Abstract
Aluminum trichloride acts as readily available, inexpensive, and efficient catalyst for one-pot three-component condensation reaction of aldehydes, dicarbonyl, and 2-amino benzothiazole under the solvent-free conditions to afford the 4H-pyrimido[2,1-b][1,3]benzothiazole derivatives 4 with good yield. The compounds synthesized in this study were evaluated for their antibacterial activities against gram-positive and gram-negative bacteria, viz., Staphylococcus aureus, Pseudomonas aeruginosa, Salmonella typhi, Escherichia coli, Bacillus cereus, and Providencia rettegeri. Compounds 4c, 4d, 4f, 4g, and 4h showed their good activities against tested bacterial species. Pyrimidine derivatives 4d, 4f, and 4g have shown good antifungal activities against tested fungal strains, such as Aspergillus niger, Aspergillus fumigates, Aspergillus flavus, etc.
Graphical abstract

Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ability for designing new and synthesis of potent, selective, and less toxic anti-bacterial agents remains a major challenge for medicinal chemistry researchers. Pharmacologically, pyrimidines have attracted considerable interest because of their wide range of biological activities such as antihypertensive activities (Atwal et al., 1991 and Rovnyak et al., 1992), anti-tuberculosis (Khoshneviszadeh et al., 2009), antimicrobial (Chitra et al., 2010), antifilarial activities (Singh et al., 2008). Thiazoles are an important class of pharmaceutical compounds which exhibit wide spectra of biological activities (Vicini et al., 2003). Various substituted thiazoles have been synthesized and examined for their antifungal and antibacterial activities (Lanjewar et al., 2009; Bansal, 2003, and Miwatashi et al., 2008). Multicomponent reactions (MCRs) (Kappe, 2000, 2002; Zhu and Bienayme, 2005; Ramon and Yus, 2005, and Dallinger and Kappe, 2007) have attracted a large amount of attention of synthetic organic chemists. Simple procedure, high bond-forming efficiency, time and energy savings, and low expenditures are among the advantages of these reactions. The use of MCRs is the need of present century and is primarily driven by pharmaceutical industries.
Shaabani et al. have synthesized 4H-pyrimido[2,1-b]benzazoles using ionic liquid at 100°C in 5 h (Shaabani et al., 2005). Ionic liquids suffer from limitations because of poor solubility, toxicity to aquatic organism, as well as being unable to be separated by distillation (Matzke et al., 2007; Kralisch et al., 2005; Pham et al., 2010). The literature search shows that 2-amino benzothiazole containing pyrimidine synthesis exhibits poor yield and long reaction time, and hence, there is a need of rapid and higher yielding process. The present article reports a rapid, efficient, step with economic and green synthesis of biological active 4H-pyrimido[2,1-b]benzazoles derivatives by three-component reaction of ethyl acetoacetate, 2-amino benzothiazole, and aldehydes under solvent-free conditions, using AlCl3 as a catalyst target to green approach.
Materials and methods
Experimental
The 1H NMR spectra were measured using BRUKER AVANCE II 400 NMR spectrometer with tetramethylsilane as an internal standard at 20–25°C; data for 1H NMR are reported as follows: chemical shift (ppm), integration, multiplicity (s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet, and br, broad), coupling constant (Hz). IR spectra were recorded by SHIMADZU; IR spectrometer of sample dispersed in KBr pellet or Nujol is reported in terms of frequency of absorption (cm−1). E-Merck pre-coated TLC plates, RANKEM silica gel G for preparative thin-layer chromatography were used. Melting points were determined in open capillaries and are uncorrected. Brain heart infusionTM, Mueller–Hinton agar, Sabouraud dextrose agar, and Sabouraud dextrose broth were purchased from OXOID LTD., Basingstoke, Hampshire, England. 96 Microwell with lid was purchased from IWAKI Brand SCITECH DIV. Asahi Techno Glass, Japan. Ethyl acetoacetate, aldehydes, and aluminum trichloride were purchased from Himedia Laboratory Ltd., Mumbai, India. 2-amino benzothiazole was purchased from Sigma Aldrich.
