Abstract
A series of new 1,3,4-oxadiazoles and 1,2,4-triazoles were synthesized in order to obtain new compounds with potential analgesic activity. Compounds were evaluated for their analgesic activities by formalin-induced nociception test. Mefenamic acid (as the reference drug) did not show any activity in the early phase of the formalin test, while compounds 7b, 7c, 8c, and 9a significantly reduced the nociception in this phase. However in the late phase of formalin test all of the target compounds and mefenamic acid showed analgesic activity in comparison to control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Arachidonic acid (AA) contained in cellular membranes is released by phospholipase A2 (PLA2) and further metabolized by two major enzymatic pathways: 5-lipoxygenase (5-LO) and cyclooxygenase (COX), leading to pro-inflammatory leukotriens (LTs) and prostanoids, respectively. LTB4 synthesized by 5-LO potently activates cell migration and chemotaxis, as well as superoxide production and lysosomal enzyme secretion in neutrophils. Increased production of this eicosanoid has been involved in hyperalgesic response and related with phathological conditions such as rheumatoid arthritis and inflammatory bowel disease (Nikfar et al., 1997; Hosseini-Tabatabaei and Abdollahi 2008). It is interesting to note that COX inhibition by conventional non-steroidal anti-inflammatory drugs (NSAIDs) or by new COX-2 inhibitors leads to an up-regulation of the 5-LO pathway, yielding various adverse effects such as increase of gastrointestinal damage and asthma (Celotti and Laufer 2001; Rainsford 1999; Fosslien 1998). As a result, a new strategy is to minimize gastric toxicity of NSAIDs considering the dual inhibition of 5-LO and COX enzymes. In this scenario, various structural families of dual inhibitors have been designed and several compounds are currently undergoing preclinical or clinical development (Charlier and Michaux 2003; Moreau et al., 2006; Rao et al., 2005). Among these compounds, some potent NSAIDs, fenamates, resulting from the bioisosteric replacement of the carboxylic acid moiety by a tetrazole, were first reported to inhibit COX and to some extent, 5-LO. Then, several fenamates, among which flufenamic acid 1, were converted to potent dual COX/5-LOX inhibitors, after substitution of their carboxylate moiety with other acidic heterocycles, namely 1,3,4-oxadizole, 1,3,4-thiadiazole and 1,2,4-triazole. Compound 2 (X=O, Y=S) was the most potent synthesized analogue in these series (Fig. 1). (Boschelli et al., 1992; Boschelli et al., 1993).
As a part of our ongoing research program to find novel anti-inflammatory, analgesic, and anticonvulsant compounds, herein, we describe the synthesis and analgesic activity of new analogues of compound 2 by bioisosteric replacement of NH with S and oxidation of S to SO and SO2 in order to make this moiety as a real hydrogen bond acceptor similar to NH in compound 2 and in the hope of obtaining additional inhibitors of AA metabolism (Almasirad et al., 2005; Almasirad et al., 2006; Rineh et al., 2007; Shafiee et al., 2009).
Results and discussion
Chemistry
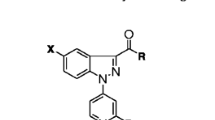
The designed compounds were synthesized according to Schemes 1 and 2. Compounds 4 and 5 were prepared by oxidation of ester 3a with hydrogen peroxide (Shafiee et al., 1998; Brannigan et al., 1976). The key intermediate hydrazides 6a–6d were prepared from the reaction of hydrazine hydrate with compounds 3a, 3b, 4, 5, respectively. 5-Aryl-1,3,4-oxadiazole-2(3H)-thiones 7a–7d were prepared by reaction of hydrazides 6a–6d with KOH and CS2. Reaction of hydrazides 6a–6d with cyanogen bromide gave 5-aryl-2-amino-1,3,4-oxadiazoles 8a–8d. 5-aryl-1,2,4-triazole-3-thiones 9a–9d were synthesized via reaction of 6a–6d with potassium thiocyanate and hydrochloric acid followed by cyclization of thiosemicarbazide intermediate with aqueous sodium hydroxide (Almasirad et al., 2007).
