Abstract
The aim of this work was to investigate the antibacterial and antifungal activities of novel d-amino acid-Schiff bases including fluorine atom and their Cr(III) and Ni(II) complexes. All these substances have been examined for antibacterial activity against pathogenic strains Listeria monocytogenes 4b ATCC19115, Staphylococcus aureus ATCC25923, Escherichia coli ATCC1280, Salmonella typhi H NCTC 901.8394, Brucella abortus (A.99, UK-1995) RSKK03026, Staphylococcus epidermis sp., Micrococus luteus ATCC9341, and Shigella dysenteria typ 10 NCTC 9351, and antifungal activity against Candida albicans Y-1200-NIH, Tokyo. The antimicrobial test results of these amino acid-Schiff base complexes exhibited better activity than some known antibiotics. In particular, diamagnetic Ni(II) complexes were more potent bactericides than all of the substances synthesized.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Schiff bases appear to be important intermediates in a number of enzymatic reactions involving enzyme interaction with an amino or a carbonyl group of a substrate. In bioinorganic chemistry, the interest in the Schiff base complexes derives from their ability to provide synthetic models for metal-containing sites in metalloproteins and to contribute to developments in medicinal chemistry. Thus, Schiff bases and their complexes have a variety of applications in biological, clinical, and analytical fields (Eglof et al., 2009; Ellis et al., 1997). Recently, there has been considerable interest in the chemistry of amino acid-Schiff base compounds due to their potential in nuclear medicine applications (Abram and Alberto, 2006; Gharagozlou and Boghaei, 2008).
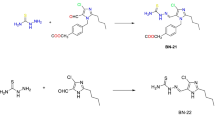
However, the drug resistances against antibacterial agents may pose a problem in their use with medical purpose (Singh et al., 2006). The problem could be overcome by the preparation of metal complexes, by a process of chelation with the coordination of transition metal ions. It is well known that N and O atoms play a key role in the coordination of metals. Amino acid-Schiff bases have N and O atoms as their basic elements. Schiff base derivatives containing donor atom can act as good chelating agents for the transition of metal ions (Avaji et al., 2008; Takeuchi et al., 1999; Sari et al., 2008). Amino acid-Schiff base metal complexes have found wide applications in corrosion inhibitors (Negm and Zaki, 2009), tumor radio-imaging agents (Wang et al., 2005), and pharmacologically active compounds (Wang et al., 2007). However, no studies have been carried out on Schiff bases and their metal complexes derived from amino acid-Schiff bases including fluorine. This study aimed to fill in this gap. Novel amino acid-Schiff bases derivatives were investigated to find out the antibacterial properties of Schiff bases and their complexes (Scheme 1). Amino acid-Schiff base derivatives were synthesized by condensation methods. And then, Cr(III) and Ni(II) complexes were synthesis by template methods.
Chemicals and methods
5-Fluoro-2-hydroxy benzaldehyde, 3-chloro-5-fluoro-2-hydroxbenzaldehyde, d-glycine, d-alanine, Ni(II) chloride, and Cr(III) chloride were provided by Sigma-Aldrich Company. 1H and 13C NMR spectra of the Schiff bases were recorded using a Bruker DPX-300 MHz and 100 MHz using TMS as an internal standard and CDCl3 as solvent. FTIR spectra in the 4000–400 cm−1 range were measured using KBr disks on a Mattson 1000 FTIR spectrophotometer. Carbon, hydrogen, and nitrogen content were obtained using LECO-9320 analyzer. Conductivity measurements were carried out at 20 °C in 10−3 M DMSO using a Siemens WPA CM 35 apparatus. The room temperature magnetic moments were measured using MK-1 model Gouy Balance of Christison Scientific Equipment Ltd. Metal contents were determined using a Philips PU 9285 atomic absorption instrument. Mass spectra were recorded on a Micro Mass-UK Platform II mass spectrometer.