One-pot three-component reaction
Typical procedure A mixture of aldehydes (0.005 mol), ethyl acetoacetate (0.005 mol), and 2-amino benzothiazole (0.005 mol) were heated at 60–70°C under solvent-free conditions using aluminum chloride as a catalyst (10 mol%). The reaction was monitored by TLC. After completion of the reaction, the reaction mixture was cooled to room temperature, and the content was transferred into water with the help of methanol and filtered to obtain the solid mass. The crude product was purified by column chromatography with chloroform and methanol (1:1). Pure compounds 4 were collected as pale yellow colored solid.
Ethyl-2-methyl-4-(phenyl)-4H-pyrimido[2,1-b][1,3]benzothiazole-3-carboxylate (4a)
Reaction was carried out according to typical procedure with benzaldehyde (0.005 mol), ethyl acetoacetate (0.005 mol), and 2-amino benzothiazole (0.005 mol) to give compound 4a. Pale-yellow crystals, mp 178–180°C, R f = 0.47 (DCM:Toluene; 3:2); IR (KBr) (υmax, cm−1): 3043 (C–Hstr), 2968 (C–Hstr in CH2CH3), 1670 (C=Ostr), 1589 (C=Nstr), 1462 (C=Cstr), 744 (C–Hdef); 1H NMR (400 MHz, CDCl3): δH 1.27 (3H, t, J HH = 14.24 Hz, CH2CH3), 2.46 (3H, s, CH3), 4.11–4.21 (2H, m, CH2CH3), 6.39 (1H, s, –CH), 7.07–7.43 (9H, m, ArH); 13C NMR (100 MHz, DMSO): 165.44, 162.59, 154.04, 141.26, 137.43, 128.26, 126.79, 122.22, 111.65, 102.56, 59.35, 56.82, 23.17, 13.99; ESI–MS: m/z Calculated for C20H18N2O2S 350.44, Found [M + H]+ 351.2.
Ethyl-2-methyl-4-(4-hydroxy-3-methoxy phenyl)-4H-pyrimido[2,1-b][1,3]benzothiazole-3-carboxylate (4b)
Reaction was carried out according to typical procedure with vanillin (0.005), ethyl acetoacetate (0.005), and 2-amino benzothiazole (0.005) to give compound 4b. Pale-yellow powder, mp 192–194°C, R f = 0.53 (DCM:Toluene; 3:2); IR (KBr) (υmax, cm−1): 3059 (C–Hstr), 2983 (C–Hstr in CH2CH3), 1703 (C=Ostr), 1597 (C=Nstr), 1504 (C=Cstr), 740 (C–Hdef); 1H NMR (400 MHz, CDCl3): δH 1.29 (3H, t, J HH = 14.24 Hz, CH2CH3), 2.47 (3H, s, CH3), 3.8 (3H, s, Ar–OCH3), 4.13–4.22 (2H, m, CH2CH3), 6.36 (1H, s, –CH), 6.78 (1H, d, J HH = 5.6 Hz, ArH), 6.89–6.92 (2H, m, ArH), 7.13–7.59 (4H, m, ArH), 9.82 (1H, s, OH); 13C NMR (100 MHz, DMSO): 165.60, 162.34, 153.51, 147.12, 146.42, 137.61, 132.52, 126.37, 122.21, 115.18, 111.92, 110.88, 59.28, 55.43, 23.09, 14.08; ESI–MS: m/z Calculated for C21H20N2O4S 396.49, Found [M + H]+ 397.2.
Ethyl-2-methyl-4-(4-dimethylamino phenyl)-4H-pyrimido[2,1-b][1,3]benzothiazole-3-carboxylate (4c)
Reaction was carried out according to typical procedure with p-dimethylamino benzaldehyde (0.005 mol), ethyl acetoacetate (0.005 mol), and 2-amino benzothiazole (0.005 mol) to give compound 4c. Pale-yellow powder, mp 175–178°C, R f = 0.57 (DCM:Toluene; 3:2); IR (KBr) (υmax, cm−1): 3059 (C–Hstr), 2897 (C–Hstr in CH2CH3), 1612 (C=Ostr), 1581 (C=Nstr), 1431 (C=Cstr), 815–754 (C–Hdef); 1H NMR (400 MHz, CDCl3): δH 1.29 (3H, t, J HH = 14.14 Hz, CH2CH3), 2.46 (3H, s, CH3), 3.06 (6H, s, N(CH3)2), 4.12–422 (2H, m, CH2CH3), 6.66–7.92 (8H, m, ArH); 13C NMR (100 MHz, DMSO): 189.35, 166.39, 165.44, 153.95, 152.63, 133.43, 127.71, 125.91, 124.45, 121.63, 120.57, 111.53, 111.29, 110.74, 23.13, 13.60; ESI–MS: m/z Calculated for C22H23N3O2S 393.54, Found [M + H]+ 394.2.
