Abstract
Acinetobacter baumannii is an increasingly nosocomial pathogen throughout the world, and the occurrence of multidrug-resistant (MDR) species is increasing. The aim of this study is to present the antimicrobial effects of a newly synthesized imidazoacridine, 11-chloro-3-methyl-3H-imidazo(4,5-a)acridine (CMIA), against MDR clinical isolates of A. baumannii. Standard dilution tube-test assay was performed to determine the MBC of CMIA for 91 clinical isolates of highly antibiotic-resistant bacteria with 28 of A. baumannii in them. The MBCs of CMIA ranged from 2.0 to 10.9 mg/l for Acinetobacter isolates while it was more than 47.9 mg/l for other clinical strains. The findings demonstrate that CMIA is a potent and selective antimicrobial agent against clinical strains of antibiotic-resistant A. baumannii.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Acinetobacter is a common type of bacteria found in many places, including water, soil, and sewage. There are at least 25 different types of Acinetobacter. Acinetobacter baumannii is the particular type that is often associated with hospital infections.
The multidrug-resistant (MDR) types of Acinetobacter spp. are a series of the most important pathogens causing the nosocomial infections in communities (Abbo et al., 2005; Jain and Danziger, 2004). The Centers for Disease Control and Prevention recently highlighted the enormity and gravity of MDR A. baumannii infections in military medical facilities treating civilians and service personnel injured in Iraq, Kuwait and Afghanistan (Hujer et al., 2006). Antimicrobial evaluation of the mentioned species showed that more than half of them were resistant to three or more classes of antibiotics.
Another significant problem is the infection of large number of military personnel by MDR Acinetobacter spp. and finally widely disseminated types of bacteria over the world (Hujer et al., 2006). With the appearance of MDR strains of A. baumannii in ICUs, and burn units, as well as in soldiers returning from overseas, new treatments for such infections are necessary (Osterburg et al., 2009; Insa et al., 2007).
In this study, we present a recently synthesized analog of imidazoacridine as a selective growth inhibitor of MDR A. baumannii in comparison with common pathogenic strains of bacteria.
Results and discussions
Our interest in imidazoacridine derivatives as bactericide agents emerges from the early research down by Rahimizadeh et al., in which the synthesis and antibacterial activity of some imidazo(4,5-a)acridines was reported (Rahimizadeh et al., 2009). It was shown that these series of compounds have growth-inhibitory activity against four standard strains of Escherichia coli, Pseudomonas aeruginosa, Staphylococcus aureus, and Bacillus subtilis. Herein, the minimum bactericidal concentrations (MBCs) of three 11-chloro-3-alkyl-3H-imidazo[4,5-a]acridines possessing methyl, n-propyl and n-pentyl substituents (5a–5c) (Scheme 1), were evaluated against S. aureus PTCC 1074, P. aeruginosa PTCC 1431, Klebsiella pneumonia PTCC 1053, and E. coli PTCC 1338. The MBCs were determined by using the dilution tube-test method, introduced by the National Committee for Clinical Laboratory Standards (Finegold and Garrod, 1995).
The results showed that the compounds with more lipophilic character exert less antibacterial activity. The octanol–water distribution coefficient (Log D) of 5a–5c was measured by using shake flask method (Table 1) (Berthod and Carda-broch, 2004). Among the synthetic compounds, 5a (11-chloro-3-methyl-3H-imidazo[4,5-a]acridine: CMIA), which exhibited the best bactericidal activity, was evaluated against some antibiotic-resistant gram-negative and gram-positive bacteria. 247 strains were isolated from different organs of patients at the Microbiological Laboratory of Ghaem Hospital (Medical University of Mashhad, Iran) and tested for antibiotic resistancy by disc diffusion method (Prescott et al., 2002). Among the isolates, 91 cases with highest antibiotic resistancy: A. baumannii (28 isolates), E. coli (13 isolates), S. aureus (15 isolates), S. epidermidis (4 isolates), S. saprophyticus (5 isolates), K. pneumonia, and P. aeruginosa (13 isolates) were evaluated. The experiments were also done for four groups of standard antibiotics:
Beta-lactam: Amoxicillin, Ceftazidime, Cefixime, and Cefotaxime; Fluoroquinolone: Nalidixic acid and Norfloxacine; aminoglycoside: Tobramycin, Kanamycin, and Tetracycline.
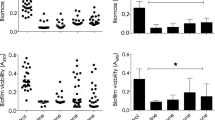
Table 2 shows the in vitro activities of CMIA in comparison with the reference agents against clinical and standard strains. These results are expressed as MBC values. Among the clinical isolates, CMIA showed potent activity against A. baumannii isolates. Among all A. baumannii cases, the MBCs of CMIA for bronchi and wound isolates were most potent (2.0–4.7 mg/l). The A. baumannii MBC values of the reference antibiotics were more than 70 mg/l except for Norfloxacine. CMIA inhibited the rest of gram-negative and gram-positive strains at or above 47.9 mg/l. The data of Table 1 shows that the higher concentration of CMIA is needed for effective growth inhibition of A. baumannii urinary isolates. Unlike the A. baumannii isolates, many other strains were completely resistant to CMIA at the doses tested.
