Abstract
A new class of 2-azetidinyl-4-quinazolinones 6a–k was synthesized by multi-step process, starting from 2-[2-(2,6-dichlorophenyl)amino]phenyl acetic acid 1. Acid 1 was easily converted to acid chloride 2, which on cyclization reaction with 5-bromo anthranilic acid yielded benzoxazinone 3. The condensation reaction of 3 with benzene-1,4-diamine afforded 4-quinazolinone 4. Finally the title compound 6a–k was synthesized from 4-quinazolinone 4 by Schiff base formation 5a–k with aromatic aldehyde and then cyclization reaction with chloroacetylchloride. The in vitro antimicrobial activity of compounds 5a–k and 6a–k were tested. These compounds showed pronounced antimicrobial activity when 4-Cl and 4-OCH 3 groups were present.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The synthetic studies of 4-quinazolinone derivatives have been presented due to the chemical and biological interest. 4-Quinazolinone derivatives possesses antibacterial, antifungal (Grover and Kini, 2006), hypolipidemic (Kurogi et al., 1996), antitumor (Baek et al., 2004), anti-inflammatory (Kumar et al., 2007), antimalarial (Jiang et al., 2005), CNS depressant, anticonvulsant (Jatav et al., 2008), analgesic (Alagarsamy et al., 2007), antitubercular (Mosaad et al., 2004) and antiviral (Saleh et al., 2002) activities. Schiff base has good antibacterial (Parekh et al., 2005), antifungal (Gursoy et al., 2005; Mishra et al., 2005), antitubercular (Joshi et al., 2008), antioxidant (Yuksek et al., 2006), antitumor (Ren et al., 2002) and pharmacological applications. β-Lactam (4-membered cyclic amide) ring structure constitutes the dominant class of agents currently employed for the chemotherapy of bacterial infections. Various 2-azetidinones have been reported to possess antibacterial (Halve et al., 2006; Suryavanshi and Pai, 2006), antifungal (Havaldar and Khatri, 2006; Panwar et al., 2006), anti-inflammatory (Gurupadayya et al., 2008), anthelminthic (Srivastava et al., 2004), antiviral (Pandey et al., 2005) and anticonvulsant (Rajasekaran and Murugesan, 2005) activities.
Diclofenac is available as generic drug in a number of formulations. Over the counter use is approved in some countries for minor aches and pains and fever associated with common infections. In the United Kingdom, India and the United States, it may be supplied as either the sodium or potassium salt, in China most often as the sodium salt, while in some other countries only as the potassium salt. Diclofenac is often used to treat chronic pain associated with cancer, particularly if inflammation is also present. Diclofenac has been found to be effective against all strains of multi-drug resistant Escherichia coli. Therefore, it may be suggested that diclofenac has the capacity to treat UTI (uncomplicated urinary tract infections) caused by E. coli (Mazumdar et al., 2006). The diclofenac analogue compounds displayed antibacterial (Dutta et al., 2000), antimycobacterial (Sriram et al., 2006), anti-inflammatory, analgesic, ulcerogenic, lipid peroxidation (Amir and Shikha, 2004; Bhandari et al., 2008), antitumor (Barbaric et al., 2007) and transthyretin amyloid fibril formation inhibitor (Oza et al., 2002) activities.
We have synthesized 2,3-disubstituted 4-quinazolinone derivatives in which C-2 position was occupied by 2-[(2,6-dichlorophenyl)amino]benzyl unit from lead molecule diclofenac and 3rd position was replaced with various substituted aryls (Patel and Lilakar, 2001), aryl acetamides (Patel and Chaudhari, 2006), 4-thiazolidinones (Patel and Patel, 2007a, b), 2-azetidinones (Patel and Patel, 2008) and aryl sulfonamides (Patel and Chaudhari, 2008). We observed that these synthesized compounds showed very good antimicrobial activity and henceforth we enhanced this work with diclofenac analogue of 4-quinazolinone 5a–k and 6a–k. The synthetic route is shown in Scheme 1. The structure of 5a–k and 6a–k was firmly established by elemental analyses, IR and NMR spectral data. Preliminary microbiological test showed that most of the compounds possessed good antimicrobial activity in vitro.
Results and discussion
Chemistry
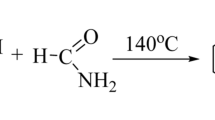
We have synthesized a series of heterocyclic compounds, 2-[2-(2,6-dichlorophenyl) amino]benzyl-3-[4-(2-substitutedarylidene)aryl]-6-bromoquinazolin-4(3H)ones 5a–k and 2-[2-(2,6-dichlorophenyl)amino]benzyl-3-{4-[4-(2-substitutedphenyl)-3-chloro-2-oxo-azetidinyl]aryl}-6-bromoquinazolin-4(3H)one 6a–k as illustrated in Scheme 1. Structures of all the compounds were established on the basis of elemental analyses, IR, 1H NMR and 13C NMR spectral data. The required benzoxazinone 3 was prepared by the cyclization between 5-bromoanthranilic acid and 2-[(2,6-dichlorophenyl)amino]phenyl acetyl chloride 2 using pyridine (Gao et al., 2007). Formation of the product was confirmed by a sharp band at 1695 cm−1 for C=O group along with a band at 1140 cm−1 for C–O–C stretching in IR spectrum. Compound 3 was converted to quinazolin-4(3H)one 4, by its condensation with benzene-1,4-diamine (Laddha et al., 2006). Insertion of the nitrogen atom of benzene-1,4-diamine in benzoxazinone ring was confirmed by the disappearance of C–O–C stretching band at 1140 cm−1 and also gave a strong C=O stretching vibration of quinazolinone at 1685 cm−1 instead in gave a band at 1695 cm−1 of benzoxazinone. Further confirmed by 13C NMR spectrum, which showed C=O and C=N signals of quinazolinone at δ 160.4 ppm and δ 164.5 ppm, respectively. When compound 4 was treated with substituted aromatic aldehydes in the presence of glacial acetic acid as a catalyst, Schiff bases 5a–k were formed (Archana et al., 2002), which were confirmed by the presence of strong –N=CH– stretching vibration of Schiff bases at around 1632 cm−1 and 1H NMR spectra showed singlet at around δ 6 ppm due to one proton of Schiff base. Further cyclization reaction of Schiff bases 5a–k with chloroacetylchloride in presence of triethylamine as a catalyst at 0–5 °C gave the desired compounds azetidinyl-quinazolin-4(3H)ones 6a–k (Halve et al., 2006). IR spectra of compounds 6a–k showed strong stretching vibration at around 1750 cm−1 due to C=O group of β-lactam ring. 1H NMR spectra of 6a–k showed doublet at around δ 3.35 ppm and δ 3.25 ppm equivalent to one proton due to >CH–Ar and >CH–Cl of β-lactam ring respectively.