Ethyl-2-methyl-4-(4-nitro phenyl)-4H-pyrimido[2,1-b][1,3]benzothiazole-3-carboxylate (4d)
Reaction was carried out according to typical procedure with p-nitro benzaldehyde (0.005 mol), ethyl acetoacetate (0.005 mol), and 2-amino benzothiazole (0.005 mol) to give compound 4d. Yellow powder, mp 170–172°C, R f = 0.52 (DCM:Toluene; 3:2); IR (KBr) (υmax, cm−1): 3348 (C–Hstr), 2933 (C–Hstr in CH2CH3), 1625 (C=Ostr), 1510 (C=Nstr), 1267 (C=Cstr), 962–812 (C–Hdef); 1H NMR (400 MHz, CDCl3): δH 1.31 (3H, s, CH2CH3), 2.46 (3H, s, CH3), 4.18–4.21 (2H, m, CH2CH3), 6.52 (1H, s, –CH), 7.03–8.12 (8H, m, ArH); 13C NMR (100 MHz, CDCl3): 166.25, 163.56, 155.83, 147.95, 147.62, 137.44, 128.06, 126.88, 124.43, 124.01, 123.73, 122.47, 111.36, 102.01, 60.42, 57.03, 23.97, 14.40; ESI–MS: m/z Calculated for C20H17N3O4S 395.46, Found [M + H]+ 396.4.
Ethyl-2-methyl-4-(2-hydroxy phenyl)-4H-pyrimido[2,1-b][1,3]benzothiazole-3-carboxylate (4e)
Reaction was carried out according to typical procedure with salicylaldehyde (0.005 mol), ethyl acetoacetate (0.005 mol), and 2-amino benzothiazole (0.005 mol) to give compound 4e. Pale-yellow powder, mp 212–215°C, R f = 0.53 (DCM:Toluene; 3:2); IR (KBr) (υmax, cm−1): 3028 (C–Hstr), 2810 (C–Hstr in CH2CH3), 1668 (C=Ostr), 1575 (C=Nstr), 1485 (C=Cstr), 837–750 (C–Hdef); 1H NMR (400 MHz, DMSO): δH 1.28 (3H, t, J HH = 14.16, CH2CH3), 2.45 (3H, s, CH3), 4.10–4.19 (2H, m, CH2CH3), 6.33 (1H, s, –CH), 6.66–7.92 (8H, m, ArH), 9.89 (1H, s, OH); 13C NMR (100 MHz, DMSO): 190.23, 171.88, 165.92, 162.99, 157.23, 151.33, 133.73, 128.14, 126.08, 122.92, 116.07, 155.70, 111.03, 59.26, 56.23, 23.11, 14.04; ESI–MS: m/z Calculated for C20H18N2O3S 367.34, Found [M]+ 367.2.
