Abstract
Tubulins, an αβ heterodimers, the major component of microtubules, are important molecular target of numerous small molecule ligands for anticancer therapy. In this study, the molecular modeling studies were performed to develop predictive 3D-QSAR models using set of 32 compounds of benzoyl urea derivatives as tubulin-binding agents for antiproliferative activity. A five-point common pharmacophore hypotheses with one hydrogen bond acceptor (A), two hydrogen bond donors (D), one hydrophobic (H), and one ring (R) vector features were selected for alignment of all compounds. The 3D-QSAR models generated using training set of 21 compounds and test set of 11 compounds showed good partial least squares statistical results. The developed CPHs and 3D-QSAR models were validated further externally by predicting the activity of database of compounds from literature and comparing it with actual activity. Docking studies were also carried out for all compounds on colchicine-binding site of β-tubulin for studying of binding affinity of compounds for antiproliferative activity. The results of these molecular modeling studies are helpful to refine the pharmacophore for design of new potential compounds for antiproliferative activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Microtubules (MTs), major structural components in cells, are the target of a large and diverse group of natural product anticancer drugs (Jordan and Wilson, 2004). MTs are polymeric protein complexes constructed from a heterodimer of two highly homologous proteins known as α- and β-tubulin. The assembly of tubulin heterodimers into a macromolecular MT complex is a tightly regulated and dynamic process. MTs have essential roles in vital cellular functions, such as motility, division, shape maintenance, and intracellular transport (Verhey and Gaertig, 2007; Pellegrini and Budman, 2005). Tubulin is therefore target of numerous small molecule ligands that interfere with MT dynamics, several of which are of clinical use, in particular for cancer treatment (Kiselyov et al., 2007; Jeffrey et al., 2007; Giannakakou et al., 2000). A structurally diverse collection of ligands, such as vinblastine sulfate, colchicine, combretastatin A-4 (Pettit et al., 1989), the epothilones (Goodin et al., 2004) and paclitaxel (Wani et al., 1971; Schiff and Horwitz, 1980) as well as some synthetic molecules (Yoshimatsu et al., 1997; Arora et al., 2009) are known to exert cytotoxic activities through binding to tubulin. Recently, much effort has been devoted to the identification of new compounds from both natural and synthetic sources that bind at the colchicine site of tubulin (Li and Sham, 2002; Kingston, 2009; Beckers and Mahboobi, 2003). Mutations in β-tubulin that affect MT polymer mass and/or drug binding are associated with resistance to tubulin-binding agents such as paclitaxel (Kavallaris, 2010). Also, independent of the precise mechanism of action, clinical use of antitubulin drugs is associated with problems of drug toxicity and bioavailability (Sridhare et al., 2004). Therefore, there is a need to find new tubulin-binding agents for anticancer activity. Recently, the benzoyl urea analogs have shown good anticancer activity and become new family of tubulin-binding agents (Song et al., 2008; Ling et al., 2002; Ling et al., 2003; Jiang et al., 2002; Li et al., 2003; Hwang et al., 2002).
We have previously demonstrated the use of energetic pharmacophore for the designing of cyclooxygenase-2 inhibitors (Lokwani et al., 2012) and used docking study for predicting the binding affinity of targets such as non-nucleoside reverse transcriptase inhibitors (Mokale et al., 2012). In search of new targets using computational studies, we present here the correlation of structures of benzoyl urea analogs with antiproliferative activity. Thus, to optimize this pharmacophore and for further improving the activity, we developed atom-based 3D-QSAR models using pharmacophore alignment and scoring engine (PHASE) and performed docking study on colchicine-binding site of β-tubulin using grid-based ligand docking with energetics (GLIDE). PHASE is self contained system for pharmacophore perception, QSAR model development, and 3D database screening (Dixon et al., 2006). We validated developed 3D-QSAR models internally by predicting the activity of test set and externally by predicting the activity of compounds obtained from literature. The GLIDE docking module approximated a complete systematic search of the conformational, orientational, and positional space of the docked ligand molecules into the receptor-binding pocket (Friesner et al., 2004). The developed atom-based 3D-QSAR model and docking studies highlight the structural features of benzoyl urea analogs for binding to colchicine-binding site of β-tubulin which is useful for further design of more potent tubulin-binding agent having antiproliferative activity.