Antimicrobial activity
The results of preliminary antibacterial testing of the compounds 5a–k and 6a–k are shown in Tables 1 and 2. All these compounds were compared with control drug penicillin-G. It can be seen from the calculated potency that the Schiff base derivatives showed good antibacterial activity but when they were converted to 2-azetidinone derivatives, the results found different depending upon the substituents. Schiff bases 5e, 5f, 5g, 5h and 5i possessed moderate activities (50–53.85%) against Staphylococcus aureus while 2-azetidinone derivatives 6e, 6f, 6g, 6h and 6i displayed very good activities (58.33–66.67%) against S. aureus. On the other hand, Schiff bases 5f and 5g were found very active (59.79 and 63.91%, respectively) while 2-azetidinone derivatives 6f and 6g showed decreased activities (57.44 and 57.14%, respectively) against Bacillus subtilis. Schiff base derivatives 5d, 5e, 5f, 5g and 5h showed moderate activities (44.44–52.88%) against Pseudomonas aeruginosa while 2-azetidinone derivatives 6d, 6e, 6f, 6g and 6h exhibited very good activities (58.33–65.76%). Schiff base derivatives 5f, 5g, 5h and 5i showed moderate to very good activities (42.86–64.33%) as compared to 2-azetidinone derivatives 6f, 6g, 6h and 6i which showed moderate activities (50–53.33%) against E. coli. In addition, Schiff base 5g (64.33%) was found very active than 2-azetidinone 6g (53.33%); on the other hand, 2-azetidinones 6f and 6i exhibited higher activities (51.09 and 50%, respectively) than Schiff bases 5f and 5i (49.68 and 42.86%, respectively) against E. coli.


The results of antifungal testing of compounds 5a–k and 6a–k are shown in Table 3. The results of antifungal activity revealed that Schiff base derivatives 5f and 5g as well as 2-azetidinone derivatives 6f and 6g exhibited comparatively good activity (2 mm at 50 μg/mL and 3–5 mm at 100 μg/mL) against fungi Candida albicans as compared to control drug amphotericine-B. The remaining compounds showed moderate to poor activities.

Conclusion
A series of 2-azetidinyl-4-quinazolinones 6a–k exhibited comparatively good antimicrobial activity, most in case when they restrain chloro and methoxy group. Schiff base derivative with 4-methoxy group showed good activity against gram positive as well as gram negative bacteria among the series but the result seems different after cyclization of Schiff base to 2-azetidinone. It shows that the activity got increase against S. aureus and P. aeruginosa, while decrease against B. subtilis and E. coli. 2-Azetidinone derivatives with 2-chloro and 4-chloro group displayed good activity against S. aureus. 4-Chloro and 4-methoxy group containing 2-azetidinone derivatives possessed good activity against P. aeruginosa. Whereas Schiff base derivative with 4-methoxy group exhibited good activity against B. subtilis and E. coli. Schiff base as well as 2-azetidinone derivatives containing 4-chloro and 4-methoxy group showed good antifungal activity against C. albicans. 2-Azetidinone derivatives were found more active than that of Schiff base derivatives.
Experimental
General
Melting points (m.p.) were determined in one-end-open capillary tubes on a Mel-Temp apparatus and are uncorrected. Infrared (IR) absorption spectra were recorded on Perkin-Elmer RX-1 FTIR spectrometer using potassium bromide (KBr) pellet and the wave numbers were given in cm−1. The 1H NMR spectra were recorded in deutero chloroform (CDCl3) on a Bruker Avance II 400 NMR spectrometer (400 MHz). The 13C NMR spectra were recorded in deutero chloroform (CDCl3) on a Bruker Avance II 400 NMR spectrometer operating at 100 MHz. The chemical shifts are reported in part per million (δ ppm) using tetramethylsilane (TMS) as an internal standard. The microanalyses were performed on a Perkin-Elmer 240C elemental analyzer. The purities of the compounds were checked by thin layer chromatography (TLC) using ready-made silica gel plates (Merck) and benzene:methanol (8:2) as a solvent system. The spots were developed in an iodine chamber and visualized under ultraviolet (UV) lamp. 2-[(2,6-Dichlorophenyl)amino]phenyl acetyl chloride 2 was synthesized by literature procedure (Furniss et al., 1989).
Synthesis of 2-[2-(2,6-dichlorophenyl)amino]benzyl-6-bromo-3,1-benzoxazin-4(H)one (3)
A mixture of 2-[(2,6-dichlorophenyl)amino]phenyl acetyl chloride (2) (0.02 mol) and 5-bromo anthranilic acid (0.02 mol) in pyridine (40 mL) were stirred at 0–5 °C for 1 h, further stirred for 1 h at room temperature. A pasty mass obtained which was washed thoroughly with sodium bicarbonate (5%) to remove unreacted acid. A solid separated was filtered, dried and recrystallized from methanol to give (3). Yield = 55%, m.p. 194–198 °C; IR (KBr) cm−1: 3445 (NH), 2928, 2852 (CH2), 1695 (C=O of benzoxazinone), 1615 (C=N), 1320 (C–N), 1140 (C–O–C), 784 (C–Cl), 630 (C–Br); 1H NMR (CDCl3): δ 9.25 (s, 1H, NH), 6.15–7.58 (m, 10H, ArH), 3.58 (s, 2H, CH2); 13C NMR (CDCl3): δ 164.3, 159.2, 148.5, 138.3, 138.1, 135.3, 135.2, 129.2, 129.1, 127.6, 127.4, 126.7, 124.1, 121.5, 121.2, 119.1, 118.5, 118.2, 32.5; Anal. Calcd. for C21H13N2O2BrCl2: C, 52.97; H, 2.75; N, 5.88. Found: C, 52.95; H, 2.78; N, 5.85.
2-[2-(2,6-Dichlorophenyl)amino]benzyl-3-(4-aminoaryl)-6-bromoquinazolin-4(3H)ones (4)
A mixture of (3) (0.01 mol) and benzene-1,4-diamine (0.01 mol) in pyridine (20 mL) was refluxed on an oil bath for 5–6 h. After completion of the reaction, the oily mass obtained, which was slowly poured onto crushed ice-cold water contained concentrated HCl (36% 10 mL) with occasional stirring. The product obtained was filtered and washed several times with water. The crushed product was dried and crystallized from ethanol to provide (4). Yield = 58%, m.p. 209–211 °C; IR (KBr) cm−1: 3505 (NH2), 3438 (NH), 2922, 2849 (CH2), 1685 (C=O of quinazolinone), 1613 (C=N), 1317 (C–N), 784 (C–Cl), 627 (C–Br); 1H NMR (CDCl3): δ 9.30 (s, 1H, NH), 6.30–7.60 (m, 14H, ArH), 5.73 (s, 2H, NH2), 3.62 (s, 2H, CH2); 13C NMR (CDCl3): δ 164.5, 160.4, 146.2, 144.1, 138.6, 136.4, 135.4, 132.4, 129.8, 129.5, 127.9, 127.8, 126.8, 124.7, 123.1, 122.8, 122.5, 121.8, 121.2, 119.1, 118.3, 116.6, 28.9; Anal. Calcd. for C27H19ON4BrCl2: C, 57.27; H, 3.38; N, 9.89. Found: C, 57.22; H, 3.32; N, 9.83.
General procedure for the preparation of 2-[2-(2,6-dichlorophenyl)amino]benzyl-3-[4-(substitutedarylidene)aryl]-6-bromoquinazolin-4(3H)ones (5a–k)
A mixture of (4) (0.005 mol) and substituted aromatic aldehyde (0.005 mol) was taken in absolute ethanol (40 mL) and added few drops of glacial acetic acid. Then the mixture was refluxed for 4–6 h on water bath. The excess solvent was distilled off, poured in to ice cold water. The separated solid was filtered, washed and recrystallized from ethanol to provide (5a–k).