Pharmacology
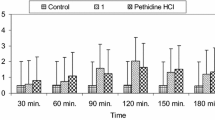
The analgesic effect of target compounds 7a–9c is shown in Tables 1 and 2. As seen in Table 1, compounds 7b, 7c, 8c, and 9a showed significant reduction in the early phase of formalin-induced nociception. Mefenamic acid was ineffective on this phase (neurogenic pain). As can be observed from Table 1, oxidation of sulfur in compound 7a to sulfoxide 7b or sulfone 7c increased the activity. However, in triazole series 9, oxidation of sulfur decreased the activities (compounds 9b and 9c). In addition bioisosteric replacement of oxygen atom in compound 7a with NH increased the activity. In fact, compound 9a was the most active compound. The effect of synthesized compounds on inflammatory pain is summarized in Table 2. All of the target compounds 7a–9c as well as mefenamic acid showed significant analgesic activity in the late phase of formalin test whereas compound 7b was more active than mefenamic acid. The synthesized compounds generally exhibited more activity during the inflammatory phase (57.95–86.12, inhibition range) in comparison to neurogenic phase (13.13–49.38, inhibition range).
The pharmacological evaluation of this study showed a good analgesic profile in comparison to control and mefenamic acid. Our results suggest that compounds 7b, 7c, 8c and 9a, unlike mefenamic acid as the reference drug and most of other NSAIDs have an additional and interesting analgesic action, in that these compounds were effective on neurogenic pain (Vaz et al., 1996; Correa and Calixto 1993; Malmberg and Yaksh 1992). The analgesic effect of these compounds during the neurogenic phase of the formalin test suggests that they have a different or additional mechanism of action than classical NSAIDs. However, further studies are needed to determine that mechanism(s) responsible for the analgesic action of compounds 7b, 7c, 8c and 9a. These four compounds are among the 1,3,4-oxadiazole-2(3H)-thiones, 2-amino-1,3,4-oxadiazoles and 1,3,4-triazole-2-thiones respectively, so all of these scaffolds can be active in the early phase but according to the results, sulfoxide and sulfone moieties are the common functional groups between three of these four compounds. In contrast to the early phase, all of the target compounds and mefenamic acid were active in the late phase that can be explained by inhibition of the arachidonic acid metabolic pathway (Hunskaar et al., 1985).The most active compound 7b in the late phase, similar to the compound 2 (X=O, Y=S) as a dual COX/5-LO inhibitor, has the 1,3,4-oxadiazole-2-(3H)-thione heterocyclic ring so the dual inhibition of COX/5-LO by these compounds is likely to happen (Boschelli et al., 1992).
Various oxadiazole and triazole derivatives were prepared with the objective of developing new analgesic agents. In summary, synthesized compounds showed analgesic activity in both phases of formalin test indicating involvement of different mechanisms of action. Neurogenic pain generally responds poorly to conventional analgesics and its treatment can be difficult (Davies et al., 1994; Niv and Devor 2006). Therefore, discovery of new drugs for treatment of this kind of pain is clinically relevant.
Experimental
Chemicals were purchased from Merck chemical company (Tehran, Iran), Compounds 3a, 3b, 6a, 7a, 8a, 9a were prepared according to the previously described methods (Almasirad et al., 2007; Bentrude and Martin 1962; Dubuisson and Dennis 1977).
Melting points were taken on a Kofler hot stage apparatus (Reichert, Vienna, Austria) and are uncorrected. 1H-NMR spectra were obtained using a Brucker FT-80 spectrometer (Brucker, Rheinstetten, Germany). Tetramethylsilane was used as an internal standard. Mass spectra were obtained using a Finnigan Mat TSQ-70 spectrometer at 70 eV (Finnigan Mat, Bremen, Germany). The IR spectra were obtained using a Nicolet FT-IR Magna 550 spectrographs (KBr disks) (Nicolet, Madision, WI, USA). The purity of compounds was confirmed by TLC using different mobile phases. The results of the elemental analyses (C, H, N) were within ±0.4% of theoretical values for C, H, and N.