Ethyl-2-methyl-4-(4-hydroxy phenyl)-4H-pyrimido[2,1-b][1,3]benzothiazole-3-carboxylate (4f)
Reaction was carried out according to typical procedure with p-hydroxy benzaldehyde (0.005 mol), ethyl acetoacetate (0.005 mol), and 2-amino benzothiazole (0.005 mol) to give compound 4f. Pale-yellow powder, mp 210–212°C, R f = 0.59 (DCM:Toluene; 3:2); IR (KBr) (υmax, cm−1): 3288 (OHstr), 3059 (C–Hstr), 2897 (C–Hstr in CH2CH3), 1612 (C=Ostr), 1581 (C=Nstr), 1431 and 1377 (C=Cstr), 815–754 (C–Hdef); 1H NMR (400 MHz, CDCl3): δH 1.25 (3H, t, CH3CH2, J HH = 14.16 Hz), 2.35 (3H, s, CH3), 4.04–4.14 (m, 2H, CH3CH2), 6.30 (1H, s, –CH), 6.66 (2H, d, ArH, J HH = 8.40 Hz), 7.13–7.29 (5H, m, ArH), 7.59 (1H, d, ArH, J HH = 7.76 Hz), 9.26 (1H, s, OH); 13C NMR (100 MHz, DMSO): 165.55, 162.27, 157.22, 153.46, 137.56, 131.95, 128.13, 126.34, 123.57, 122.94, 122.20, 115.02, 111.80, 102.96, 59.27, 56.37, 23.09, 14.03; ESI–MS: m/z Calculated for C20H18N2O3S 367.34, Found [M]+ 367.2.
Ethyl-2-methyl-4-(4-methoxy phenyl)-4H-pyrimido[2,1-b][1,3]benzothiazole-3-carboxylate (4g)
Reaction was carried out according to typical procedure with anisaldehyde (0.005 mol), ethyl acetoacetate (0.005 mol), and 2-amino benzothiazole (0.005 mol) to give compound 4g. Pale-yellow powder, mp 130–132°C, R f = 0.53 (DCM:Toluene; 3:2); IR (KBr) (υmax, cm−1): 2941 (C–Hstr in CH2CH3), 1627 (C=Ostr), 1508 (C=Nstr), 1280 (C=Cstr), 962–813 (C–Hdef); 1H NMR (400 MHz, CDCl3): δH 1.28 (3H, t, CH3CH2, J HH = 14.24 Hz), 2.45 (3H, s, CH3), 3.71 (3H, s, Ar–OCH3), 4.11–4.21 (2H, m, CH3CH2), 6.34 (1H, s, –CH), 6.78 (2H, d, ArH, J HH = 8.64 Hz), 7.21–7.58 (6H, m, ArH); 13C NMR (100 MHz, DMSO): 166.67, 163.27, 159.42, 154.51, 152.07, 138.06, 133.81, 128.51, 126.57, 123.88, 123.82, 122.14, 120.89, 119.04, 113.90, 111.80, 103.24, 60.09, 57.20, 55.18, 23.73, 14.40; ESI–MS: m/z Calculated for C21H20N2O2S 380.47, Found [M + H]+ 381.3.
Ethyl-2-methyl-4-(2, 6-dichloro phenyl)-4H-pyrimido[2,1-b][1,3]benzothiazole-3-carboxylate (4h)
Reaction was carried out according to typical procedure with 2,6-dichloro benzaldehyde (0.005 mol), ethyl acetoacetate (0.005 mol), and 2-amino benzothiazole (0.005 mol) to give compound 4h. Pale-yellow powder, mp 150–152°C, R f = 0.56 (DCM:Toluene; 3:2); IR (KBr) (υmax, cm−1): 3012 (C–Hstr), 2922 (C–Hstr in CH2CH3), 1625 (C=Ostr), 1500 (C=Nstr), 1278 (C=Cstr), 960–812 (C–Hdef); 1H NMR (400 MHz, DMSO): δH 1.09 (3H, t, J HH = 14.16 Hz, CH2CH3), 2.29 (3H, s, CH3), 4.01–4.05 (2H, m, CH2CH3), 7.01–7.66 (7H, m, Ar–H); 13C NMR (100 MHz, DMSO): 171.88, 165.92, 162.99, 151.33, 137.57, 133.73, 132.41. 129.14, 126.08, 125.57, 124.52, 122.97, 121.69, 117.66, 116.07, 59.26, 23.11, 14.04; ESI–MS: m/z Calculated for C20H16N2Cl2O2S 419.41, Found [M]+ 419.6.