5-Fluoro-2-hydroxybenzaldehyde-d-glycine
A solution of 5-fluoro-2-hydroxy benzaldehyde 0.35 g (2.5 × 10−3 mol) in MeOH (10 ml) was added drop wise to the 4 ml hot aqueous solution of the d-glycine 0.19 g (2.5 × 10−3 mol) and heated under reflux for 4 h in nitrogen atmosphere. After cooling, the mixture was filtered and allowed to stand. On standing for a further 4 days in nitrogen atmosphere, the solid crude formed was collected by filtration, dried in desiccators over CaCl2. Yields: 52.
5-Fluoro-3-chloro-2-hydroxybenzaldehyde-d-glycine
A solution of 3-chloro-5-fluoro-2-hydroxbenzaldehyde 0.44 g (2.5 × 10−3 mol) in MeOH (10 ml) was added drop wise to the 4 ml hot aqueous solution of the d-glycine 0.19 g (2.5 × 10−3 mol) and heated under reflux for 4 h in nitrogen atmosphere. After cooling, the mixture was filtered and allowed to stand. On standing for a further 6 days in nitrogen atmosphere, the solid crude formed was collected by filtration, dried in desiccators over CaCl2. Yields: 56.
5-Fluoro-2-hydroxybenzaldehyde-d-alanine
A solution of 5-fluoro-2-hydroxy benzaldehyde 0.35 g (2.5 × 10−3 mol) in MeOH (10 ml) was added drop wise to the 4 ml hot aqueous solution of the d-glycine 0.19 g (2.5 × 10−3 mol) and heated under reflux for 4 h in nitrogen atmosphere. After cooling, the mixture was filtered and allowed to stand. On standing for a further 4 days in nitrogen atmosphere, the solid crude formed was collected by filtration, dried in desiccators over CaCl2. Yields: 68.
5-Fluoro-3-chloro-2-hydroxybenzaldehyde-d-alanine
A solution of 3-chloro-5-fluoro-2-hydroxbenzaldehyde 0.44 g (2.5 × 10−3 mol) in MeOH (10 ml) was added drop wise to the 4 ml hot aqueous solution of the d-alanine 0.22 g (2.5 × 10−3 mol) and heated under reflux for 4 h in nitrogen atmosphere. After cooling, the mixture was filtered and allowed to stand. On standing for a further 4 days in nitrogen atmosphere, the solid crude formed was collected by filtration, washed with a small volume of ethanol and dioxan, and then, finally crystallized from methanol, dried in desiccators over CaCl2. Yields: 58.
General synthesis of complexes
Cr(III) and Ni(II) complexes of Schiff bases resulting from the condensation of 1 mol of aldeyhde with 1 mol of d-amino acid has been isolated by metal template reaction. A sample of CrCl3·6H2O and NiCl2·6H2O (for Cr(III) and Ni(II); 0.67 and 0.59 g, respectively, 2.5 × 10−3 mol) was dissolved in methanol (25 ml). To the solution, amino acid (d-glycine 0.19 g, d-alanine 0.22 g; 2.5 × 10−3 mol) and aldehyde (5-fluoro-2-hydroxy benzaldehyde 0.35 g, 3-chloro-5-fluoro-2-hydroxbenzaldehyde 0.44 g; 2.5 × 10−3 mol) in methanol (25 ml) was added, immediately giving a colored solution. The resulting solution was stirred for ca. 5 h in nitrogen atmosphere, filtered, and allowed to stand. On standing for a further 10–20 days, the solid complexes formed was collected by filtration, washed with a small volume of ethanol and acetone, and then, dried in a desiccator over CaCl2. Yields: 61–73.
Detection of antimicrobial activity
The bacterial subcultures chosen were Listeria monocytogenes 4b ATCC19115, Staphylococcus aureus ATCC25923, Escherichia coli ATCC1280, Salmonella typhi H NCTC-901.8394, Brucella abortus (A.99, UK-1995) RSKK03026, Staphylococcus epidermis sp., Micrococus luteus ATCC9341, and Shigella dysenteria type 10 NCTC 9351. An antifungal susceptibility test was used by Candida albicans Y-1200-NIH, Tokyo.
