Abstract
A series of 3-chloro-1-(5H-dibenz[b,f]azepine-5yl)propan-1-one derivatives (2a–k) bearing different amino acids were synthesized by base condensation reaction. 3-Chloro-1-(5H-dibenz[b,f]azepine-5yl)propan-1-one(2) was obtained by N-acylation of 5H-dibenz[b,f]azepine (1). All the synthesized compounds were evaluated for their potential over antioxidant activities against inhibition of lipid peroxidation by β-carotene and linoleic acid assay and inhibition of human low-density lipoprotein (LDL) oxidation assay. Typically, compound 2 showed weak antioxidant activity, whereas coupling of different amino acids enhances the antioxidant activities based on the presence of different functional groups. Among the derivatives, compound 2d showed significant antioxidant activities followed by 2h, 2i, 2j and 2k.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Free radicals and active oxygen species have been related with cardiovascular and inflammatory diseases and even with a role in cancer and ageing (Beckman and Ames, 1998; Halliwel and Gutteridge, 1989). Efforts to counteract the damage caused by these species are gaining acceptance as a basis for novel therapeutic approaches, and the field of preventive medicine is experiencing an upsurge of interest in medically useful antioxidants (Block, 1992; Rice-Evans et al., 1996). Recent evidence (Yen and Chen, 1995) suggests that free radicals, which are generated in many bioorganic redox processes, may induce oxidative damage in various components of the body (e.g. lipids, proteins and nucleic acids) and may also be involved in processes leading to the formation of mutations. Furthermore, radical reactions play a significant role in the development of life-limiting chronic diseases such as cancer, ageing, diabetes, arteriosclerosis and others (Moskovitz et al., 2000).
It has been suggested that oxidative modification of low-density lipoproteins (LDLs) may play a role in the development of atherosclerosis (Jialal and Devaraj, 1996). The oxidative modification depends on a common initiating step—the peroxidation of polyunsaturated fatty acid components in the LDLs (Steinberg et al., 1989). Such modification of LDLs can be inhibited by antioxidants (Frankel and Kanner, 1993).
In the literature, some tricyclic amines and their chemical structure showed antioxidant neuroprotective activity in vitro (Chirtian Beh and Moosmann, 2000). Nowadays, the free-radical scavenging mechanism of aromatic amines (Ar2NHs) has been discussed from the view of chemical kinetics (Lucarini et al., 1999). 5H-dibenz[b,f]azepine, i.e. iminostilbene, is a common basic fused tricyclic amine. It is used as an intermediate for the synthesis of the registered anticonvulsant drug oxcarbazepine (Krichka and Ledwith, 1974), the structure of which has recently been reported (Hempel et al., 2005). Dibenz[b,f]azepine and its derivatives has been variously reported as having antiallergic activity, specifically antihistaminic activity, spansmolytic, serotonin antagonistic, anticonvulsive, antiemetic, antiepileptic, anti-inflammatory, sedative and fungicidal action (Fouche and Leger, 1971).
The research on free radicals provides theoretical information for the medicinal development and supplies some in vitro methods for quick-optimizing drugs; it attracts more scientific attention from bioorganic and medicinal chemists. In addition to the traditional O–H bond type antioxidant, tricyclic amines having N–H bond functions as the antioxidant have attracted much research attention, because aromatic amines (Ar2NHs) have always been the central structure in many currently used drugs (You-Zhi and Zai-Qun, 2007). Usually phenolic compounds were found to have antioxidant and radical scavenging activities and they also inhibit LDL oxidation (Vinson et al., 1995; Teissedre and Waterhouse, 2000).
Recently, the antioxidant properties of 5H-dibenz[b,f]azepine and some of its analogues were reported, and their structure–activity relationships was established based on the different substituents and positions (Vijay Kumar et al., 2008).
Herein, we have reported the synthesis of new 3-chloro-1-(5H-dibenz[b,f]azepine-5-yl)propan-1-one analogues obtained by coupling of different amino acids. The antioxidant properties of 3-chloro-1-(5H-dibenz[b,f]azepine-5-yl)propan-1-one derivatives obtained were evaluated by their inhibition of lipid peroxidation using the β-carotene-linoleic model system and inhibition of human LDL oxidation assay.