Methyl 2-(phenylsulfinyl)benzoate (4, R=H)
To a stirring solution of compound 3a (7 g, 28.7 mmol) in acetic acid (4 ml) was added 30% hydrogen peroxide (3 ml) at room temperature. Four additional portions of 30% hydrogen peroxide (3 ml) were added after 2, 4, 6, and 8 h. The stirring was continued for 1 h, and then the mixture was diluted with water and neutralized with 10% aqueous solution of sodium hydroxide. The organic layer was dried (sodium sulfate) and concentrated in vacuo to give 6.39 g (85%) of 4 (R=H), mp 76–77°C; IR (KBr) ν cm−1 1710 (C=O), 1031 (S=O); 1H-NMR(CDCl3): 8.78 (dd, 1H, J = 7.8, 1.3 Hz, aromatic), 8.09–7.26 (m, 8H, aromatic), 3.87 (s, 3H, CH3). MS: m/z (%) 260 (M+, 15), 211(16), 180 (10), 166 (100), 151 (32), 77 (12). Anal. Calcd. for C14H12O3S: C, 64.60; H, 4.65. Found: C, 64.26; H, 4.87.
Methyl 2-(phenylsulfonyl)benzoate (5, R=H)
Compound 3a (4.52 g, 18.5 mmol) was dissolved in 65 ml of acetic acid, and H2O2 (12 ml of 30% solution) was added. The solution was heated under reflux for 20 h. The mixture was cooled and diluted with water and worked up similar to compound 4 to give compound (5, R=H). The yield was 4.5 g (88%), mp 57–59°C; IR (KBr) ν cm−1 1738 (C=O), 1311, 1158 (SO2); 1H-NMR (CDCl3): 8.19–7.92 (m, 3H, aromatic), 7.71–7.26 (m, 6H, aromatic), 3.92 (s, 3H, CH3). MS: m/z (%) 276 (M+, 10), 244 (93), 212 (100), 182 (95), 152 (85), 104 (30), 77 (34). Anal. Calcd. for C14H12O4S: C, 60.86; H, 4.38. Found: C, 60.98; H, 4.17.
General procedure for the preparation of hyrazides (6b–6d)
To a stirring solution of compounds 3b, 4, or 5 (14 mmol) in 7 ml ethanol at room temperature hydrazine hydrate (14 ml, 280 mmol) was added. After 8 h, the content were poured into water (15 ml), filtered and crystallized from suitable solvents.
2-(Phenylsulfinyl)benzoic acid hydrazide (6b)
Yield 92%; mp 145–146°C (ethanol). IR (KBr) ν cm−1 3482, 3380 (NH2), 3288 (NH), 1623 (C=O), 1024 (S=O). 1H-NMR (DMSO-d6): 9.95 (bs, 1H, NH), 8.12 (dd, 1H, J = 7.9, 1.4 Hz, aromatic), 7.81–7.38 (m, 8H, aromatic), 4.55 (bs, 2H, NH2). MS: m/z (%) 260 (M+, 5), 229 (100), 184 (36), 152 (6), 77 (4). Anal. Calcd. for C13H12N2O2S: C, 59.98; H, 4.65; N, 10.76. Found: C, 60.12; H, 4.52; N, 10.92.
2-(Phenylsulfonyl)benzoic acid hydrazide (6c)
Yield 70%; mp 165–167°C (ether). IR (KBr) ν cm−1 3365, 3170 (NH2, NH), 1631 (C=O), 1324, 1160 (SO2). 1H-NMR (DMSO-d6): 9.81 (bs, 1H, NH), 8.20 (dd, 1H, J = 8.1, 1.8 Hz, aromatic), 7.92–7.25 (m, 8H, aromatic), 4.65 (bs, 2H, NH2). MS: m/z (%) 276 (M+, 18), 245 (99), 181 (10), 152 (100), 77 (41). Anal. Calcd. for C13H12N2O3S: C, 56.51; H, 4.38; N, 10.14. Found: C, 56.73; H, 4.52; N, 10.36.
2-(2-Chlorophenylthio)benzoic acid hydrazide (6d)
Yield 91%; mp 85–86 (ether). IR (KBr) ν cm−1 3303, 3127 (NH2, NH), 1665 (C=O). 1H-NMR (CDCl3): 8.78 (bs, 1H, NH), 7.67–7.17 (m, 8H, aromatic), 4.05 (bs, 2H, NH2). MS: m/z (%) 280 (M++2, 5) 278 (M+, 15), 247 (100), 212 (27), 184 (48), 142 (24). Anal. Calcd. for C13H11ClN2OS: C, 56.01; H, 3.98; N, 10.05. Found: C, 56.19; H,3.17; N, 10.26.