Antibacterial assay
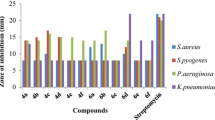
Different concentrations 50, 25, 12.5, 6.25, 3.12, 1.56, and 0.78 μg/ml of the compounds (4a–4h) were prepared in sterile microwell to determine minimum inhibitory concentration (MIC). Nutrient broth was adjusted to pH 7.0 used for the determination of MIC. The inoculum of the test microorganisms was prepared by using 16-h-old cultures adjusted by reference to the 0.5 McFarland standards (1.5 × 108 CFU/ml). Brain heart infusion broth was prepared; 150 μl of it was taken in each well and 10 μl of each compound was added in broth with different concentrations; then 10 μl of bacterial culture broth was added. The plate was shaken to uniformly mix the inoculum with the broth. Optical density was taken by photo spectrometer (μQuant, Biotek Ltd. USA), and then incubated for 24 h at 37°C. Appearance of any turbidity shows that the compound is not able to inhibit the growth of the bacteria, while no turbidity indicates the inhibition of microorganism by the sample (Table 3).
In vitro antifungal test
For antifungal testing, the pyrimidine derivatives and fluconazole (standard drug) solution was prepared in DMSO to make effective concentrations of 50, 25, 12.5, 6.25, 3.12, 1.56, and 0.78 μg/ml. Isolated fungal species Aspergillus niger, Aspergillus fumigates, and Aspergillus flavus were selected. Sabouraud dextrose agar was prepared; 150 μl of it was taken in each well; 10 μl of each compound was added in broth with different concentrations; and then 10 μl of fungi culture broth was added. The plate was shaken to uniformly mix the inoculum with the broth and note the optical density. The well was incubated for 72 h at 28°C. Appearance of any turbidity shows that the compound is not able to inhibit the growth of the bacteria, while no turbidity indicates the inhibition of microorganism by the sample. To ensure that solvent had no effect on fungal growth, a control test was performed with test medium supplemented with DMSO at the same dilutions as used in the experiment. The results are incorporated in Table 4.
To determine zone of inhibition, sterilized filter disks were dipped in these solutions and subsequently dried to remove DMSO. Sabouraud dextrose agar was prepared and allowed to solidify. One of these disks was kept free from antifungal drug (fluconazole) and served as growth control. Fungi selected were viz., A. niger, A. fumigates, A. flavus, and 1 ml of each fungus culture was added in the Sabouraud dextrose agar plates and spread with the help of sterile spreader. The filter paper disks soaked in the above mentioned dilutions of fluconazole and pyrimidine derivatives were placed aseptically over the inoculated plates using sterile forceps. The plates were incubated at 28°C for 72 h, in upright position. The zone of inhibition was measured using scale (Table 4).
Results and discussion
The present methodology involves the synthesis of 4H-pyrimido[2,1-b]benzazole ring system using aldehydes 1 (0.005 mol) and ethyl acetoacetate 2 (0.005 mol) in the presence of 2-amino benzothiazole 3 (0.005 mol) using aluminum chloride as a catalyst under solvent-free conditions (Scheme 1). Reaction was completed within 70–120 min with good yield. The structures of 4H-pyrimido[2,1-b]benzazole derivatives were characterized by mp, IR, 1H NMR, 13C NMR, and mass spectral studies. IR spectra of all pyrimidine derivatives showed peaks between 740 and 754 cm−1 due to C–Hdef, peak around 1,500 cm−1 (C=Nben), band between 1,431 and 1,504 (C=Cstr in ring), strong band between 1,600 and 1,700 cm−1 (C=O), band between 1,240 and 1,274 (C–Hben) weak band between 2,800 and 3,000 cm−1 (C–Hstr of benzene ring). 1H NMR spectra of compound 4a showed triplet at δ 1.29 (OCH2CH3), singlet at δ 2.47 (CH3), multiplet at δ 4.17 (OCH2CH3), singlet at δ 6.39 (C–H), multiplet at δ 7.07–7.43 (benzene rings); compound 4b showed singlet at δ 3.8 for methoxy group; 4c showed singlet at δ 3.06 for p-dimethylamino group; and 4f showed singlet at δ 8.9 for hydroxyl group. Mass spectra of all compound showed corresponding molecular ion peaks in mass spectra.