2-[2-(2,6-Dichlorophenyl)amino]benzyl-3-[4-(2-nitroarylidene)aryl]-6-bromoquinazolin-4(3H)one (5a)
Yield = 56%, m.p. 167–170 °C; IR (KBr) cm−1: 3443 (NH), 2925, 2852 (CH2), 1674 (C=O of quinazolinone), 1635 (N=CH), 1615 (C=N), 1542, 1360 (NO2), 1342 (C–N), 785 (C–Cl), (C–Br); 1H NMR (CDCl3): δ 9.30 (s, 1H, NH), 6.55–7.84 (m, 18H, ArH), 6.20 (s, 1H, N=CH), 3.60 (s, 2H, CH2); 13C NMR (CDCl3): δ 165.3, 161.2, 160, 148.8, 147.8, 146.3, 138.5, 136.5, 135.3, 135.2, 132.4, 132.3, 131.2, 130.1, 129.8, 129.4, 128.3, 127.8, 127.7, 126.8, 124.6, 124.1, 123.2, 122.9, 122.5, 121.7, 121.3, 119.2, 118.4, 28.6; Anal. Calcd. for C34H22O3N5BrCl2: C, 58.39; H, 3.17; N, 10.01. Found: C, 58.31; H, 3.09; N, 9.96.
2-[2-(2,6-Dichlorophenyl)amino]benzyl-3-[4-(3-nitroarylidene)aryl]-6-bromoquinazolin-4(3H)one (5b)
Yield = 57%, m.p. 157–161 °C; IR (KBr) cm−1: 3441 (NH), 2928, 2855 (CH2), 1678 (C=O of quinazolinone), 1632 (N=CH), 1613 (C=N), 1545, 1358 (NO2), 1340 (C–N), 787 (C–Cl), (C–Br); 1H NMR (CDCl3): δ 9.25 (s, 1H, NH), 6.53–7.85 (m, 18H, ArH), 6.17 (s, 1H, N=CH), 3.58 (s, 2H, CH2); 13C NMR (CDCl3): δ 164.6, 162.1, 161.2, 148.7, 148.2, 146.4, 138.4, 136.6, 135.4, 135.3, 134.7, 132.4, 131.3, 129.8, 129.7, 129.3, 127.7, 127.6, 126.8, 126.2, 124.5, 123.3, 122.8, 122.7, 122.6, 121.8, 121.4, 119.1, 118.3, 28.5; Anal. Calcd. for C34H22O3N5BrCl2: C, 58.39; H, 3.17; N, 10.01. Found: C, 58.35; H, 3.08; N, 9.98.
2-[2-(2,6-Dichlorophenyl)amino]benzyl-3-[4-(2-hydroxyarylidene)aryl]-6-bromo quinazolin-4(3H)one (5c)
Yield = 55%, m.p. 155–160 °C; IR (KBr) cm−1: 3450 (NH), 3255 (OH), 2928, 2853 (CH2), 1680 (C=O of quinazolinone), 1635 (N=CH), 1618 (C=N), 1335 (C–N), 784 (C–Cl), (C–Br); 1H NMR (CDCl3): δ 9.25 (s, 1H, NH), 6.57-7.86 (m, 18H, ArH), 6.15 (s, 1H, N=CH), 4.85 (s, 1H, OH), 3.62 (s, 2H, CH2); 13C NMR (CDCl3): δ 164.2, 161.1, 160.2, 159.3, 148.8, 146.5, 138.6, 136.6, 135.5, 132.6, 132.5, 131.3, 130.6, 129.6, 129.2, 127.8, 127.6, 126.9, 124.3, 123.5, 122.7, 122.5, 121.7, 121.5, 121.3, 119.3, 118.5, 118.2, 116.3, 28.5; Anal. Calcd. for C34H23O2N4BrCl2: C, 60.92; H, 3.46; N, 8.36. Found: C, 60.83; H, 3.37; N, 8.29.
2-[2-(2,6-Dichlorophenyl)amino]benzyl-3-[4-(4-hydroxyarylidene)aryl]-6-bromo quinazolin-4(3H)one (5d)
Yield = 57%, m.p. 163–165 °C; IR (KBr) cm−1: 3448 (NH), 3245 (OH), 2918, 2852 (CH2), 1673 (C=O of quinazolinone), 1630 (N=CH), 1615 (C=N), 1345 (C–N), 785 (C–Cl), (C–Br); 1H NMR (CDCl3): δ 9.28 (s, 1H, NH), 6.52–7.84 (m, 18H, ArH), 6.20 (s, 1H, N=CH), 4.88 (s, 1H, OH), 3.67 (s, 2H, CH2); 13C NMR (CDCl3): δ 164.4, 160.8, 160.3, 159.5, 148.5, 146.3, 138.4, 136.7, 135.3, 132.5, 131.4, 130.6, 129.5, 129.3, 127.7, 127.5, 126.5, 126.4, 124.4, 123.6, 122.8, 122.7, 121.6, 121.4, 119.2, 118.3, 116.3, 28.6; Anal. Calcd. for C34H23O2N4BrCl2: C, 60.92; H, 3.46; N, 8.36. Found: C, 60.85; H, 3.38; N, 8.31.
2-[2-(2,6-Dichlorophenyl)amino]benzyl-3-[4-(2-chloroarylidene)aryl]-6-bromo quinazolin-4(3H)one (5e)
Yield = 54%, m.p. 142–145 °C; IR (KBr) cm−1: 3444 (NH), 2922, 2848 (CH2), 1677 (C=O of quinazolinone), 1632 (N=CH), 1617 (C=N), 1335 (C–N), 787 (C–Cl), (C–Br); 1H NMR (CDCl3): δ 9.18 (s, 1H, NH), 6.57-7.87 (m, 18H, ArH), 6.25 (s, 1H, N=CH), 3.78 (s, 2H, CH2); 13C NMR (CDCl3): δ 165.1, 161.2, 160.1, 148.7, 146.4, 138.3, 136.3, 135.4, 133.9, 133.4, 132.5, 132.3, 131.3, 130.6, 129.5, 129.2, 128.9, 127.9, 127.6, 127.2, 126.6, 124.7, 123.3, 122.8, 122.4, 121.5, 121.2, 119.3, 118.3, 29.1; Anal. Calcd. for C34H22ON4BrCl3: C, 59.28; H, 3.22; N, 8.13. Found: C, 59.24; H, 3.14; N, 8.08.
2-[2-(2,6-Dichlorophenyl)amino]benzyl-3-[4-(4-chloroarylidene)aryl]-6-bromo quinazolin-4(3H)one (5f)
Yield = 56%, m.p. 160–163 °C; IR (KBr) cm−1: 3446 (NH), 2925, 2845 (CH2), 1679 (C=O of quinazolinone), 1636 (N=CH), 1619 (C=N), 1332 (C–N), 783 (C–Cl), (C–Br); 1H NMR (CDCl3): δ 9.20 (s, 1H, NH), 6.51-7.85 (m, 18H, ArH), 6.28 (s, 1H, N=CH), 3.75 (s, 2H, CH2); 13C NMR (CDCl3): δ 165, 162.2, 160.8, 148.5, 146.2, 138.5, 136.7, 136.6, 135.4, 132.3, 131.8, 131.3, 130.5, 129.6, 129.4, 128.7, 127.8, 127.6, 126.7, 124.3, 123.5, 122.7, 122.5, 121.5, 121.3, 119.1, 118.2, 28.7; Anal. Calcd. for C34H22ON4BrCl3: C, 59.28; H, 3.22; N, 8.13. Found: C, 59.28; H, 3.16; N, 8.06.