General procedure for the preparation of 5-aryl-1,3,4-oxadiazole-2(3H)-thiones (7b–7d)
A mixtue of hyrazide (6b–6d) (12.2 mmol), potassium hydroxide (12.2, mmol), carbon disulfide (2.5 ml) and ethanol (14 ml) was heated under reflux for 7 h. The solvent was removed in vacuo and the residue was dissolved in water and acidified with dilute hydrochloric acid. The resulting precipitate was removed by filtration and purified by crystallization or TLC.
5-[2-(Phenylsulfinyl)phenyl]-1,3,4-oxadiazole-2-(3H)-thione (7b)
Yield 57%; mp 247–249°C (ethanol). IR (KBr) ν cm−1 3121 (NH), 2530 (weak, SH), 1342 (C=S). 1H-NMR (DMSO-d6): 14.22 (bs, 1H, NH), 8.22-7.52 (m, 9H, aromatic). MS: m/z (%) 302 (M+, 11), 213 (100), 184 (31), 152 (10). Anal. Calcd. for C14H10N2O2S2: C, 55.61; H, 3.33; N, 9.26. Found: C, 55.42; H, 3.14; N, 9.47.
5-[2-(Phenylsulfonyl)phenyl]-1,3,4-oxadiazole-2(3H)-thione (7c)
Yield 86%; mp 140–142°C (ethanol). IR (KBr) ν cm−1 3272 (NH), 1306 (C=S). 1H-NMR (DMSO-d6): 14.30 (bs, 1H, NH), 8.34 (d, 1H, J = 7.1 Hz, aromatic), 7.90–7.60 (m, 8H, aromatic). MS: m/z (%) 318 (M+, 93), 252 (61), 228 (75), 182 (100), 151 (38), 77 (33). Anal. Calcd. for C14H10N2O3S2: C, 52.82; H, 3.17; N, 8.80. Found: C, 52.96; H, 3.07; N, 8.65.
5-[2-(2-Chlorophenylthio)phenyl]-1,3,4-oxadiazole-2-(3H)-thione (7d)
Compound 7d was purified by TLC eluting with petroleum ether-ethyl acetate (1:1) and then crystallized from ethanol, yield 30%; mp 206–208°C. IR (KBr) ν cm−1 3277 (NH), 1332 (C=S). 1H-NMR (DMSO-d6): 14.49 (bs, 1H, NH), 7.88–7.37 (m, 7H, aromatic), 6.97–6.85 (m, 1H, aromatic); MS: m/z (%) 322 (M++2, 40), 320 (M+, 100), 285 (16), 246 (18), 224 (32), 182 (23). Anal. Calcd. for C14H9ClN2OS2: C, 52.41; H, 2.83; N, 8.73. Found: C, 52.60; H, 2.71; N, 8.64.
General procedure for the preparation of 2-amino-5-aryl-1,3,4-oxadiazoles (8b–8d)
To a stirring solution of hydrazide (6b–6d) (3.71 mmol) in dioxane (12 ml), sodium bicarbonate (3.71 mmol) in water (4 ml) was added at room temperature. The mixture was stirred at room temperature for 5 min and cyanogen bromide (3.85 mmol) was added. After 4 h, water (30 ml) was added to the mixture and the precipitate was removed by filtration and purified by crystallization or TLC.
2-Amino-5-[2-(phenylsulfinyl)phenyl]-1,3,4-oxadiazole (8b)
Yield 45%; mp 228-231°C (ethanol). IR (KBr) ν cm−1 3320, 3275 (NH2), 1670 (NH2). 1H-NMR (DMSO-d6): 8.22 (dd, 1H, J = 7.4, 1.7 Hz, aromatic) 7.88–7.40 (m, 8H, aromatic). MS: m/z (%) 285 (M+, 10), 213 (100), 183 (57), 151 (24). Anal. Calcd. for C14H11N3O2S : C, 58.93; H, 3.89; N, 14.73. Found: C, 59.12; H, 3.95; N, 14.55.
2-Amino-5-[2-(Phenylsulfonyl)phenyl]-1,3,4-oxadiazole (8c)
Yield 50%; mp 166–168°C (ethanol). IR (KBr) ν cm−1 3441, 3344 (NH2), 1644 (NH2). 1H-NMR (DMSO-d6): 8.34–8.23 (m, 1H, aromatic), 7.98–7.52 (m, 8H, aromatic), 7.10 (bs, 2H, NH2). MS: m/z (%) 301 (M+, 10), 235 (100), 182 (95), 152 (60), 77 (32). Anal. Calcd. for C14H11N3O3S: C, 55.80; H, 3.68; N, 13.95. Found: C, 55.96; H, 3.54; N, 13.76.