Materials and methods
Biological data
A set of 32 benzoyl urea analogs (Table 1) with available IC50 data for antiproliferative activity were taken from literature (Song et al., 2008) for the development of ligand-based CPHs. The negative logarithm of the measured IC50 value (pIC50) was used in this study. For 3D-QSAR studies, these 32 compounds were divided into a training set (21 compounds) and a test set (11 compounds). The training set molecules were selected randomly in such a way that they contained information in terms of both their structural features and biological activity ranges. The most active molecules, moderately active, and less active molecules were included in training set to spread out the range of activities. In order to assess the predictive power of the model, a set of 11 compounds was arbitrarily set aside as the test set. The test compounds were selected in such a way that they truly represent the training set.

Ligand preparation
The structure of each compounds were cleaned and optimized using Ligprep v2.4 (Schrödinger, LLC, New York, NY, 2009). The cleanup and optimization process include conversion of structures from 2D to 3D, addition of hydrogen atoms, removal of counter ions, ionization state at the pH 7.0, generation of stereoisomers, removal of noncompliant structures, and energy minimization. Conforms of all ligands were generated using ConfGen macromodel search method (Watts et al., 2010) with maximum number of conformers 1,000 per structure and minimization steps 100 and minimized using OPLS_2005 force field (Jorgensen et al., 1996). Each minimized conformer was further filtered to eliminate redundant conformations. For each molecule, a set of conformers with maximum energy difference of 10 kcal/mol relative to global energy minimum conformers were retained. Also, conformers with the RMSD of lower than 1.0 Å between all pairs of corresponding heavy atoms were considered identical and discarded.
Generation of the common pharmacophore hypotheses (CPHs)
Pharmacophore features; hydrogen bond acceptor (A), hydrogen bond donor (D), hydrophobic group (H), negatively charged group (N), positively charged group (P), and aromatic ring (R) were defined by a set of chemical structure patterns as SMARTS queries. Common pharmacophoric features were then identified from a set of variants (set of feature types) that define a possible pharmacophore using a tree-based partitioning algorithm with maximum tree depth of four with the requirement that all actives must match. After applying default feature definitions to each ligand, CPHs were generated using a final box of 1 Å.
All generated CPHs were examined and selected based on a scoring function to yield the best alignment of the active ligands using an overall maximum root mean square deviation (RMSD) value of 1.2 Å with default options for distance tolerance. The quality of alignment was measured by a survival score, defined as
where W is the weight and the S is the score; S site is based on alignment score which is RMSD in the site point position as
S align (i) represents alignment score and C align (i) is RMSD cutoff (default value 1.2 Å); S vec represents vector score which is average cosine of the angles formed by corresponding pairs of vector features (acceptor, donors, and aromatic rings) in aligned structures; S vol represents volume score based on overlap of van der Waals model of non-hydrogen atoms in each pair of structures
where V common (i) is common or overlapping volume between ligand i and the reference ligand, while V total (i) is the total volume occupied by both ligand; S sel is the selectivity score which is an empirical estimate of the rarity of hypothesis, and accounts for what fraction of molecules are likely to match the hypothesis regardless of their activity toward the receptor; W mrev is the reward weights defined by m − 1, where m is the number of actives that match the hypothesis; ΔE is a penalty for high energy structures by subtracting a multiple of the relative energy from final score; A is the penalty for hypotheses for which the reference ligand activity is lower than the highest activity, by adding a multiple of the reference ligand activity to the score; W site, W vec, W vol, W rew, and W act, have default values of 1.0, while W sel and W E has a default value of 0.0. In hypothesis generation, default values have been used.
The CPHs with high survival score were chosen for alignment of molecules and used for further 3D-QSAR studies.