2-[2-(2,6-Dichlorophenyl)amino]benzyl-3-[4-(4-methoxyarylidene)aryl]-6-bromo quinazolin-4(3H)one (5g)
Yield = 62%, m.p. 161–164 °C; IR (KBr) cm−1: 3425 (NH), 2920, 2840 (CH2), 1670 (C=O of quinazolinone), 1628 (N=CH), 1610 (C=N), 1310 (C–N), 1196, 1100 (C–O–C), 778 (C–Cl), 630 (C–Br); 1H NMR (CDCl3): δ 9.30 (s, 1H, NH), 6.58–7.85 (m, 18H, ArH), 5.93 (s, 1H, N=CH), 3.80 (s, 3H, OCH3), 3.60 (s, 2H, CH2); 13C NMR (CDCl3): δ 164.8, 162.7, 161.2, 160.9, 148.2, 146.4, 138.3, 136.5, 135.3, 132.4, 131.4, 130.5, 129.5, 129.3, 127.7, 127.4, 126.5, 126.3, 124.4, 123.6, 122.5, 122.3, 121.7, 121.3, 119.2, 118.3, 114.5, 55.3, 28.4; Anal. Calcd. for C35H25O2N4BrCl2: C, 61.42; H, 3.68; N, 8.19. Found: C, 61.33; H, 3.59; N, 8.13.
2-[2-(2,6-Dichlorophenyl)amino]benzyl-3-[4-(3,4,5-trimethoxyarylidene)aryl]-6-bromo quinazolin-4(3H)one (5h)
Yield = 59%, m.p. 150–155 °C; IR (KBr) cm−1: 3430 (NH), 2923, 2845 (CH2), 1675 (C=O of quinazolinone), 1630 (N=CH), 1615 (C=N), 1313 (C–N), 1195, 1105 (C–O-C), 780 (C–Cl), 632 (C–Br); 1H NMR (CDCl3): δ 9.32 (s, 1H, NH), 6.44–7.79 (m, 16H, ArH), 5.95 (s, 1H, N=CH), 3.85 (s, 6H, OCH3), 3.68 (s, 3H, OCH3), 3.63 (s, 2H, CH2); 13C NMR (CDCl3): δ 164.2, 161.5, 160.2, 150.9, 148.4, 146.3, 141.5, 138.2, 136.5, 135.3, 132.3, 131.2, 129.5, 129.3, 128.1, 127.7, 127.5, 126.6, 124.4, 123.6, 122.6, 122.4, 121.7, 121.2, 119.3, 118.3, 106.6, 55.1, 54.6, 29.5; Anal. Calcd. for C37H29O4N4BrCl2: C, 59.69; H, 3.93; N, 7.53. Found: C, 59.62; H, 3.84; N, 7.47.
2-[2-(2,6-Dichlorophenyl)amino]benzyl-3-[4-(2-hydroxy-4-methoxyarylidene)aryl]-6-bromoquinazolin-4(3H)one (5i)
Yield = 63%, m.p. 171–174 °C; IR (KBr) cm−1: 3425 (NH), 3250 (OH), 2925, 2848 (CH2), 1678 (C=O of quinazolinone), 1632 (N=CH), 1614 (C=N), 1317 (C–N), 1198, 1109 (C–O–C), 775 (C–Cl), 625 (C–Br); 1H NMR (CDCl3): δ 9.25 (s, 1H, NH), 6.49–7.73 (m, 17H, ArH), 5.85 (s, 1H, N=CH), 4.75 (s, 1H, OH), 3.91 (s, 3H, OCH3), 3.65 (s, 2H, CH2); 13C NMR (CDCl3): δ 164.9, 164.3, 162.1, 161.5, 160.8, 148.5, 146.4, 138.5, 136.5, 135.4, 132.4, 131.6, 131.4, 129.5, 129.3, 127.8, 127.5, 126.7, 124.2, 123.6, 122.9, 122.6, 121.6, 121.4, 119.2, 118.4, 110.8, 107.2, 102.1, 55.8, 28.6; Anal. Calcd. for C35H25O3N4BrCl2: C, 60.02; H, 3.60; N, 8.00. Found: C, 59.94; H, 3.51; N, 7.94.
2-[2-(2,6-Dichlorophenyl)amino]benzyl-3-[4-(4-N-dimethylaminoarylidene)aryl]-6-bromoquinazolin-4(3H)one (5j)
Yield = 65%, m.p. 174–177 °C; IR (KBr) cm−1: 3435 (NH), 2920, 2845 (CH2), 1686 (C=O of quinazolinone), 1633 (N=CH), 1615 (C=N), 1317 (C–N), 784 (C–Cl), 614 (C–Br); 1H NMR (CDCl3): δ 9.20 (s, 1H, NH), 6.45–7.81 (m, 18H, ArH), 5.95 (s, 1H, N=CH), 3.62 (s, 2H, CH2), 2.89 (s, 6H, N–(CH3)2); 13C NMR (CDCl3): δ 165.8, 161.4, 160.2, 151.8, 148.3, 146.5, 138.2, 136.4, 135.2, 132.5, 131.3, 130.1, 129.4, 129.3, 127.6, 127.5, 126.6, 124.6, 123.7, 123.3, 122.4, 122.2, 121.6, 121.4, 119.3, 118.2, 114.4, 41.1, 28.4; Anal. Calcd. for C36H28ON5BrCl2: C, 62.00; H, 4.05; N, 10.04. Found: C, 61.91; H, 3.96; N, 9.97.
2-[2-(2,6-Dichlorophenyl)amino]benzyl-3-[4-(2-hydroxy-4-N-diethylaminoarylidene) aryl]-6-bromoquinazolin-4(3H)one (5k)
Yield = 58%, m.p. 144–148 °C; IR (KBr) cm−1: 3445 (NH), 3244 (OH), 2923, 2847 (CH2), 1685 (C=O of quinazolinone), 1637 (N=CH), 1618 (C=N), 1316 (C–N), 785 (C–Cl), 620 (C–Br); 1H NMR (CDCl3): δ 9.23 (s, 1H, NH), 6.15–7.85 (m, 17H, ArH), 5.84 (s, 1H, N=CH), 3.57 (s, 2H, CH2), 3.44 (q, 4H, N–(CH2)2), 1.28 (t, 6H, CH3); 13C NMR (CDCl3): δ 164.9, 162.2, 161.5, 160.2, 153.3, 148.3, 146.3, 138.4, 136.4, 135.5, 132.5, 131.5, 131.3, 129.7, 129.6, 127.8, 127.5, 126.7, 124.3, 123.5, 122.6, 122.4, 121.6, 121.4, 119.3, 118.2, 108.2, 107.3, 99.1, 44.6, 28.9, 13.2; Anal. Calcd. for C38H32O2N5BrCl2: C, 61.55; H, 4.35; N, 9.44. Found: C, 61.47; H, 4.26; N, 9.39.