2-Amino-5-[2-(2-Chlorophenylthio)phenyl]-1,3,4-oxadiazole (8d)
Compound 8d was purified by TLC eluting with petroleum ether-ethyl acetate (1:5) and then crystallized from ethanol, yield 76%; mp 158–159°C; IR(KBr) ν cm−1 3344, 3252 (NH2), 1640 (NH2). 1H-NMR (CDCl3): 7.89–7.78 (m, 1H, aromatic), 7.51–6.92 (m, 7H, aromatic), 5.81 (bs, 2H, NH2). MS: m/z (%) 305 (M++2, 5), 303 (M+, 12), 268 (100), 355 (28), 198 (64), 184 (40), 152 (31). Anal. Calcd. for C14H10ClN3OS: C, 55.35; H, 3.32; N, 13.83. Found: C, 55.54; H, 3.26; N, 13.65.
General procedure for the preparation of 5-aryl-1,2,4-triazole-3-thiones (9b–9c)
A suspension of hydrazide (6b–6d) (8.2 mmol), potassium thiocyanate (41 mmol), hydrochloric acid (8 ml) and water 100 ml was refluxed for 3 h. After cooling the resulting thiosemicarbazide was removed by filtration and washed with water. It was dissolved in 4% sodium hydroxide solution (5 ml) and refluxed for 4 h. The mixture after charcoal treatment and filtration was acidified with hydrochloric acid to pH 5–6 and the resulting precipitate was filtered and purified by crystallization or TLC.
5-[2-(Phenylsulfinyl)phenyl]-2,4-dihydro-3H-1,2,4-triazole-3-thione (9b)
Yield 47%; mp 247–249°C (ethanol). IR (KBr) ν cm−1 3128 (NH), 2539 (weak, SH), 1320 (C=S). 1H-NMR (DMSO-d6): 13.72 (bs, 1H, NH), 7.76 (dd, 1H, J = 7.3, 2.9 Hz, aromatic), 7.51-7.33 (m, 7H, aromatic), 7.05 (dd, 1H, J = 7.1, 2.4 Hz, aromatic). MS: m/z (%) 301 (M+, 12), 285 (16), 212 (100), 184 (43), 108 (23). Anal. Calcd. for C14H11N3OS2: C, 55.79; H, 3.68; N, 13.94. Found: C, 55.93; H, 3.79; N, 13.78.
5-[2-(Phenylsulfonyl)phenyl]-2,4-dihydro-3H-1,2,4-triazole-3-thione (9c)
Compound 9c was purified by TLC eluting with chloroform-ethanol (5:1) and then crystallized from ethanol, yield 30%; mp 133–135°C. IR (KBr) ν cm−1 3132 (NH), 1306 (C=S). 1H-NMR (DMSO-d6): 13.4 (bs, 1H, NH), 8.26–7.53 (m, 9H, aromatic). MS: m/z (%) 317 (M+, 45), 252 (100), 220 (20), 165 (17), 77 (10). Anal. Calcd. for C14H11N3O2S2: C, 52.98; H, 3.49; N, 13.24. Found: C, 52.76; H, 3.65; N, 13.46.
5-[2-(2-Chlorophenylthio)phenyl]-2,4-dihydro-3H-1,2,4-triazole-3-thione (9d)
Compound 9d was purified by TLC eluting with chloroform-ethanol (12:1) and then crystallized from ethanol, yield 45%; mp 233–235°C. IR (KBr) ν cm−1 3201, 3172 (NH), 2543 (weak, SH), 1303 (C=S). 1H-NMR (DMSO-d6): 13.15 (bs, 1H, NH), 7.75–7.21 (m, 8H, aromatic). MS: m/z (%) 321 (M++2, 35), 319 (M+, 100) 284 (90), 225 (16), 209 (10). Anal. Calcd. for C14H9ClN2S2: C, 55.16; H, 2.98; N, 9.19. Found: C, 55.37; H, 3.14; N, 9.37.