Building of 3D-QSAR models
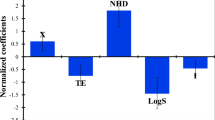
Atom-based 3D-QSAR models were developed using partial least squares (PLS) regression analysis for the top four CPHs based on their survival score. In atom-based 3D-QSAR in PHASE, each molecule is treated as a set of overlapping van der Waals spheres. Each atom (and hence each sphere) is placed into one of six categories according to a simple set of rules: hydrogens attached to polar atoms are classified as hydrogen bond donors (D); carbons, halogens, and C–H hydrogens are classified as hydrophobic/non-polar (H); atoms with an explicit negative ionic charge are classified as negative ionic (N); atoms with an explicit positive ionic charge are classified as positive ionic (P); non-ionic atoms are classified as electron withdrawing (W); and all other types of atoms are classified as miscellaneous (X). For construction of atom-based 3D-QSAR model, a rectangular grid of cubes (1 Å on each side) were defined for aligned training set for occupation of all atoms. Each occupied cubes were allotted one or more volume bits to represent the molecules by string of zero and ones. This representation gives rise to binary-valued occupation patterns that was used as independent variables to create PLS QSAR models (Dixon et al., 2006). The PLS regression was carried out with maximum of N/5 PLS factors, where N is the number of ligands in training set.
Docking method
The molecular docking tool, Glide v5.6 (Schrödinger, LLC, New York, NY, 2009) was used for docking studies of all compounds on colchicine-binding site of β-tubulin. The crystal structure of β-tubulin was obtained from protein data bank (PDB code: 1SA0) and was prepared for docking using “protein preparation wizard” in Maestro wizard v9.1 (Schrödinger, LLC, New York, NY, 2009). Water molecules in the crystal structures were deleted and termini were capped by adding ACE and NMA residue. The protein preparation was carried out in two steps, preparation and refinement. In preparation component, after ensuring chemical correctness, the hydrogens were added where hydrogen atoms were missing. Side chains that are not close to the binding cavity and do not participate in salt bridges were neutralized. In the refinement component, a restrained impact minimization of the co-crystallized complex was carried out. This helps in reorientation of side chain hydroxyl group. It uses the OPLS_2005 force field for this purpose. Grids were defined by centering them on the ligand in the crystal structure using the default box size.
The ligands were built using maestro build panel and prepared by Ligprep v2.4 (Schrödinger, LLC, New York, NY, 2009) which produces the low energy conformer of ligands using OPLS_2005 force field. The lower energy conformations of the ligands were selected and were docked into the grid generated from protein structures using standard precision (SP) docking mode.
Results and discussion
Generation of 3D-QSAR models
Different variant CPHs were generated by common pharmacophore identification process. All CPHs were examined and scored to identify the pharmacophore that yields the best alignment of the active compounds (pIC50 > 0.0). All CPHs were validated by aligning and scoring the inactive compounds (pIC50 < −0.3). We have selected top four CPHs models whose survival-inactive scores ranked in the top 1 % for alignment of all compounds and 3D-QSAR studies. The survival score for these CPHs is shown in Table 2. All top four CPHs were found to be associated with the five-point hypotheses, which consists of one hydrogen bond acceptor (A), two hydrogen bond donors (D), one hydrophobic (H), and one aromatic ring (R) vector features. All compounds were aligned using CPH ADDHR.102 for 3D-QSAR study (Fig. 1).