General procedure for the preparation of 2-[2-(2,6-dichlorophenyl)amino]benzyl-3-{4-[4-(2-substitutedphenyl)-3-chloro-2-oxo-azetidinyl]aryl}-6-bromoquinazolin-4(3H)ones (6a–k)
A solution of (5) (0.0025 mol) in dry dioxane (20 mL) was added to a well-stirred mixture of chloroacetylchloride (0.0025 mol) and triethylamine (0.0025 mol) in dry dioxane at 0–5 °C. The reaction mixture was stirred for 10–12 h and kept for 2 days at room temperature. The product was isolated and recrystallized from ethanol to provide (6a–k).
2-[2-(2,6-Dichlorophenyl)amino]benzyl-3-{4-[4-(2-nitrophenyl)-3-chloro-2-oxo-azetidinyl]aryl}-6-bromoquinazolin-4(3H)one (6a)
Yield = 58%, m.p. 174–176 °C; IR (KBr) cm−1: 3440 (NH), 2926, 2853 (CH2), 1746 (C=O of azetidinone), 1682 (C=O of quinazolinone), 1613 (C=N), 1545, 1355 (NO2), 1335 (C–N), 788 (C–Cl), (C–Br); 1H NMR (CDCl3): δ 9.25 (s, 1H, NH), 6.51–7.85 (m, 18H, ArH), 3.52 (s, 2H, CH2), 3.32 (d, 1H, CH–Ar), 3.23 (d, 1H, CH–Cl); 13C NMR (CDCl3): δ 164.3, 162.6, 160.5, 147.3, 146.2, 138.4, 137.4, 137.3, 136.4, 135.3, 134.6, 132.3, 129.7, 129.5, 128.4, 127.9, 127.7, 127.6, 127.4, 126.7, 124.5, 123.5, 123.3, 121.7, 121.5, 121.2, 119.1, 118.3, 62.3, 55.4, 28.1; Anal. Calcd. for C36H23O4N5BrCl3: C, 55.73; H, 2.99; N, 9.03. Found: C, 55.66; H, 2.91; N, 8.95.
2-[2-(2,6-Dichlorophenyl)amino]benzyl-3-{4-[4-(3-nitrophenyl)-3-chloro-2-oxo-azetidinyl]aryl}-6-bromoquinazolin-4(3H)one (6b)
Yield = 56%, m.p. 165–168 °C; IR (KBr) cm−1: 3445 (NH), 2925, 2850 (CH2), 1740 (C=O of azetidinone), 1680 (C=O of quinazolinone), 1615 (C=N), 1543, 1351 (NO2), 1338 (C–N), 786 (C–Cl), 628 (C–Br); 1H NMR (CDCl3): δ 9.35 (s, 1H, NH), 6.53–7.87 (m, 18H, ArH), 3.48 (s, 2H, CH2), 3.31 (d, 1H, CH–Ar), 3.22 (d, 1H, CH–Cl); 13C NMR (CDCl3): δ 164.2, 162.2, 160.3, 147.7, 146.5, 144.5, 138.5, 137.4, 136.5, 135.4, 133.2, 132.3, 129.6, 129.5, 129.4, 128.5, 127.6, 127.5, 126.9, 124.4, 123.5, 123.4, 121.8, 121.7, 121.6, 121.5, 119.3, 118.4, 62.6, 55.6, 28.6; Anal. Calcd. for C36H23O4N5BrCl3: C, 55.73; H, 2.99; N, 9.03. Found: C, 55.67; H, 2.93; N, 8.99.
2-[2-(2,6-Dichlorophenyl)amino]benzyl-3-{4-[4-(2-hydroxyphenyl)-3-chloro-2-oxo-azetidinyl]aryl}-6-bromoquinazolin-4(3H)one (6c)
Yield = 55%, m.p. 163–167 °C; IR (KBr) cm−1: 3448 (NH), 3250 (OH), 2923, 2852 (CH2), 1753 (C=O of azetidinone), 1681 (C=O of quinazolinone), 1613 (C=N), 1338 (C–N), 785 (C–Cl), 625 (C–Br); 1H NMR (CDCl3): δ 9.28 (s, 1H, NH), 6.58–7.93 (m, 18H, ArH), 4.85 (s, 1H, OH), 3.63 (s, 2H, CH2), 3.36 (d, 1H, CH–Ar), 3.28 (d, 1H, CH–Cl); 13C NMR (CDCl3): δ 164.5, 163.1, 160.9, 154.3, 146.4, 138.5, 137.5, 136.6, 135.4, 132.5, 130.8, 129.5, 129.2, 128.4, 128.3, 128.1, 127.8, 127.5, 126.8, 124.3, 123.4, 121.7, 121.5, 121.4, 121.2, 119.2, 118.3, 115.6, 61.7, 55.6, 28.7; Anal. Calcd. for C36H24N4O3Cl3Br: C, 57.89; H, 3.24; N, 7.50. Found: C, 57.81; H, 3.16; N, 7.43.
2-[2-(2,6-Dichlorophenyl)amino]benzyl-3-{4-[4-(4-hydroxyphenyl)-3-chloro-2-oxo-azetidinyl]aryl}-6-bromoquinazolin-4(3H)one (6d)
Yield = 61%, m.p. 170–175 °C; IR (KBr) cm−1: 3450 (NH), 3247 (OH), 2920, 2853 (CH2), 1755 (C=O of azetidinone), 1675 (C=O of quinazolinone), 1618 (C=N), 1340 (C–N), 787 (C–Cl), 621 (C–Br); 1H NMR (CDCl3): δ 9.25 (s, 1H, NH), 6.55–7.90 (m, 18H, ArH), 4.90 (s, 1H, OH), 3.60 (s, 2H, CH2), 3.35 (d, 1H, CH–Ar), 3.30 (d, 1H, CH–Cl); 13C NMR (CDCl3): δ 164.7, 162.3, 160.3, 156.5, 146.4, 138.5, 137.7, 136.7, 136.1, 135.3, 132.6, 129.4, 129.3, 128.5, 128.3, 127.6, 127.4, 126.5, 124.5, 123.7, 121.8, 121.7, 121.5, 119.1, 118.2, 115.7, 61.9, 55.8, 28.5; Anal. Calcd. for C36H24N4O3Cl3Br: C, 57.89; H, 3.24; N, 7.50. Found: C, 57.85; H, 3.18; N, 7.45.
2-[2-(2,6-Dichlorophenyl)amino]benzyl-3-{4-[4-(2-chlorophenyl)-3-chloro-2-oxo-azetidinyl]aryl}-6-bromoquinazolin-4(3H)one (6e)
Yield = 63%, m.p. 157–160 °C; IR (KBr) cm−1: 3448 (NH), 2923, 2850 (CH2), 1740 (C=O of azetidinone), 1675 (C=O of quinazolinone), 1615 (C=N), 1330 (C–N), 784 (C–Cl), 635 (C–Br); 1H NMR (CDCl3): δ 9.28 (s, 1H, NH), 6.52–7.85 (m, 18H, ArH), 3.52 (s, 2H, CH2), 3.36 (d, 1H, CH–Ar), 3.29 (d, 1H, CH–Cl); 13C NMR (CDCl3): δ 164.7, 163.2, 161.2, 146.5, 143.5, 138.3, 137.6, 136.3, 135.4, 132.4, 132.2, 129.6, 129.2, 128.6, 128.4, 128.3, 128.2, 127.8, 127.5, 126.7, 126.6, 124.7, 123.4, 121.7, 121.5, 121.3, 119.3, 118.4, 62.4, 54.3, 29.2; Anal. Calcd. for C36H23O2N4BrCl4: C, 56.50; H, 3.03; N, 7.32. Found: C, 56.44; H, 2.94; N, 7.27.