Pharmacology
Male NMRI mice weighing 20–25 g (from the animal house of the Faculty of Pharmacy, TUMS) were used. The animals were housed in colony cages, condition of constant temperature (22 ± 2°C), a 12 h light/dark schedule, and allowed free access to standard diet and tap water except during the experiment. The animals were allowed to habituate to the laboratory environment for 2 h before the experiments were initiated. All ethical manners for use of laboratory animals were considered carefully and the protocol of study was approved by TUMS ethical committee. The compounds were administered intra-peritoneally (ip), 30 μmol/kg; 0.2 ml/20 g as a suspension in saline and tween 80 (4% w/v) 30 min before formalin injection. Mefenamic acid (Hakim Pharmaceutical Co., Tehran, Iran) (30 μmol/kg, ip) was used as standard drug under the same conditions. The control group received vehicle (0.2 ml/20 g, ip) alone (Rineh et al., 2007).
Formalin-induced test
Final compounds (7a–9c) were tested in the formalin-induced pain test. Twenty-five microliters of formalin (0.5%) was injected subcutaneously into the dorsal surface of the right hind paw of the mouse using a micro syringe with a 26-gauge needle. Immediately after the formalin injection, animals were placed individually in glass cylinder (20 cm wide, 25 cm long) and a glass door and a mirror were arranged at a 45° angle under the cylinder to allow clear observation of the paws of the animals. The total time in seconds that the animal spent licking or biting the injected paw during the period of 0–10 min was considered as indicator of neurogenic pain (early phase) and during the 10–30 min (late phase) represented the inflammatory pain (Rineh et al., 2007; Dubuisson and Dennis 1977; Monsef et al., 2004).
Statistics
The results were expressed as the mean ± SEM of 6 animals per group. The data were statistically analyzed by one-way analysis of variance (ANOVA) followed by Tukey multi-comparison test. Differences with P < 0.05 between experimental groups were considered statistically significant.
References
Almasirad A, Tajik M, Bakhtiari D, Shafiee A, Abdollahi M, Zamani MJ, Khorasani R, Esmaily H (2005) Synthesis and analgesic activity of N-arylhydrazone derivatives of mefenamic acid. J Pharm Pharm Sci 8(3):419–425
Almasirad A, Hosseini R, Jalalizadeh H, Rahimi-Moghaddam Z, Abaeian N, Janafrooz M, Abbaspour A, Ziaee V, Dalvandi A, Shafiee A (2006) Synthesis and analgesic activity of 2-phenoxybenzoic acid and N-phenylanthranilic acid hydrazides. Biol Pharm Bull 26(6):1180–1184
Almasirad A, Vousooghi N, Tabatabai SA, Kebriaeezadeh A, Shafiee A (2007) Synthesis, anticonvulsant and muscle relaxant activites of substituted 1,3,4-oxadiazole, 1,3,4-thiadiazole and 1,2,4-triazole. Acta Chim Slov 54:317–324
Bentrude WG, Martin JC (1962) Anchimerically accelerated bond homolysis. II. Neighboring Iodide and sulfide groups in t-butyl perester decomposition. J Am Chem Soc 84:156–157
Boschelli D, Connor DI, Hoefle M, Bornemeier DA, Dyere RD (1992) Conversion of NSAIDS into balanced dual inhibitors of cyclooxygense and 5-lipoxygenase. Bioorg Med Chem Lett 2:69–72
Boschelli D, Connor DI, Hoefle M, Bornemeier DA, Dyere RD, Kennedy JA, Kupiers PJ, Okonkowo GC, Schrier DJ, Wright CD (1993) 1,3,4-Oxadiazole, 1,3,4-thiadiazole and 1,2,4-triazole analogus of the fenamates: in vitro inhibition of cyclooxygenase and 5-lipoxygenase activities. J Med Chem 36:1802–1810
Brannigan LH, Hodge RB, Field LB (1976) Biologically oriented organic sulfur chemistry. 14. Anti-inflammatory properties of some aryl sulfides, sulfoxides, and sulfones. J Med Chem 19(6):798–802
Celotti F, Laufer S (2001) Anti-inflammatory drugs: new multitarget compounds to face an old problem. The dual inhibition concept. Pharmacol Res 43:429–436