All top CPHs were used for atom-based 3D-QSAR model generation. The CPH ADDHR.102 yielded a 3D-QSAR model with good value of regression coefficient, low standard deviation, and high variance ratio with good stability, but showed diminished predictive power along with high RMSE value and low Pearson R value which stand for correlation between predicted and observed activity for test set. Similarly, CPH ADDHR.232 yielded a 3D-QSAR model with good statistical values but showed low predictive power (Q 2 = 0.4927) (Table 3). The CPHs ADDHR.11 and ADHHR.245 yielded 3D-QSAR models with good PLS statistical values. Both these hypotheses showed good internal as well as external predictive power. The training set correlation in both CPHs is characterized by PLS factors (R 2 = 0.7217, SD = 0.4705, F = 14.7, P = 5.641e−005, Q 2 = 0.5982 for CPH ADDHR.11 and R 2 = 0.7282, SD = 0.4651, F = 15.2, P = 4.641e−005, Q 2 = 0.5297 for CPH ADDHR.245). The test set correlation is characterized by PLS factors (RMSE = 0.5304, Pearson R = 0.7962 for CPH ADDHR.11 and RMSE = 0.4902, Pearson R = 0.769 for CPH ADDHR.245).
We have selected the 3D-QSAR models generated by CPHs ADDHR.11 and ADDHR.245 for correlating the structure with activity. Graph of observed versus predicted biological activity of training and test sets are shown in Figs. 2 and 3, respectively. Residuals values obtained by subtraction of predicted activities from observed biological activities are near to zero which indicated that error in prediction of biological activity is low and predicting ability of QSAR models developed by CPHs ADDHR.11 and ADDHR.245 is good (Table 4). Mean of residual in predicting the activity of compounds was calculated by average of summation of all residual values. 3D-QSAR models associated with hypotheses ADDHR.11 and ADDHR.245 showed 0.3659 and 0.3167, respectively, as the mean of residual which also support the predictability of both QSAR models. The pharmacophore hypothesis showing distance between pharmacophoric sites is depicted in Fig. 4.
Both 3D-QSAR models developed using CPHs ADDHR.11 and ADDHR.245 were applied to database of compounds taken from literature (Song et al., 2008, 2009a, b; Hu et al., 2007) for validation purpose. The activity of these reported compounds having benzoyl urea or similar pharmacophoric group were predicted and compared with its actual activity (Supplementary Data). The residual values obtained by subtraction of predicted activity from reported activity was found to be near zero for number of compounds. The mean of residual was also calculated and found as 0.529 and 0.484 for 3D-QSAR models associated with CPHs ADDHR.11 and ADDHR.245, respectively. These results suggested that correctness and capability of 3D-QSAR models developed by CPHs ADDHR.11 and ADDHR.245 for prediction of activity of compounds which can be applied to newly designed compounds for the prediction of their activity prior to synthesis.
Based on overall statistical results, 3D-QSAR model developed using CPHs ADDHR.11 were applied to each compound in the series for the establishment of structure–activity correlation. A pictorial representation of the cubes generated in the present 3D-QSAR is shown in Figs. 5 and 6. In these generated cubes, the blue cubes indicate favorable features, while red cubes indicate unfavorable features for biological activity. The blue cubes around the hydrogen bond acceptor and hydrogen bond donor group of benzoyl urea suggest the importance of core ring for activity.
Pictorial representation of the cubes generated using the QSAR model developed using CPH ADDHR.11 for a most active compound 31 and b least active compound 30 in the training set. Blue cubes indicate favorable regions, while red cubes indicate unfavorable region for the activity (Color figure online)
The substitutions around aromatic ring were observed to have small hydrophobic group and electron withdrawing group alone or both. The comparison of the most significant favorable and unfavorable interactions were observed when the 3D-QSAR model was visualized in the context of most active ligand (compound 31) and the least active ligand (compound 30) (Fig. 5). The blue cubes were observed at the position C6 at phenyl ring near hydrogen bond acceptor (A2) vector which indicated that for better activity the ring should be substituted with halogen atom (Fig. 5a). Thus, compounds having substituted ring at position C6 with halogen atom (compounds 7, 16, 18, 19, 31) are more active than compounds having substituted ring with other groups (compounds 14, 30). Moreover, Figs. 5 and 6 showed the importance of fluorine atom for activity among the all halogen atom as hydrophobic group. However, some compounds containing fluorine atom at the position C6 at phenyl ring were shown the moderate or lower activity, but this may be due to the replacement of hydrophobic group (H9) –Br or –I by –Cl near to Hydrogen bond acceptor (A1). This is proved in Fig. 6b, where blue cubes were observed around fluorine atom at the position C6 and red cubes around hydrophobic group (H9) –Cl near to hydrogen bond acceptor (A1) in compound 26.