2-[2-(2,6-Dichlorophenyl)amino]benzyl-3-{4-[4-(4-chlorophenyl)-3-chloro-2-oxo-azetidinyl]aryl}-6-bromoquinazolin-4(3H)one (6f)
Yield = 57%, m.p. 172–176 °C; IR (KBr) cm−1: 3445 (NH), 2926, 2852 (CH2), 1748 (C=O of azetidinone), 1685 (C=O of quinazolinone), 1612 (C=N), 1328 (C–N), 786 (C–Cl), 630 (C–Br); 1H NMR (CDCl3): δ 9.22 (s, 1H, NH), 6.50–7.87 (m, 18H, ArH), 3.55 (s, 2H, CH2), 3.37 (d, 1H, CH–Ar), 3.28 (d, 1H, CH–Cl); 13C NMR (CDCl3): δ 164.7, 163.5, 160.2, 146.3, 141.5, 138.6, 137.7, 136.6, 135.4, 132.7, 132.4, 129.5, 129.4, 128.6, 128.5, 128.3, 127.8, 127.6, 126.7, 124.3, 123.4, 121.8, 121.5, 121.4, 119.2, 118.3, 62.1, 54.6, 29.3; Anal. Calcd. for C36H23O2N4BrCl4: C, 56.50; H, 3.03; N, 7.32. Found: C, 56.46; H, 2.95; N, 7.28.
2-[2-(2,6-Dichlorophenyl)amino]benzyl-3-{4-[4-(4-methoxyphenyl)-3-chloro-2-oxo-azetidinyl]aryl}-6-bromoquinazolin-4(3H)one (6g)
Yield = 65%, m.p. 168–170 °C; IR (KBr) cm−1: 3446 (NH), 2923, 2848 (CH2), 1740 (C=O of azetidinone), 1685 (C=O of quinazolinone), 1618 (C=N), 1349 (C–N), 1205, 1104 (C–O–C), 778 (C–Cl), 630 (C–Br); 1H NMR (CDCl3): δ 9.35 (s, 1H, NH), 6.55–7.84 (m, 18H, ArH), 3.82 (s, 3H, OCH3), 3.50 (s, 2H, CH2), 3.36 (d, 1H, CH–Ar), 3.28 (d, 1H, CH–Cl); 13C NMR (CDCl3): δ 164.1, 163.5, 159.8, 158.5, 146.3, 138.4, 137.6, 136.5, 135.7, 135.3, 132.5, 129.7, 129.5, 128.3, 127.9, 127.4, 127.2, 126.5, 124.4, 123.5, 121.8, 121.6, 121.4, 119.1, 118.2, 114.3, 63.5, 56.4, 54.5, 28.6; Anal. Calcd. for C37H26O3N4BrCl3: C, 58.40; H, 3.44; N, 7.36. Found: C, 58.32; H, 3.36; N, 7.28.
2-[2-(2,6-Dichlorophenyl)amino]benzyl-3-{4-[4-(3,4,5-trimethoxyphenyl)-3-chloro-2-oxo-azetidinyl]aryl}-6-bromoquinazolin-4(3H)one (6h)
Yield = 59%, m.p. 165–167 °C; IR (KBr) cm−1: 3448 (NH), 2925, 2850 (CH2), 1745 (C=O of azetidinone), 1675 (C=O of quinazolinone), 1613 (C=N), 1320 (C–N), 1189, 1095 (C–O–C), 780 (C–Cl), 632 (C–Br); 1H NMR (CDCl3): δ 9.28 (s, 1H, NH), 6.44–7.79 (m, 16H, ArH), 3.92 (s, 6H, OCH3), 3.65 (s, 3H, OCH3), 3.57 (s, 2H, CH2), 3.34 (d, 1H, CH–Ar), 3.25 (d, 1H, CH–Cl); 13C NMR (CDCl3): δ 164.2, 162.4, 161.4, 150.5, 146.2, 138.3, 137.7, 137.5, 137.3, 136.4, 135.3, 132.3, 129.4, 129.3, 128.4, 127.5, 127.3, 126.6, 124.5, 123.6, 121.7, 121.6, 121.3, 119.3, 118.3, 104.3, 64.2, 56.6, 55.2, 54.5, 28.7; Anal. Calcd. for C39H30O5N4BrCl3: C, 57.06; H, 3.68; N, 6.82. Found: C, 56.97; H, 3.59; N, 6.76.
2-[2-(2,6-Dichlorophenyl)amino]benzyl-3-{4-[4-(2-hydroxy-4-methoxyphenyl)-3-chloro-2-oxo-azetidinyl]aryl}-6-bromoquinazolin-4(3H)one (6i)
Yield = 54%, m.p. 184–188 °C; IR (KBr) cm−1: 3435 (NH), 3248 (OH), 2928, 2852 (CH2), 1748 (C=O of azetidinone), 1678 (C=O of quinazolinone), 1615 (C=N), 1316 (C–N), 1195, 1110 (C–O–C), 778 (C–Cl), 627 (C–Br); 1H NMR (CDCl3): δ 9.28 (s, 1H, NH), 6.45–7.75 (m, 17H, ArH), 4.81 (s, 1H, OH), 3.85 (s, 3H, OCH3), 3.58 (s, 2H, CH2), 3.36 (d, 1H, CH-Ar), 3.26 (d, 1H, CH-Cl); 13C NMR (CDCl3): δ 164.6, 162.2, 161.4, 160.1, 155.2, 146.3, 138.4, 137.6, 136.5, 135.3, 132.5, 129.4, 129.3, 129.2, 128.5, 127.8, 127.6, 126.6, 124.2, 123.5, 123.2, 121.7, 121.6, 121.4, 119.1, 118.3, 106.7, 101.8, 63.6, 57.1, 55.8, 28.7; Anal. Calcd. for C37H26O4N4BrCl3: C, 57.20; H, 3.37; N, 7.21. Found: C, 57.12; H, 3.28; N, 7.15.
2-[2-(2,6-Dichlorophenyl)amino]benzyl-3-{4-[4-(4-N-dimethylaminophenyl)-3-chloro-2-oxo-azetidinyl]aryl}-6-bromoquinazolin-4(3H)one (6j)
Yield = 52%, m.p. 187–190 °C; IR (KBr) cm−1: 3437 (NH), 2923, 2851 (CH2), 1752 (C=O of azetidinone), 1686 (C=O of quinazolinone), 1614 (C=N), 1318 (C–N), 785 (C–Cl), 620 (C–Br); 1H NMR (CDCl3): δ 9.30 (s, 1H, NH), 6.50–7.83 (m, 18H, ArH), 3.50 (s, 2H, CH2), 3.35 (d, 1H, CH–Ar), 3.30 (d, 1H, CH–Cl) 2.89 (s, 6H, N–(CH3)2); 13C NMR (CDCl3): δ 165.1, 163.3, 160.7, 147.6, 146.4, 138.3, 137.5, 136.5, 135.3, 133.2, 132.5, 129.6, 129.4, 128.4, 127.9, 127.8, 127.6, 126.6, 124.4, 123.6, 121.7, 121.5, 121.2, 119.2, 118.2, 114.1, 63.3, 56.3, 41.2, 28.6; Anal. Calcd. for C38H29O2N5BrCl3: C, 58.97; H, 3.78; N, 9.05. Found: C, 58.89; H, 3.68; N, 8.96.