The ligands and the complexes were tested for their antimicrobial activity by the well-diffusion method. Each ligand and complex was kept dry at room temperature and dissolved (0.25 μg/ml) in DMF. DMF was used as solvent and also for control. It was found to have no antimicrobial activity against any of the tested organisms. 1% (v/v) of 24-h broth culture containing 106 CFU/ml was placed in sterile Petri dishes. Mueller-Hinton Agar (MHA) (15 ml) kept at 45 °C was then poured into the Petri dishes and allowed to solidify. Then 6-mm diameter wells were punched carefully using a sterile cork borer and were entirely filled with the test solutions. The plates were incubated for 24 h at 37 °C. On completion of the incubation period, the mean value obtained for the three holes was used to calculate the zone of growth inhibition of each sample. Bacterial and fungal subcultures were tested for resistance to six antibiotics (produced by Oxoid Lt., Basingstoke, UK): ampicillin (preventing the growth of gram-negative bacteria), nystatin (binding to sterols in the fungal cellular membrane, altering the permeability, and allowing leakage of the cellular contents), kanamycin (used in molecular biology as agent to isolate bacteria), sulfamethoxazol (bacteriostatic antibacterial agent that interferes with folic acid synthesis in susceptible bacteria), amoxycillin (b-lactam antibiotic used to treat bacterial infections caused by susceptible microorganisms), Sulbactam.
Results and dissociations
Analytical data and some of the physical properties of the Schiff bases and their complexes are summarized in Table 1. The complexes are only soluble in DMF and DMSO, but insoluble in organic solvents like CCl4 and benzene. Molar conductance values of the Cr(III) and Ni(II) complexes were found to be ca. 7 μS/cm in 10−3 M DMF solutions, indicating the 1:1 electrolytic nature of the compounds. This state indicated the nonelectrolytic behavior for Cr(III) and Ni(II) complexes (Altundas et al., 2010). The results obtained showed that the structure of complexes is the ML complexes according to the LC-mass spectroscopy of [M-3H2O]+ 253 (m/z: %11.2), [M-3H2O]+ 267 (m/z: %9.8), [M-H2O]+ 287.6 (m/z: %18.2), [M-H2O]+ 301.6 (m/z: %11.2), [M-2H2O]+ 281.7 (m/z: %11.2), [M-2H2O]+ 295.7 (m/z: %11.2), [M-2H2O]+317.1 (m/z: %11.2), and [M-2H2O]+ 330.1 (m/z: %11.2); for [Ni(5F-Gly)(H2O)3], [Ni(5F-Ala)(H2O)3], [Ni(5F-3Cl-Gly)(H2O)], [Ni(5F-3Cl-Ala)(H2O)], [Cr(5F-Gly)Cl(H2O)2], [Cr(5F-Ala)Cl(H2O)2], [Cr(5F-3Cl-Gly)Cl(H2O)2], and [Cr(5F-3Cl-Ala)lCl(H2O)2], respectively.
Table 2 summarizes the main IR and UV–visible bands of the Schiff bases and their complexes. IR bands of Schiff bases in the 1663–1667 cm−1, 3110–3125 cm−1, 2798–2864/2446–2467 cm−1 regions are characteristic of ν(CH=N), ν(OH), and ν(CH)arom/alif, respectively (Sarı and Gürkan, 2004). The bands in the 1621–1638 cm−1 and 1579–1590 cm−1 regions may, respectively, ascribe to νCOO-(asym) and νCOO-(sym) vibrations (Sarı and Gürkan, 2004). The electronic spectra of the Schiff bases in DMF show bands ca. 340 nm are attributed to the azomethine chromospheres n → π* transition (Sari et al., 2003). The bands at higher energies (272–343 nm) are associated with the benzene π → π* and σ → σ* transition (Sari et al., 2003; Silverstein et al., 1981). The 1H-NMR and 13C-NMR data of the Schiff bases are presented in Table 3. In general, the duplets, quartet, or triplets observed at 7.10–7.85 ppm are assigned to aldehyde ring protons. The singlet at 8.27–8.41 ppm and 9.95–9.99 ppm are assigned to imine and aromatic hydroxyl protons, respectively. The protons of –CH3, –CH2, and –CH of amino acid group in the Schiff bases are also observed as expected. The 13C-NMR spectra data of the Schiff bases (Table 3) are also in accordance with the proposed structures.