Figure 6 shows features at position C4 and C5 which indicated that for better activity ring should un-substituted at these position. Therefore, compounds substituted by –Cl, CH3, and OCH3 groups at position C4 and –COOCH3 or NO2 group at position C5 showed lower and moderate activity, respectively. This also indicated that substitution by electron withdrawing groups alone or along with hydrophobic groups lower down the activity.
Docking studies
All structures were docked to colchicine-binding site of β-tubulin for studying of binding mode of compounds for antiproliferative activity. The constraint was defined as reported in literature that all compounds should bind to SH group of CYS 241 by hydrogen bond. The reliability of the docking results was first checked by comparing the best docking poses obtained for the co-crystallized inhibitor with its bound conformation. This was done by removing colchicine from their active site and subjecting again to docking into the binding pocket in the conformation found in the crystal structure. As a result, a RMSD of 1.36 and 0.25 Å were found for rigid and flexible docking which suggested that the docking procedure could be relied on to predict the binding mode of our compounds.
It was observed from docking results that all benzoyl urea analogs have a similar binding mode like colchicines in the binding pockets of β-tubulin (Fig. 7). The amino acids such as VAL 238, CYS 241, ALA 250, LYS 254, LEU 255, LYS 352, and THR 353 are involved in the interaction of all compounds. As defined in constraints, the interaction of standard compound colchicine with the residues of tubulin involved hydrogen bonding with side chain atom S of CYS 241. Similarly, out of three C=O groups, two C=O groups in all compounds except 19 and 32 showed hydrogen binding with SH of CYS 241 and NH of ALA 250 (Fig. 7). Some compounds also showed the hydrogen bonding with O atoms of THR 179, VAL 238, and LYS 352. 6. It was also found that amino acid residues formed the most of the hydrogen bonding with benzoyl urea chain.
CPHs ADDHR.11 and ADDHR.245 were also correlated with compounds docked at colchicine-binding site of β-tubulin and it was found that C=O group, hydrogen bond acceptor (A1) from CPH ADDHR.11 showed hydrogen binding with SH of CYS 241 (Fig. 8). Hydrogen bond acceptor (A2) vector feature from CPH ADDHR.245 not showed any hydrogen bonding, whereas C=O group near to hydrogen bond donor (D5) vector feature showed hydrogen bonding with NH of ALA 250. Thus, hydrogen bond acceptor (A1) feature showed importance for the formation of hydrogen bonding with SH of CYS 241.
Conclusion
The four 3D-QSAR models were developed using variant CPHs which consist of one acceptor, two donors, one hydrophobic, and one ring vector feature. The correctness and capability of both 3D-QSAR models were validated internally by applying to test set and externally by predicting the activity of compounds having benzoyl urea pharmacophore from literature. The one predictive 3D-QSAR models were selected based on statistical results. These 3D-QSAR models provide insight into the structural requirement of benzoyl urea analogs as tubulin agents for antiproliferative activity. Docking studies were also performed and also correlated with CPHs which indicated that all compounds bind in similar pose at colchicine-binding site of β-tubulin. Hydrogen bond acceptor (A1) vector feature showed the hydrogen bonding with SH of CYS 241 in all compounds suggested the importance of 3D-QSAR model developed using CPH ADDHR.11. Thus, this 3D-QSAR model gives a hypothetical image for designing of new potential compounds. The involvement of benzoyl urea core for the interaction with amino acid in colchicine-binding site of β-tubulin suggests its importance as common core for tubulin-binding agents. In conclusion, the overall study provides detailed structure and important binding information of benzoyl urea derivatives as tubulin-binding agents for antiproliferative activity.