Data from this study indicates that CMIA is a potent and selective antimicrobial agent against A. baumannii.
Conclusion
It is well documented that the 11-chloro-3-alkyl-3H-imidazo[4,5-a]acridines are notable bactericidal agents and their efficacies are decreased by descending the N-alkyl length. Here, it was also found that the CMIA is the most potent and selective bactericidal analog against clinical isolates of highly antibiotic-resistant A. baumannii. The observed bactericidal activity of CMIA represents a potentially attractive alternative for topical treatment of A. baumannii infections.
Materials and methods
Determination of MBCs
The MBCs were determined by dilution tube-test method, introduced by National Committee for Clinical Laboratory Standards (Finegold and Garrod, 1995). A serial dilution of tested compounds (final concentration of 200–0.4 mg/l), were added to the test bacteria in Mueller–Hinton broth and were incubated at 37°C for 24 h (5 × 105 CFU/ml). After sufficient incubation (24 h), the tubes were examined for turbidity, indicating growth of the microorganism. For further confidence, the samples were cultured in Petri dishes containing Muller–Hinton agar (24 h at 37°C). The lowest drug concentration that prevents the test organism growth (<99.9%) is introduced as MBC (Pachon-Ibanez et al., 2004). Growth was observed in medium control but not in the inoculum control (Finegold and Garrod, 1995).
Determination of Log D
One milligram of solute 5a–5c was independently deposited in a test tube. 5 ml of each saturated layer (octanol and aqueous phosphate buffer 50 mM, pH 7.4) was added to the sample, and the tube was capped and equilibrated for 6 h on a mechanical shaker. For each of the compounds, a blank solution was prepared. The test was repeated four times for each compound. The absorbance values of the equilibrated layers and the blanks were read at 402 nm, and the partition coefficient was calculated with the equation: Log D = Log [(A oct − A blank)/(A wat − A blank)].
Chemistry
Compounds 5a–5c were synthesized according to the procedure reported by Rahimizadeh et al. by starting from N-Alkyl-5-nitrobenzimidazoles and phenylacetonitrile (Rahimizadeh et al., 2009). The compounds 3a–3c were obtained via the nucleophilic substitution of hydrogen of N-alkyl-5-nitrobenzimidazoles 1a–1c with phenylacetonitriles in KOH/MeOH under reflux condition (4 h). Compounds 3a–3c rearranged to their corresponding imidazo[4,5-a]acridones 4a–4c in concentrated sulfuric acid containing nitrous acid after 24 h at room temperature. Treatment of imidazo[4,5-a]acridones 4a–4c in boiling POCl3 gave imidazo[4,5-a]acridines 5a–5c (Scheme 1). The structure of the new synthetic compound, 5c, was confirmed by 1H NMR spectroscopy and CHN analysis.
General procedure for the synthesis of 5a–5c
Compounds 1a–1c (5 mmol) and phenyl acetonitrile (6 mmol) were added with stirring to a solution of 10 g KOH in 40 ml methanol. After the mixture was refluxed with stirring for 4 h, it was then poured into water. The precipitate was collected by filtration, washed with water, and air-dried to give 3a–3c.
Sodium nitrite (2.5 g, 75 mmol) was slowly added with stirring to a solution of 3a–3f (3.5 mmol) in 50 ml sulfuric acid 98% at −10°C. After the addition was completed, the mixture was allowed to warm to room temperature and left at room temperature (24 h). After pouring the mixture into 300 ml ice-water, precipitated solid was separated, washed with water, and dried to give 4a–4c.
A mixture of 4a–4c (2 mmol) and 3 ml POCl3 was refluxed (3 h). After cooling to room temperature, the mixture was poured on to crushed ice and neutralized with NaOH (10%). The product was extracted with ethyl acetate (2 × 25 ml). The extract was dried and evaporated to give 5a–5c.
11-Chloro-3-methyl-3H-imidazo[4,5-a]acridine (5a)
Yellow crystals (acetonitrile), yield 75%, mp: 240–241°C; 1H NMR (500 MHz, CDCl3): δ = 4.02 (s, 3H), 7.61 (d, J = 8.9 Hz, 1H), 7.65–8.05 (m, 5H), 8.65 (dd, J = 9.0 Hz, J = 2.0 Hz, 1H) ppm; 13C NMR (125 MHz, CDCl3): δ = 157.9, 156.7, 150.3, 142.6, 134.6, 131.6, 131.3, 130.7, 127.4, 125.8, 122.0, 119.0, 111.7, 110.6, 33.2 ppm; MS (70 eV): m/z = 267 (M+); Anal. Calcd. for C15H10ClN3: C, 67.30; H, 3.77; N, 15.70. Found: C, 67.49; H, 3.69; N, 15.81.