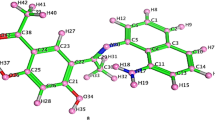
The azomethine and carboxylate bands in the IR spectra of the complexes appear in the range 1683–1691, 1651–1658, and 1549–1561 cm−1, somewhat different than observed for the free ligands. These indicate that the azomethine nitrogen and the oxygen of the carboxylate group are coordinated to metal ion. The IR spectra of all complexes exhibit characteristic bands of coordination water at ca. 3450, 885, and 774 cm−1 assigned to ν(OH), ρ r (OH), and ρ W (OH2) vibrations, respectively (Sarı and Gürkan, 2004). These observations clearly suggest that the water molecules are coordinated to the metal ion (Scheme 2). The appearance of new bands in the 503–560 and 405–411 cm−1 regions are due to ν(M–O) and ν(M–N), respectively (Altundas et al., 2010).
Since all Cr(III) complexes and octahedral Ni(II) complexes are paramagnetic, the 1H-NMR spectra could not be obtained. 1H-NMR spectra of [Ni(5F-3Cl-Gly)(H2O)] and [Ni(5F-3Cl-Gly)(H2O)] complexes were obtained due to the diamagnetic properties. The diamagnetic Ni(II) complexes exhibit signals in the range of 8.42–8.43 ppm due to –CH=N protons. These signals are observed in the lower field than ligands. The signals (1H-NMR and IR spectra) of Ni(II) complexes are different from those of the corresponding ligands, suggesting the coordination through oxygen atoms in a phenol ring and azomethine groups. The ligands showed signals at 9.95–9.99 ppm, but the complexes do not contain OH signals as the ligands can coordinate Ni(II) ions with deprotonated phenolic oxygen (Table 3). More detailed information about the structure of the ligands and their Ni(II) complexes were provided by the 13C-NMR spectra data. 13C-NMR spectra of the ligands were assigned by comparison with those of their Ni(II) complexes. The signals of carbon atoms, which neighbor to an –OH group, were different. This can be attributed to the coordination of the phenolic oxygen atom (Altundas et al., 2010) (Table 3). C9 and C4 carbon atoms in the free Schiff bases showed a significant shift after complexation. Furthermore, doublets observed at 192.70–192.64 ppm and 192.71–192.68 ppm due to complexion, are assigned to carbon of –COOH. This case may be due to the coordination of the ligand to the metal atom by the azomethine nitrogen and phenolic oxygen. Paramagnetic [Ni(5F-Gly)(H2O)3] and [Ni(5F-Ala)(H2O)3] complexes show three d–d bands in the region 865–893 nm, 636–641 nm, and 389–400 nm. First band is assigned for 3A2g(F) → 3T2g(F)(υ1), the second band for 3A2g(F) → 3T1g(F)(υ2), and third band 3A2g(F) → 3T2g(P)(υ3) transition. The UV-spectrum of Chromium (III) complexes shows three bands in the regions 537–627 nm, 329–369 nm, and 283–306 nm. The peak ca. 550, 350, and 290 nm corresponds to the 4A2g → 4T2g, 4A2g → 4T1g (F), and 4A2g → 4T1g (P) electronic transition, respectively. The magnetic measurements for Cr(III) and two Ni(II) (for [Ni(5F-Gly)(H2O)3] and [Ni(5F-Ala)(H2O)3]) complexes showed three and two unpaired electrons, and the magnetic moment values 4.10–3.81 BM and 2.84–2.88 BM, respectively, for Cr(III) and paramagnetic Ni(II) ion suggesting consistency with their octahedral environment.