References
Arora S, Wang XI, Keenan S, Andaya MC, Peng QY, Welsh WJ (2009) Novel microtubule polymerization inhibitor with potent antiproliferative and antitumor activity. Cancer Res 69:1910–1915
Beckers T, Mahboobi S (2003) Natural, semisynthetic and synthetic microtubule inhibitors for cancer therapy. Drugs Future 28:767–785
Dixon SL, Smondyrev AM, Knoll EH, Rao SN, Shaw DE, Friesner RA (2006) PHASE: a new engine for pharmacophore perception, 3D QSAR model development, and 3D database screening: 1. Methodology and preliminary result. J Comput Aided Mol Des 20:647–671
Friesner RA, Banks JL, Murphy RB, Halgren TA, Klicic JJ, Mainz DT, Repasky MP, Knoll EH (2004) Glide: a new approach for rapid, accurate docking and scoring. 1 Method and assessment of docking accuracy. J Med Chem 47:1739–1749
Giannakakou P, Sackett D, Fojo T (2000) Tubulin/microtubules: still a promising target for new chemotherapeutic agents. J Natl Cancer Inst 92:182–183
Goodin S, Kane MP, Rubin EH (2004) Epothilones: mechanism of action and biologic activity. J Clin Oncol 22:2015–2025
Hu L, Li Z, Li JN, Qu J, Jiangb JD, Boykin DW (2007) 3-(20-Bromopropionylamino)-benzamides as novel S-phase arrest agents. Bioorg Med Chem Lett 17:6847–6852
Hwang KJ, Park KH, Lee CO, Kim BT (2002) Novel benzoylurea derivatives as potential antitumor agents; synthesis, activities and structure–activity relationships. Arch Pharmacal Res 25:781–785
Jeffrey RJ, Denis RP, Mohammed MD, Pearl SH (2007) Targeted anti-mitotic therapies: can we improve on tubulin agents. Nat Rev Cancer 7:107–117
Jiang JD, Denner LY, Ling H, Li JN, Davis A, Wang Y, Li Y, Roboz J, Wang LG, Soler RP, Marcelli M, Bekesi G, Holland JF (2002) Double blockade of cell cycle at G1–S transition and M phase by 3-iodoacetamido benzoyl ethyl ester, a new type of tubulin ligand. Cancer Res 52:6080–6088
Jordan MA, Wilson L (2004) Microtubules as a target for anticancer drugs. Nat Rev Cancer 4:253–265
Jorgensen WL, Maxwell DS, Tirado-Rives J (1996) Development and testing of the OPLS all-atom force field on conformational energetics of organic liquids. J Am Chem Soc 118:11225–11236
Kavallaris M (2010) Microtubules and resistance to tubulin binding agents. Nat Rev Cancer 10:1–11
Kingston DG (2009) Tubulin-interactive natural products as anticancer agents. J Nat Prod 72:507–515
Kiselyov A, Konstantin VB, Sergey ET, Nikolay S, Alexandre VI (2007) Recent progress in discovery and development of antimitotic agents. Anti-Cancer Agents Med Chem 7:189–208
Li Q, Sham HL (2002) Discovery and development of antimitotic agents that inhibit tubulin polymerization for the treatment of cancer. Expert Opin Ther Pat 12:1663–1702
Li JN, Song DQ, Lin YH, Hu QY, Yin L, Bekesi G, Holland JF, Jiang JD (2003) Inhibition of microtubule polymerization by 3-bromopropionylamino benzoylurea (JIMB01), a new cancericidal tubulin ligand. Biochem Pharmacol 65:1691–1699
Ling YH, Liebes L, Ng B, Buckley M, Elliott PJ, Adama J, Jiang JD, Muggia FM, Perez-Soler R (2002) PS-341, a novel proteasome inhibitor, induces Bcl-2 phosphorylation and cleavage in association with G2-M phase arrest and apoptosis. Mol Cancer Ther 1:841–849
Ling YH, Liebes L, Jiang JD, Holland JF, Elliott PJ, Adama JF, Muggia M, Perez-Soler R (2003) Mechanisms of proteasome inhibitor PS-341-induced G2-M-phase arrest and apoptosis in human non-small cell lung cancer cell lines. Clin Cancer Res 9:1145–1154
