesis and in vitro antiproliferative activity of new 11-aminoalkylamino-substituted 5H- and 6H-indolo[2,3-b]quinolines; structure-activity relationships of neocryptolepines and 6-methyl congeners. Bioorg Med Chem 20:4820–4829
Wang L, Lu WJ, Odawara T, Misumi R, Mei ZW, Peng W, El-Sayed IE, Inokuchi T (2013) Improved synthesis and reaction of 11-chloroneocryptolepines, strategic scaffold for antimalaria agent, and their 6-methyl congener fromindole-3-carboxylate. J Heterocyclic Chem 49 (in press)
Wender PA, Hinkle KW (2000) Synthesis and biological evaluation of a new class of bryostatin analogues: the role of the C20 substituent in protein kinase C binding. Tetrahedron Lett 41:6725–6729
Wright CW, Phillipson JD, Awe SO, Kirby GC, Warhurst DC, Quetin-Leclercq J, Angenot L (1996) Antimalarial activity of cryptolepine and some other anhydronium bases. Phytother Res 10:361–363
Xiong J, Zhu HF, Zhao YJ, Lan YJ, Jiang JW, Yang JJ, Zhang SF (2009) Synthesis and antitumor activity of amino acid ester derivatives containing 5-fluorouracil. Molecules 14:3142–3152
Yang LX, Pan XD, Wang HJ (2002) Novel camptothecin derivatives. Part 1: oxyalkanoic acid esters of camptothecin and their in vitro and in vivo antitumor activity. Bioorg Med Chem Lett 12:1241–1244
Acknowledgments
We gratefully acknowledge supports by Okayama University and the Advanced Science Research Center for NMR and EA. We thank MEXT for the scholarship to L.W. We are thankful to Prof. S. Nakashima and Prof. X.-Q.Yu, Sichuan University, for HRMS analyses, and to Prof. J. Futami for UV measurements. Support by Adaptable and Seamless Technology Transfer Program of JST and generous gift of various indoles from Air Water Inc. are highly appreciated.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lu, WJ., Świtalska, M., Wang, L. et al. In vitro antiproliferative activity of 11-aminoalkylamino-substituted 5H-indolo[2,3-b]quinolines; improving activity of neocryptolepines by installation of ester substituent. Med Chem Res 22, 4492–4504 (2013). https://doi.org/10.1007/s00044-012-0443-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-012-0443-x