To optimize the reaction conditions and catalyst loading, a model reaction of benzaldehyde (0.005 mol), 2-amino benzothiazole (0.005 mol), and ethyl acetoacetate (0.005 mol) was carried out. The results show that in the absence of catalyst, the reaction took a long time with poor yield. The highest yield (79%) of 4H-pyrimido[2,1-b][1,3]benzazole was obtained when 10 mol% of aluminum trichloride was used (Fig. 1). The reaction was extended to perform the evaluation of various catalysts (Table 1). Out of the various catalyst evaluations, AlCl3 showed the highest yield with the shortest reaction time. Copper chloride and lithium chloride gave moderate yield. Nickel nitrate, cerium chloride, zinc chloride, acetic acid, and silver nitrate gave slightly lower yields compared with other catalysts. The study was further extended to other substrate materials having electron-donating and electron-withdrawing groups. The electronic effects due to substituent do not show any significant deviation. The highest yield was obtained with substituent –OH. Para-substituted aldehydes gave better yield as compared with the ortho- and meta-substituted aldehydes (Table 2); it may be due to the steric hindrance. The results exhibit that this reaction is better for those reactions carried out using solvents and ionic liquids.
Antibacterial activity
All the synthesized compounds were screened for their antibacterial activities based on micro dilution broth susceptibility test method. The stock solution of pyrimidine derivatives (4a–4h) was prepared in DMSO. The stock solution (μg/ml) of each of these pyrimidines was serially diluted and added to Brain Heart Infusion broth, after which a standardized bacterial suspension was added. The lowest concentration of pyrimidine derivatives in μg/ml that prevented in vitro growth of microorganism has been represented as MIC (minimum inhibitory concentration) shown in Table 3. Susceptibility test in vitro was done on multiresistant bacteria Staphylococcus aureus (ATCC 11632), Pseudomonas aeruginosa (ATCC 15499), Salmonella typhi (ATCC 23564), Escherichia coli (ATCC 35218), Bacillus cereus (MTCC 7350), and Providencia rettegeri isolated from infected fish. The results have been tabulated in Table 3. Each was performed in triplicate, and the MICs reported represent the best of at least two repetitions. Compounds 4c, 4d, 4f, 4g, and 4h showed very promising activities on multiresistant microorganism. The compound 4g having methoxy substitution at C-4 aryl ring has shown better activity against all the tested bacterial strain (6.25 μg/ml) except S. typhi. Substitution at para position as hydroxyl group (4f) shows good activity against E. coli (6.25 μg/ml) and B. cereus (6.25 μg/ml). Introduction of the chloro group at ortho position of C-2 aryl ring (4f) also has shown potential activity against E. coli and B. cereus (6.25 μg/ml). Surprisingly, nitro group introduction at C-4 aryl ring shows potential activity against S. typhi and B. cereus. Results show that all 4H-pyrimido[2,1-b][1,3]benzothiazole derivatives exhibited good activity against B. cereus (Table 3). Most encouraging result was found against P. aeruginosa, E. coli, B. cereus, and P. rettegeri. MIC was compared with one of the best marketed antibiotic, viz., ciprofloxacin. p-dimethylamino benzaldehyde and p-nitro benzaldehyde have shown good result. Derivatives 4f, 4g, and 4f have shown encouraging results against all the tested bacteria strain. Pyrimidine derivatives have shown encouraging results against B. cereus. The literature survey suggests that pyrimidine derivatives, synthesized by three-component reaction of aldehydes, ethyl acetoacetate, and 2-amino benzothiazole, have not been evaluated so far for their antibacterial activity.
Antifungal activity
The literature survey, however, suggests that these types of pyrimidine derivatives have not been evaluated for their antifungal activity so far. We have now evaluated the antifungal activity of pyrimidine derivatives against fungi, viz., A. niger, A. fumigates, and A. flavus. From the results shown in Table 4, we can observe that pyrimidine derivatives 4a (phenyl), 4b (4-hydroxy-3-methoxy), and 4e (2-hydroxy) show moderate antifungal activities against A. fumigates, A. niger, and A. flavus, respectively. Analogues 4d (4-nitro) and 4f (4-hydroxy) derivatives show good activities A. niger and A. fumigates; derivatives 4c and 4h do not show any activity against tested fungal strain. 4g (4-methoxy) derivative of pyrimidine has shown excellent antifungal activity against all the tested fungal strains. The antifungal activity of pyrimidine derivatives was compared with the standard antifungal drugs fluconazole. It was found that p-methoxy derivative (4g) exhibits excellent activity against all the tested fungal strains (Table 4). This has thrown open a new era for exploring suitably designed, new scaffold in molecules as potential antifungal/antibacterial drugs.