2-[2-(2,6-Dichlorophenyl)amino]benzyl-3-{4-[4-(2-hydroxy-4-N-diethylamino phenyl)-3-chloro-2-oxo-azetidinyl]aryl}-6-bromoquinazolin-4(3H)one (6k)
Yield = 67%, m.p. 158–162 °C; IR (KBr) cm−1: 3446 (NH), 3247 (OH), 2924, 2848 (CH2), 1750 (C=O of azetidinone), 1685 (C=O of quinazolinone), 1619 (C=N), 1317 (C–N), 784 (C–Cl), 625 (C–Br); 1H NMR (CDCl3): δ 9.25 (s, 1H, NH), 6.23–7.88 (m, 17H, ArH), 3.58 (s, 2H, CH2), 3.42 (q, 4H, N–(CH2)2), 3.34 (d, 1H, CH–Ar), 3.28 (d, 1H, CH–Cl), 1.25 (t, 6H, CH3); 13C NMR (CDCl3): δ 164.5, 162.5, 161.5, 154.9, 149.2, 146.4, 138.5, 137.6, 136.5, 135.4, 132.5, 129.7, 129.5, 129.2, 128.3, 127.7, 127.5, 126.6, 124.4, 123.3, 121.8, 121.5, 121.4, 120.4, 119.2, 118.1, 106.7, 98.7, 62.4, 55.6, 44.7, 28.9, 13.3; Anal. Calcd. for C40H33O3N5BrCl3: C, 58.73; H, 4.07; N, 8.56. Found: C, 58.67; H, 3.98; N, 8.48.
Antimicrobial activity
The compounds 5a–k and 6a–k were tested for in vitro antimicrobial activity by cup-plate method (Barry, 1976). Antibacterial activity was screened against two gram positive bacteria (Staphylococcus aureus ATCC 9144 and Bacillus subtilis ATCC 6633), two gram negative bacteria (Pseudomonas aeruginosa ATCC 9027 and Escherichia coli ATCC 25922) whereas antifungal activity was screened against fungi Candida albicans ATCC 10231. Penicillin-G and Amphotericine-B were used as standard drugs. Microbial cultures were obtained from National Collection of Industrial Microorganisms (NCIM), National Chemical Laboratory, Pune. The potency of the compounds for antibacterial activity was calculated by using the following formula (Edwin and Marion, 1945) and antifungal activity was noted as the zone of inhibition only.
where F = dilution factor = 1 (same dilution used for standard and test); M = potency of standard = 100; I = log(SH/SL); D = (UH + UL) − (SH + SL); B = (UH − UL) + (SH − SL); SH = zone of inhibition of standard at 100 μg/mL; SL = Zone of inhibition of standard at 50 μg/mL; UH = zone of inhibition of unknown at 100 μg/mL; UL = zone of inhibition of unknown at 50 μg/mL.
References
Alagarsamy V, Solomon VR, Dhanabal K (2007) Synthesis and pharmacological evaluation of some 3-phenyl-2-substituted-3H-quinazolin-4-one as analgesic, anti-inflammatory agents. Bioorg Med Chem 15:235–241
Amir M, Shikha K (2004) Synthesis and anti-inflammatory, analgesic, ulcerogenic and lipid peroxidation activities of some new 2-[(2,6-dichloroanilino)phenyl]acetic acid derivatives. Eur J Med Chem 39:535–545
Archana, Srivastava VK, Kumar A (2002) Synthesis of newer thiadiazolyl quinazolin-4(3H)-ones as potential anticonvulsant agents. Eur J Med Chem 37:873–882
Baek D, Kang T, Kim HJ (2004) Synthesis of nonclassical quinazolinone antifolates as thymidylate synthase inhibitors and their antitumor activity in vitro. Bull Korean Chem Soc 25:1898–1906
Barbaric M, Kralj M, Marjanovic M, Husnjak I, Pavelic K, Filipovic-Grcic J, Zorc D, Zorc B (2007) Synthesis and in vitro antitumor effect of diclofenac and fenoprofen thiolated and nonthiolated polyaspartamide-drug conjugates. Eur J Med Chem 42:20–29
Barry AL (1976) The antimicrobial susceptibility test, principles and practices. Illus lea and Febiger, Philadelphia, PA, p 180
Bhandari SV, Bothara KG, Raut MK, Patil AA, Sarkate AP, Mokale VJ (2008) Design, synthesis and evaluation of antiinflammatory, analgesic and ulcerogenicity studies of novel S-substituted phenacyl-1,3,4-oxadiazole-2-thiol and Schiff bases of diclofenac acid as nonulcerogenic derivatives. Bioorg Med Chem 16:1822–1831
Dutta NK, Annadurai S, Mazumdar K, Dastidar SG, Kristiansen JE, Molnar J, Martins M, Amaral L (2000) The antibacterial action of diclofenac shown by inhibition of DNA synthesis. Int J Antimicrob Agents 14(3):249–251
Edwin JD, Marion BS (1945) Assay of antibiotic substances, p 459
Furniss BS, Hannaford AJ, Smith PWG, Tatchell AR (1989) Vogel’s textbook of practical organic chemistry, 5th edn. Wiley, New York, p 692
Gao X, Cai X, Yan K, Song B, Gao L, Chen Z (2007) Synthesis and antiviral bioactivities of 2-aryl- or 2-methyl-3-(substituted-benzalamino)-4(3H)-quinazolinone derivatives. Molecules 12:2621–2642
Grover G, Kini S (2006) Synthesis and evaluation of new quinazolone derivatives of nalidixic acid as potential antibacterial and antifungal agents. Eur J Med Chem 41:256–262
Gursoy A, Unal B, Karali N, Otuk G (2005) Synthesis, characterization and primary antimicrobial activity evaluation of 3-phenyl-6-methyl-4(3H)-quinazolinone-2-yl-mercaptoacetic acid arylidenehydrazides. Turk J Chem 29:233–245
Gurupadayya BM, Gopal M, Padmashali B, Manohara YN (2008) Synthesis and pharmacological evaluation of azetidin-2-ones and thiazolidin-4-ones encompassing benzothiazole. Indian J Pharm Sci 70(5):572–577
Halve AK, Dubey R, Bhadauria D, Bhaskar B, Gour P (2006) Synthesis of some new azetidin-2-ones as potential antimicrobial agents. J Ind Chem Soc 83:386–388
Havaldar FH, Khatri NK (2006) Synthesis of 2-trifluoromethyl-phenothiazinoazetidin-2-ones as antibacterial and antifungal agents. Ind J Heterocycl Chem 16:87–88
Jatav V, Mishra P, Kashaw S, Stables JP (2008) CNS depressant and anticonvulsant activities of some novel 3-[5-substituted-1,3,4-thiadiazole-2-yl]-2-styryl quinazoline-4(3H)-ones. Eur J Med Chem 43:1945–1954
Jiang S, Zeng Q, Gettayacamin M, Tungtaeng A, Wannaying S, Lim A, Hansukjariya P, Okunji CO, Zhu S, Fang D (2005) Antimalarial activities and therapeutic properties offebrifugine analogs. Antimicrob Agents Chemother 49:1169–1176