11-Chloro-3-n-propyl-3H-imidazo[4,5-a]acridine (5b)
Yellow crystals (acetonitrile), yield 79%, mp 185–187°C; 1H NMR (500 MHz, CDCl3): δ = 1.01 (t, J = 7.2 Hz, 3H), 1.88–2.11 (m, 2H), 4.30 (t, J = 7.2 Hz, 2H), 7.68–8.32 (m, 6H), 8.67 (dd, J = 9.0 Hz, J = 2.0 Hz, 1H) ppm; 13C NMR (125 MHz, CDCl3): δ = 157.7, 156.6, 148.3, 142.3, 134.6, 131.7, 130.8, 130.3, 127.0, 125.6, 121.5, 118.8, 111.5, 110.5, 52.3, 21.6, 10.1 ppm; MS (70 eV): m/z = 295 (M+); Anal. Calcd. for C17H14ClN3: C, 69.03; H, 4.77; N, 14.21. Found: C, 69.39; H, 4.65; N, 14.17.
11-Chloro-3-n-pentyl-3H-imidazo[4,5-a]acridine (5c)
Yellow crystals (acetonitrile), yield 75%, mp: 175–176°C; 1H NMR (500 MHz, CDCl3) δ 0.98 (t, J = 6.8 Hz, 3H), 1.21–1.70 (m, 4H), 1.73–2.01 (m, 2H), 4.31 (t, J = 6.8 Hz, 2H), 7.63-8.31 (m, 6H), 8.66 (dd, J = 9.0 Hz, J = 2.0 Hz, 1H) ppm; 13C NMR (125 MHz, CDCl3): δ = 157.7, 156.5, 147.7, 141.9, 134.5, 131.6, 130.8, 130.3, 126.9, 125.6, 121.4, 118.7, 111.2, 110.1, 49.7, 30.8, 20.4, 20.1, 11.1 ppm; MS (70 eV): m/z = 321 (M+); Anal. Calcd. for C19H18ClN3: C, 70.47; H, 5.60; N, 12.98. Found: C, 70.35; H, 5.65; N, 12.91.
References
Abbo A, Navon-Venezia S, Hammer-Muntz O, Krichali T, Siegman-Igra Y, Carmeli Y (2005) Multidrug-resistant Acinetobacter baumannii. Emerg Infect Dis 11:22–29
Berthod A, Carda-broch S (2004) Determination of liquid–liquid partition coefficients by separation methods. J Chromatogr 1037:3–14
Finegold SM, Garrod L (1995) Bailey & Scott’s diagnostic microbiology, Chap. 13, 8th edn. C.V. Mosby Co., Toronto, pp 171–193
Hujer KM, Hujer AM, Hulten EA, Bajaksouzian S, Adams JM (2006) Analysis of antibiotic resistance genes in multidrug-resistant Acinetobacter sp. isolates from military and civilian patients treated at the Walter Reed Army Medical Center. Antimicrob Agents Chemother 50:4114–4123
Insa R, Cercenado E, Goyanes MJ, Morente A, Bouza E (2007) In vitro activity of tigecycline against clinical isolates of Acinetobacter baumannii and Stenotrophomonas maltophilia. J Antimicrob Chemother 59:583–585
Jain R, Danziger LH (2004) Multidrug-resistant Acinetobacter infections: an emerging challenge to clinicians. Ann Pharmacother 38:1449–1459
Osterburg A, Gardner J, Hyon SH, Neely A, Babcock G (2009) Highly antibiotic-resistant Acinetobacter baumannii clinical isolates are killed by the green tea polyphenol (−)-epigallocatechin-3-gallate (EGCG). Clin Microbiol Infect 215:341–346
Pachon-Ibanez ME, Jimenez-Mejias ME, Pichardo C (2004) Activity of tigecycline (GAR-936) against Acinetobacter baumannii strains, including those resistant to imipenem. Antimicrob Agents Chemother 48:4479–4481
Prescott LM, Harley JP, Klein DA (2002) Microbiology, Chap. 3, 5th edn. McGraw-Hill, New York, pp 809–810
Rahimizadeh M, Pordel M, Bakavoli M, Bakhtiarpoor Z, Orafaie A (2009) Synthesis of imidazo(4,5-a)acridones and imidazo(4,5-a)acridines as potential antibacterial agents. Monatsh Chem 140:633–638
Acknowledgments
We express our sincere gratitude to Mohammad Naser Shafiee (Faculty of Health Sciences, Mashhad University of Medical Sciences) for reviewing the manuscript. We thank Mr. Mohammad Ramezanpour and Mr. Mohammad Jafar Arjmand (Microbiology Laboratory, Ghaem Hospital) for their assistance in MIC determination. We are also grateful to Mashhad University of Medical Sciences for financial support of this work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sadeghian, A., Pordel, M., Safdari, H. et al. 11-Chloro-3-methyl-3H-imidazo[4,5-a]acridine (CMIA) as a potent and selective antimicrobial agent against clinical isolates of highly antibiotic-resistant Acinetobacter baumannii . Med Chem Res 21, 3897–3901 (2012). https://doi.org/10.1007/s00044-011-9933-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-011-9933-5