Cytotoxicity against L123 (human lung cells)
Cytotoxicity was performed by MTT assay method (Mosman, 1983 and Heilmann et al., 2001). A 96-well flat bottomed tissue culture plate was seeded with 2 × 103 cells in 0.1 ml of MEM medium supplemented with 10% FBS and allowed to attach for 24 h. After 24 h of incubation, cells were treated with test compounds to get a concentration of 5, 10, 20, 50, and 100 μg/ml after being incubated for 48 h. The cells in the control group received only the medium containing the 0.2% DMSO. Each treatment was performed in duplication. After the treatment, drug containing media was removed and washed with 200 μl of PBS. To each well of the 96 well plate, 100 μl of MTT reagent (Stock: 1 mg/ml in serum-free medium) was added and incubated for 4 h at 37°C. After 4 h of incubation, the plate was inverted on tissue paper to remove the MTT reagent. To solubilize formazan crystals in the wells, 100 μl of 100% DMSO was added to each well. The optical density was measured by microtiter plate reader at 590 nm. The compound concentrations (μg) required to reduce the viability of mock-infected cells by 50% as determined by MTT method are summarized in Table 4.
Conclusion
In conclusion, the present method employing AlCl3 is an efficient, one-pot procedure for preparation of 4H-pyrimido[2,1-b]benzazole derivatives in good yield. The assumed structure of pyrimidines was confirmed by the IR, 1H NMR, 13C NMR, and mass spectra interpretation. The antibacterial activity study revealed that all the tested compounds showed good to moderate antibacterial activities against P. aeruginosa, E. coli, B. cereus, and P. rettegeri bacteria strain. Compounds 4c, 4d, 4f, 4g, and 4h have shown good activity against tested bacterial strain. Pyrimidine derivatives 4a (phenyl), 4b (4-hydroxy-3-methoxy), and 4e (2-hydroxy) show the moderately antifungal activities against A. fumigates, A. niger, and A. flavus, respectively. 4d (4-nitro) and 4f (4-hydroxy) derivative show good activity against A. niger and A. fumigates. Compound 4g has shown excellent antifungal activity against all the tested fungal strains. The field is further open for study of these compounds with respect to toxicity, chronic toxicity, pharmacokinetics, and clinical studies to establish these molecules as drugs in the market. The operational simplicity, and easy availability of starting material make it a rather better advanced alternative procedure than traditional multi-step methods. Green approach of aluminum chloride makes it superior over organic acids and hazardous metal-salt catalysts. The main advantages of this methodology are the short reaction times, simple catalyst system, higher yields, organic solvent-free reactions, and ease of operational procedure.