Biological activity of Schiff bases and their complexes
The ligands and their complexes were screened for antimicrobial activity in DMF solvent as a control substance. The compounds were tested with the same concentrations in DMF solution (0.25 μg/μl). All the synthesized compounds and antibiotic exhibited varying degree of inhibitory effects on the growth of different tested strains (Table 4). All the compounds were active against L. monocytogenes, Br. abortus, M. luteus, and C. albicans. Br. abortus is a gram-negative bacterium that causes premature abortion of a cattle fetus. What makes this bacterium so dangerous is that it can be transferred from an animal to a human host (Sari et al., 2006). In humans, this disease causes both acute and chronic symptoms, but can be treated with antibiotics. This research indicates that amino acid-Schiff bases and their Ni(II) and Cr(III) complexes are active against Br. abortus (Table 4). All the synthesized compounds showed moderate activity against L.monocytogenes except [5F-3Cl-Ala]. [5F-Ala] and its Cr(III) complexes were inactive in S.typhi H, whereas [5F-Gly] and their complexes were active gram (−ve), [5F-3Cl-Gly] and their complexes were active gram (+ve). In general, the metal complexes are more potent bactericides than the ligand. This enhancement in activity may be explained on the basis of chelation theory (Altundas et al., 2010). Chelation reduces the polarity of the metal ion. Hence, a complex has lipophilic character, and increases the interaction between metal ion and the lipid is favored. This lead to the breakdown of the permeability barrier of the cell, resulting in interference with the normal cellular processes (Murukan and Mohanan, 2007). As seen in Table 4, diamagnetic Ni(II) complexes showed a significant activity against gram (+ve) and gram (−ve) (Scheme 3). The investigated compound’s antimicrobial activity values in this research were higher than that reported for other amino acid-Schiff base derivatives (Sakiyan et al., 2004; Sari et al., 2003). The main difference in the amino acid-Schiff base derivatives reported in this paper is the presence of the fluorine atom. The other amino acid-Schiff base derivatives included substituted benzyl, pyridine, or naphtha groups. Coordination structure of complexes plays an important role in their biological activity mechanisms. The results show that the diamagnetic Ni(II) complexes are more active against the tested yeast and as compared to the paramagnetic Ni(II) complexes. Diamagnetic Ni(II) complexes have square planer structure (D4h). Square planer structure may be reducing the polarity of the metal ion due to the strong overlap in between the dx2−y2 orbital of metal ion and donor orbital of the ligand. Thus, the lipophilic character of the central metal atom is enhanced, which results in a higher capability to penetrate the microorganisms through the lipid layer of the cell membrane.
Furthermore, the antibacterial activity of these compounds was also compared with seven commercial antibiotics, namely Kanamycin, Sulfamethoxazol, Ampicillin, Ciprofloxacin, Amoxycillin, Sulbactam, and Nystatin. It was seen that the synthesized compounds were effective as the antibiotics mentioned.
Conclusions
In this paper, new amino acid-Schiff base derivatives were synthesized by the condensation of 5-fluoro-2-hydroxy benzaldehyde, 3-chloro-5-fluoro-2-hydroxbenzaldehyde with d-glycine, d-alanine. And then, Ni(II) and Cr(III) complexes were prepared by the template method. The structures of the prepared compound were confirmed by elemental analysis, IR 1H and 13C NMR spectral analysis. Diamagnetic Ni(II) complexes showed a significant activity against gram (+ve) and gram (−ve).