Joshi SD, Vagdevi HM, Vaidya VP, Gadaginamath GS (2008) Synthesis of new 4-pyrrol-1-yl-benzoic acid hydrazide analogs and some derived oxadiazole, triazole and pyrrole ring systems: a novel class of potential antibacterial and antitubercular agents. Eur J Med Chem 43:1989–1996
Kumar A, Rajput CS, Bhati SK (2007) Synthesis of 3-[4-(p-chlorophenyl)-thiazol-2-yl]-2-[(substitutedazetidinone/thiazolidinone)-aminomethyl]-6-bromoquinazolin-4-ones as anti-inflammatory agent. Bioorg Med Chem 15:3089–3096
Kurogi Y, Inoue Y, Tsutsumi K, Nakamura S, Nagao K, Yoshitsugu H, Tsuda Y (1996) Synthesis and hypolipidemic activities of novel 2-[4-[(diethoxyphosphoryl) methyl]phenyl]quinazolines and 4(3H)-quinazolinones. J Med Chem 39:1433–1437
Laddha SS, Wadodkar SG, Meghal SK (2006) Studies on some biologically active substituted 4(3H)-quinazolinones. Part 1. Synthesis, characterization and anti-inflammatory-antimicrobial activity of 6,8-disubstituted 2-phenyl-3-[substituted-benzothiazol-2-yl]-4(3H)-quinazolinones. Arkivoc 11:1–20
Mazumdar K, Dutta NK, Dastidar SG, Motohashi N, Shirataki Y (2006) Diclofenac in the management of E. coli urinary tract infections. In Vivo 20(5):613–619
Mishra P, Rajak H, Mehta A (2005) Synthesis of schiff bases of 2-amino-5-aryl-1,3,4-oxadiazoles and their evaluation for antimicrobial activities. J Gen Appl Microbiol 51:133–141
Mosaad SM, Mohammed KI, Ahmed MA, Abdel-Hamide SG (2004) Synthesis of certain new 6-iodoquinazolines as potential antitubercular agents. J Appl Sci 4:302–307
Oza VB, Smith C, Raman P, Koepf EK, Lashuel HA, Petrassi HM, Chiang KP, Powers ET, Sachettinni J, Kelly JW (2002) Synthesis, structure, and activity of diclofenac analogues as transthyretin amyloid fibril formation inhibitors. J Med Chem 45:321–332
Pandey VK, Gupta VD, Upadhyay M, Singh VK, Tandon M (2005) Synthesis, characterization and biological activity of 1,3,4-substituted 2-azetidinones. Ind J Chem 44B:158–162
Panwar H, Verma RS, Srivastava VK, Kumar A (2006) Synthesis of some substituted azetidinonyl and thiazolidinonyl-1,3,4-thiadiazino[6,5-b]indoles as prospective antimicrobial agents. Ind J Chem 45B:2099–2104
Parekh J, Inamdhar P, Nair R, Baluja S, Chanda S (2005) Synthesis and antibacterial activity of some schiff bases derived from 4-aminobenzoic acid. J Serb Chem Soc 70:1155–1161
Patel NB, Chaudhari RC (2006) Quinazolin-4(3H)-ones of 2-[(2,6-dichlorophenyl) amino]phenyl acetic acid with substituted arylacetamide and their microbial studies. J Ind Chem Soc 83:838–841
Patel NB, Chaudhari RC (2008) Synthesis and antimicrobial studies of 4(3H)-quinazolinones. J Saudi Chem Soc 12:251–260
Patel NB, Lilakar JD (2001) Synthesis of new substituted 4(3H)-quinazolinone and their antibacterial activity. Ind J Heterocycl Chem 11:85–86
Patel NB, Patel VN (2007a) New 2,3-disubstituted quinazolin-4(3H)-ones as antimicrobial agents. Ind J Heterocycl Chem 16:247–250
Patel NB, Patel VN (2007b) Synthesis and antimicrobial evaluation of new (4-oxo-thiazolidinyl)quinazolin-4(3H)ones of 2-[(2,6-dichlorophenyl)amino]phenyl acetic acid. Iranian J Pharm Res 6:251–258
Patel NB, Patel JC (2008) Synthesis and antimicrobial screening of 2-[2-(2,6-dichlorophenyl)amino]phenylmethyl-3-{4-[4-(substitutedphenyl)-3-chloro-2-oxo-azetidinyl]aryl}-7-chloro quinazolin-4(3H)ones. J Saudi Chem Soc 12:121–130
Rajasekaran A, Murugesan S (2005) Synthesis and characterization of some novel azetidinone derivatives as antibacterial and anticonvulsant agents. J Pharm Bioresour 2(2):162–168
Ren S, Wang R, Komatsu K, Bonaz-Krause K, Zyrianow Y, McKenna CE, Csipke C, Tokes ZA, Lien EJ (2002) Synthesis, biological evaluation and quantitative structure-activity relationship analysis of new schiff bases of hydroxysemicarbazide as potential antitumor agents. J Med Chem 45:410–419
Saleh MA, Abdel-Megged MF, Abdo MA, Shokr AM (2002) Synthesis and antiviral evaluation of some new glycosylthioureas containing a quinazolinone nucleus. Nucleosides Nucleotides Nucleic Acids 21:93–106
Sriram D, Yogeeswari P, Devakaram RV (2006) Synthesis, in vitro and in vivo antimycobacterial activities of diclofenac acid hydrazones and amides. Bioorg Med Chem 14:3113–3118
Srivastava SK, Yadav R, Srivastava SD (2004) Synthesis of some new 2-mercaptobenzothiazolyl-2-oxoazetidines as antimicrobial and anthelmintic agents. J Ind Chem Soc 81:342–343
Suryavanshi JP, Pai NR (2006) Synthesis and antibacterial screening of n-[naphthol[1,2-b]pyrano[3,4-d]thiazol-8-yl]spiroindoloazetidin-2-ones/thiazolidin-4-ones. Ind J Chem 45B:1227–1230
Yuksek H, Kolayli S, Kucuk M, Yuksek MO, Ocak U, Sahinbas E, Sivrikaya E, Ocak M (2006) Synthesis and antioxidant activities of some 4-benzylidenamino-4,5-dihydro-1H-1,2,4-triazol-5-one derivatives. Ind J Chem 45B:715–718
Acknowledgments
The authors wish to place their thanks to Professor and Head, Department of Chemistry, VNSGU, Surat. Thanks to SAIF, Punjab University, Chandigarh, for spectral analysis.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Patel, N.B., Patel, J.C. Synthesis and antimicrobial activities of 2-azetidinyl-4-quinazolinone derivatives of diclofenac analogue. Med Chem Res 20, 511–521 (2011). https://doi.org/10.1007/s00044-010-9345-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-010-9345-y