References
Atwal KS, Swanson BN, Unger SE, Floyd DM, Moreland S, Hedberg A, O’reilly BC (1991) Dihydropyrimidine calcium channel blockers. 3. 3-carbamoyl-4-aryl-1, 2, 3,4-tetrahydro-6-methyl-5-pyrimidinecarboxylic acid eaters as orally effective antihypertensive agents. J Med Chem 34(2):806–811
Bansal RK (2003) Heterocyclic chemistry, vol 3. New Age International Publishers, New Delhi, p 425
Chitra S, Devanathan D, Pandiarajan K (2010) Synthesis and in vitro microbiological evaluation of novel 4-aryl-5-isopropoxycarbonyl-6-methyl-3,4-dihydropyrimidinones. Eur J Med Chem 45:367–371
Dallinger D, Kappe CO (2007) Microwave-assisted synthesis in water as solvent. Chem Rev 107:2563–2591
Heilmann J, Wasescha MR, Schmidt TJ (2001) The influence of glutathione and cysteine levels on the cytotoxicity of helenanolide type sesquiterpene lactones against KB cells. Bioorg Med Chem 9:2189–2194
Kappe CO (2000) Recent advances in the Biginelli dihydropyrimidine synthesis. New tricks from an old dog. Acc Chem Res 33:879–888
Kappe CO (2002) High-speed combinatorial synthetics utilizing microwave irradiation. Curr Opin Chem Biol 6:314–320
Khoshneviszadeh M, Edraki N, Javidnia K, Alborzi A, Pourabbas B, Mardaneh J, Miri R (2009) Synthesis and biological evaluation of some new 1,4-dihydropyrimidines containing different ester substitute and diethyl carbamoyl group as anti-tuberculosis agents. Bioorg Med Chem 17:1579–1586
Kralisch D, Stark A, Korsten S, Kreisel G, Onodruschka B (2005) Energetic, environmental and economic balances: spice up your ionic liquid research efficiency. Green Chem 7:301–309
Lanjewar KR, Rahatgaonkar AM, Chorghade MS, Saraf BD (2009) Synthesis and antimicrobial activity of 5-(2-aminothiazol-4-yl)-3,4-dihydro-4-phenylpyrimidine-2(1H)-one. Ind J Chem 48B:1732–1737
Matzke M, Stolte S, Thiele K, Juffernholz T, Arning J, Ranke J, Welzbiermann U, Jastorff B (2007) The influence of anion species on the toxicity of 1-alkyl-3-methylimidazolium ionic liquids observed in an (eco) toxicological test battery. Green Chem 9:1198–1207
Miwatashi S, Arikawa Y, Matsumoto T, Uga K, Kanzaki N, Imai YN, Ohkawa S (2008) Synthesis and biological activities of 4-phenyl-5-pyridyl-1, 3-thiazole derivatives as selective adenosine A3 antagonists. Chem Pharm Bull (Tokyo) 56(8):1126–1137
Mosman T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Pham T, Cho C, Yun Y (2010) Environmental fate and toxicity of ionic liquids: a review. Water Res 44:352–372
Ramon DJ, Yus M (2005) Asymmetric multicomponent reaction (AMCRs): the new frontier. Angew Chem Int Ed 44(11):1602–1634
Rovnyak GC, Atwal KS, Hedberg A, Kimball SD, Moreland S, Gougoutas JZ, O’Reillly BC, Schwartz J, Malley MF (1992) Dihydropyrimidine calcium channel blockers. 4. Basic 3-substituted-4-aryl-1,4-dihydropyrimidine-5-carboxylic acid esters. Potent antihypertensive agents. J Med Chem 35(17):3254–3263
Shaabani A, Rahmati A, Naderi S (2005) A novel one-pot three-component reaction: synthesis of triheterocyclic 4H-pyrimido[2, 1-b]benzazoles ring systems. Bioorg Med Chem Lett 15:5553–5557
Singh BK, Mishra M, Saxena N, Yadav GP, Maulik PR, Sahoo MK, Gaur RL, Murthy PK, Tripathi RP (2008) Synthesis of 2-sulfanyl-6-methyl-1,4-dihydropyrimidines as a new class of antifilarial agents. Eur J Med Chem 43:2717–2723
Vicini P, Garonikaki A, Incerti M, Busonera B, Poni G, Cabras CA, La Colla P (2003) Synthesis and biological evaluation of benzo[d]isothiazole, benzothiazole and thiazole Schiff bases. Bioorg Med Chem 11:4785–4789
Zhu J, Bienayme HE (2005) Multicomponent reactions. Wiley-VCH, Wienheim
Acknowledgements
We gratefully acknowledge to Mr. Yogesh Sharma, Parabolic drugs Ltd., Chandigarh and SAIF Punjab University, Chandigarh for spectral analytical data.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sahu, P.K., Sahu, P.K., Lal, J. et al. A facile green synthesis and in vitro antimicrobial activity 4H-pyrimido[2,1-b][1,3]benzothiazole derivatives using aluminum trichloride under solvent free conditions. Med Chem Res 21, 3826–3834 (2012). https://doi.org/10.1007/s00044-011-9908-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-011-9908-6