Lokwani D, Shah R, Mokale S, Shastry P, Shinde D (2012) Development of energetic pharmacophore for the designing of of 1,2,3,4-tetrahydropyrimidine derivatives as selective cyclooxygenase-2 inhibitors. J Comput Aided Mol Des 26:267–277
Mokale SN, Lokwani D, Shinde DB (2012) Synthesis, biological activity and docking study of imidazol-5-one as novel non-nucleoside HIV-1 reverse transcriptase inhibitors. Bioorg Med Chem 20:3119–3127
Pellegrini F, Budman DR (2005) Review: tubulin function, action of antitubulin drugs and new drug development. Cancer Invest 23:264–273
Pettit GR, Singh SB, Hamel E, Lin CM, Alberts DS, Garcia-Kendall D (1989) Isolation and structure of the strong cell growth and tubulin inhibitor com-bretastatin A-4. Experientia 45:209–211
Schiff PB, Horwitz SB (1980) Taxol stabilizes microtubules in mouse fibroblast cells. Proc Natl Acad Sci USA 77:1561–1565
Song DQ, Wang Y, Wu L, Yang ZP, Wang YM, Gao LM, Li Y, Qu JR, Wang YH, Li YH, Du NN, Han YX, Zhang ZP, Jiang JD (2008) Benzoylurea derivatives as a novel class of antimitotic agents: synthesis, anticancer activity, and structure–activity relationships. J Med Chem 5:3094–3103
Song DQ, Du N, Wang YM, He WY, Jiang EZ, Cheng SX, Wang YX, Li YH, Wang YP, Li X, Jiang JD (2009a) Synthesis and activity evaluation of phenylurea derivatives as potent antitumor agents. Bioorg Med Chem 17:3873–3878
Song DQ, Wang YM, Du NN, He WY, Chen KL, Wang GF, Yang P, Wu LZ, Zhang XB, Jiang JD (2009b) Synthesis and activity evaluation of benzoylurea derivatives as potential antiproliferative agents. Bioorg Med Chem Lett 19:755–758
Sridhare M, Macapinlac MJ, Goel S, Verdier-Pinard D, Fojo T, Rothenberg M, Colevas D (2004) The clinical development of new mitotic inhibitors that stabilize the microtubule. Anticancer Drugs 15:553–558
Verhey KJ, Gaertig J (2007) The tubulin code. Cell Cycle 6:2152–2160
Wani MC, Taylor HL, Wall ME, Coggon P, McPhail AT (1971) Plant antitumor agents, VI. Isolation and structure of taxol, a novel antileukemic and antitumor agent from Taxus brevifolia. J Am Chem Soc 93:2325–2327
Watts KS, Dalal P, Murphy RB, Sherman WR, Friesner A, Shelley JC (2010) ConfGen: a conformational search method for efficient generation of bioactive conformers. J Chem Inf Model 50:534–546
Yoshimatsu K, Yamaguchi A, Yoshino H, Koyanagi N, Kitoh K (1997) Mechanism of action of E7010, an orally active sulfonamide antitumor agent: inhibition of mitosis by binding to the colchicine site of tubulin. Cancer Res 57:3208–3213
Acknowledgments
The authors are thankful to University Grant commission (UGC), New Delhi for financial assistance. The authors are also thankful to the Head, Department of Chemical Technology, Dr. Babasaheb Ambedkar Marathwada University, Aurangabad 431 004 (MS), India for providing all necessary facilities for above work.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lokwani, D.K., Sarkate, A.P. & Shinde, D.B. 3D-QSAR and docking studies of benzoyl urea derivatives as tubulin-binding agents for antiproliferative activity. Med Chem Res 22, 1415–1425 (2013). https://doi.org/10.1007/s00044-012-0139-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-012-0139-2