Charlier C, Michaux C (2003) Dual inhibition of cyclooxygenase-2 (Cox-2) and lipoxygenase (5-Lox) as a new strategy to provide safer non-steroidal anti-inflammatory drugs. Eur J Med Chem 38:645–659
Correa CR, Calixto JB (1993) Evidence for participation of B1 and B2 kinin receptors in formalin-induced nociceptive response in the mouse. Br J Pharmacol 110:193–198
Davies HTO, Crombie IK, Macrae WA (1994) Why use a pain clinic? Management of neurogenic pain before and after referral. J R Soc Med 87:382–385
Dubuisson D, Dennis SG (1977) The formalin test: a quantitative study of the analgesic effects of morphine, meperidine and brain stem stimulation in rats and cats. Pain 4:161–174
Fosslien E (1998) Adverse effects of non-steroidal anti-inflammatory drugs on the gastrointestinal system. Ann Clin Lab Sci 28:67–81
Hosseini-Tabatabaei A, Abdollahi M (2008) Potassium channel openers and improvement of toxic stress: do they have role in the management of inflammatory bowel disease? Inflamm Allergy Drug Targets 7:129–135
Hunskaar S, Fasmar OB, Hole K (1985) Formalin test in mice: a useful technique for evaluating mild analgesics. J Neurosci Methods 14:69–75
Malmberg AB, Yaksh TL (1992) Antinociceptive actions of spinal nonsteroidal anti-inflammatory agents on the formalin test in the rat. J Pharmacol Exp Ther 263:136–146
Monsef HR, Ghobadi A, Iranshahi M, Abdollahi M (2004) Antinociceptive effects of Peganum harmala L. alkaloid extract on mouse formalin test. J Pharm Pharm Sci 65:65–69
Moreau A, Chen QH, Roa PNP, Knaus EE (2006) Design, synthesis and biological evaluation of (E)-3-(4-methanesulfonylphenyl)-2-(aryl)acrylic acids as dual inhibitors of cyclooxygenases and lipoxygenase. Bioorg Med Chem 14:7716–7727
Nikfar S, Abdollahi M, Etemad F, Sharifzadeh M (1997) Effects of sweetening agents on morphine-induced antinociception in mice by formalin-test. Gen Pharmacol 29:583–586
Niv D, Devor M (2006) Refractory neuropathic pain: the nature and extent of the problem pain practice 6:3–9
Rainsford KD (1999) Inhibition by leukotriene inhibitors, and calcium and platelet-activating factor antagonists, of acute gastric and intestinal damage in arthritic rats and in cholinomimetic mice. J Pharm Pharmacol 51:331–339
Rao PNP, Chen QH, Knaus EE (2005) Synthesis and biological evaluation of 1,3-diphenylprop-2-yn-1-ones as dual inhibitors of cyclooxygenase and lipoxygenase. Bioorg Med Chem Lett 15:4842–4845
Rineh A, Mahmoodi N, Abdollahi M, Foroumadi A, Sorkhi M, Shafiee A (2007) Synthesis, analgesic and anti-inflammatory activity of 4-(2-phenoxyphenyl) semicarbazones. Arch Pharm Chem Life Sci 340:409–415
Shafiee A, Akbarzadeh T, Foroumadi A, Hadizadeh F (1998) Synthesis of 4 and 5-alkylsulfonylimidazoles. J Heterocyclic Chem 35:141–144
Shafiee A, Rineh A, Kebriaeezadeh A, Foroumadi A, Sheibani V, Afarinesh MR (2009) Synthesis and anticonvulsant activity of 4-(2-phenoxyphenyl)semicarbazones. Med Chem Res 18:725–769
Vaz ZR, Cechinel Filho V, Yunes RA, Calixto JB (1996) Antinociceptive action of 2-(4- bromobenzoyl)-3-methyl-4,6-dimethoxybenzofuran, a novel xanthoxyline derivative on chemical and thermal models of nociception in mice. J Pharmacol Exp Ther 278:304–312
Acknowledgements
This work was supported by grants from Tehran University of Medical Sciences Research Council and INSF (Iran National Science Foundation).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Almasirad, A., Shafiee, A., Abdollahi, M. et al. Synthesis and analgesic activity of new 1,3,4-oxadiazoles and 1,2,4-triazoles. Med Chem Res 20, 435–442 (2011). https://doi.org/10.1007/s00044-010-9335-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-010-9335-0