References
Abram U, Alberto R (2006) Technetium and rhenium: coordination chemistry and nuclear medical applications. J Braz Chem Soc 17:1486–1500
Altundas A, Sarı N, Colak N, Ögütcü H (2010) Synthesis and biological activity of new cycloalkylthiophene-Schiff bases and their Cr(III) and Zn(II) complexes. Med Chem Res 19:576–588
Avaji PG, Patil SA, Badami S (2008) Synthesis, spectral, thermal, solid-state DC electrical conductivity and biological studies of Co(II) complexes with Schiff bases derived from 3-substituted-4-amino-5-hydrazino-1,2,4-triazole and substituted salicylaldehydes. Trans Met Chem (Weinh). 33:275–283
Eglof R, Piotr P, Bogumil B, Franz B (2009) Schiff bases in biological systems. Curr Org Chem 13:241–249
Ellis G, Chang L, Cogionis B, Daneman D (1997) Incomplete removal of labile fraction when measuring hemoglobin A1c with bio-rad variant analyzer. Clin Chem 43:2437–2439
Gharagozlou M, Boghaei DM (2008) Interaction of water-soluble amino acid Schiff base complexes with bovine serum albumin: fluorescence and circular dichroism studies. Spectrochim Acta Mol Biomol Spectros 71:1617–1622
Murukan B, Mohanan K (2007) Synthesis, characterization and antibacterial properties of some trivalent metal complexes with [(2-hydroxy-1-naphthaldehyde)-3-isatin] bishydrazone. J Enzym Inhib Med Chem 22:65–70
Negm NA, Zaki MF (2009) Synthesis and characterization of some amino acid derived Schiff bases bearing nonionic species as corrosion inhibitors for carbon steel in 2N HCl. J Disper Sci Technol 30:649–655
Sakıyan I, Loğoğlu E, Sari N, Sakıyan N (2004) Antimicrobial activities of N-(2-hydroxy-1-naphthalidene)-aminoacid(glycine, alanine, phenylalanine, histidine, tryptophane) Schiff bases and their manganese(III) complexes. Biometals 17:115–120
Sarı N, Gürkan P (2004) Some novel amino acid-Schiff bases and their complexes synthesis, characterization, solid state conductivity behaviors and potentiometric studies. Z Naturforsch B 59(6):692–698
Sari N, Gürkan P, Cete S, Sakiyan I (2006) Synthesis, potentiometric and antimicrobial activity studies on DL-Amino Acids-Schiff Bases and their complexes Russ. J Coord Chem 32(7):511–517
Sarı N, Gürkan P, Arslan S (2003) Synthesis, potentiometric and antimicrobial activity studies on 2-pyridinilidene-DL-amino acids and their complexes. Trans Met Chem 28:468–474
Sarı N, Nartop D, Karcı F, Disli A (2008) Novel hydrazone derivatives and their tetracoordinated metal complexes. Asian J Chem 20:1975–1985
Silverstein RM, Bassler GC, Morrill TC (1981) Spectrophotometric identification of organic compounds, 4th edn. Wiley, New York, pp 125–130
Singh K, Singh DP, Barwa MS, Tyagi P, Mırza Y (2006) Antibacterial Co(II), Ni(II), Cu(II) and Zn(II) complexes of Schiff bases derived from fluorobenzaldehyde and triazoles. J Enzym Inhib Med Chem 21:557–562
Takeuchi T, Böttcher A, Quezada CM, Meade TJ, Gray HB (1999) Inhibition of thermolysin and human α-thrombin by cobalt(III) Schiff base complexes. Bioorg Med Chem 5:815–819
Wang MZ, Meng ZX, Liu BL, Cai GL, Zhang CL, Wang XY (2005) Novel tumor chemotherapeutic agents and tumor radio-imaging agents: potential tumor pharmaceuticals of ternary copper(II) complexes. Inorg Chem Commun 8:368–371
Wang RM, Mao JJ, Song JF, Huo CX, He YF (2007) Antioxidant activity of bovine serum albumin binding amino acid Schiff-bases metal complexes. Chinese Chem Lett 18:1416–1418
Acknowledgments
This work was supported by the Gazi University Research Fund (Project number: 05/2010-03 and 05/2007-02).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sarı, N., Pişkin, N., Öğütcü, H. et al. Spectroscopic characterization of novel d-amino acid-Schiff bases and their Cr(III) and Ni(II) complexes as antimicrobial agents. Med Chem Res 22, 580–587 (2013). https://doi.org/10.1007/s00044-012-0039-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-012-0039-5