Results and discussion
Chemistry
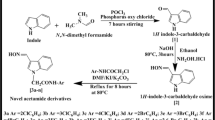
In this study, the initial compound 5H-dibenz[b,f]azepine (1) was obtained according to the literature method (Krichka and Ledwith, 1974). The model compound 3-chloro-1-(5H-dibenz[b,f]azepine-5-yl)propan-1-one (2) was prepared from N-acylation of 5H-dibenz[b,f]azepine with 3-chloro propionylchloride in the presence of triethylamine as base. Further coupling of different amino acids was done by base catalyzed condensation reaction to obtain a series of 3-chloro-1-(5H-dibenz[b,f]azepine-5-yl)propan-1-one derivatives (2a–k) with good yield. Here, the amino acids like glycine, alanine, threonine, cysteine, methionine, proline, phenylalanine, tyrosine, hydroxyproline, histidine and tryptophan were used. Selection of amino acids was done on the basis of its chemical feasibility. The reaction sequences are outlined in Schemes 1 and 2. In the present study, a series of 3-chloro-1-(5H-dibenz[b,f]azepine-5yl)propan-1-one derivatives as target compounds were synthesized. Scheme 1 illustrates the way used for the preparation of model compound 3-chloro-1-(5H-dibenz[b,f]aqepine-5yl)propan-1-one, whereas Scheme 2 illustrates the way to prepare the target compounds. The structure of the compounds was elucidated by IR, 1H NMR, mass spectral data and elemental analysis. In the IR spectra of 3-chloro-1-(5H-dibenz[b,f]azepine-5yl)propan-1-one, C=O stretching band was observed at about 1675.3 cm−1 and CH2 stretching band was observed at 2971.0–3026.1 cm−1. Absence of N–H band at 3360 cm−1 confirms the N-acylation reaction. An absorption band at 3067.9–2700 cm−1 was observed due to aromatic stretching. The structure of compound 2 was confirmed by the absence of signal at 3.5 ppm which corresponds to N–H proton signal of seven-membered ring. The signal due to seven-membered Ar–H protons was appeared as singlet at around 6.9 ppm and two methylene protons was appeared at 2.8 and 3.7 ppm as triplet, respectively. In the IR spectra of 3-chloro-1-(5H-dibenz[b,f]azepine-5yl)propan-1-one derivatives, N–H absorption band was observed at around 3315.5–3361.4 cm−1 region except in the case of compounds 2f and 2j. The carboxylic –OH stretching was observed at around 2467–3510 cm−1. The other prominent absorption bands observed in the IR spectrum are at 1667.6–1679.0 cm−1 (C=O) and 3021.3–2896 cm−1 (Ar–H). These data proved that coupling of amino acids to the intermediate has been taken place. On the other hand, in 1H NMR spectra of all the amino acid analogues, i.e. 2a–k, the seven-membered two aromatic protons was appeared as singlet at 6.9–7.0 ppm. Two methylene protons due to N-acylation were observed at 2.3–3.3 ppm as triplet. N–H proton was appeared as singlet at 1.9–2 ppm, whereas, but in case of compounds 2f and 2j, N–H proton peak was disappeared at the same position. The acid proton was observed at around 11.5–12.8 ppm as singlet. Typically, in the case of compound 2k addition to N–H proton, the N–H proton present in the imidazole ring of histidine was signalled as singlet at 13.5 ppm. In the same way, N–H group present in the indole ring of typtophan in compound 2i signalled as singlet at 7.3 ppm. All the respective aromatic protons in all analogues were observed at the expected region. Mass spectra of all the synthesized analogues showed M+ peaks in agreement with their molecular formula.
The synthesized compounds were evaluated for antioxidant activity by β-carotene–linoleic acid model system. This assay is described for rapid evaluation of antioxidant activity. The method is based on the determination of the coupled oxidation of carotene and linoleic acid. The basic principle involved here is linoleic acid, unsaturated fatty acids get oxidized by reactive oxygen species (ROS) produced by oxygenated water. The products formed initiate the β-carotene oxidation, which leads to discoloration. Bleaching of β-carotene, a free-radical mediated phenomenon resulting from hyperoxides, formed from linoleic acid. β-carotene looses its double bonds by oxidation, which results in lose of its characteristic orange colour monitored spectrophotometrically at 470 nm. Bleaching of β-carotene with respect to time for synthesized compounds at different concentrations is represented in Fig. 1.
Figure 1 indicates that due to the oxidation of linoleic acid which eventually attacks the highly unsaturated β-carotene molecule undergo rapid discoloration. In our experimental conditions, compounds 2, 2a, 2b, 2f and 2g in the suspension loose its chromophore when it allowed for oxidation during different time interval (0–180 min). Initially, the absorbance of all the compounds was more and decreased drastically with time allowed for oxidation, but in case of compounds 2d, 2h, 2i, 2j and 2k there is negligible decrease in the absorbance and the same was also observed in the case of standards like ascorbic acid (AA) and butylated hydroxy anisole (BHA). Whereas in the case of compounds 2c and 2e, average decrease in the absorbance was viewed. The control (no additive) was decolorized within 150 min, indicating that rapid oxidation occurred. The % antioxidant activity for 3-chloro-1-(5H-dibenz[b,f]azepine-5-yl)propan-1-one derivatives was measured on the basis of bleaching of β-carotene is depicted in Table 1. The presence of antioxidant compounds 2d, 2h, 2i, 2j and 2k binds the extent of β-carotene bleaching by neutralizing the linoleate free radical; hence extent of decrease in discoloration indicates higher antioxidant activity. The presence of free S–H group in 2d analogues, phenolic group in compound 2h, hydroxyl group attached to the pyrolidine ring in compound 2j and free N–H group in case of 2i and 2k may donate the hydrogen atom which decreases the extent of β-carotene bleaching by neutralizing the linoleate free radical. The extent of decrease in decoloration by these analogues possesses highest antioxidant activity. The antioxidant activity throughout this assay increases with increase in the concentrations. The increasing antioxidant activities of synthesized compounds and the standards in the assays performed are showed in the following order: AA > BHA > 2h > 2j > 2k > 2i > 2d > 2c > 2e > 2b > 2a > 2g > 2.
Antioxidant effects of 3-chloro-1-(5H-dibenz[b,f]azepine-5-yl)propan-1-one derivatives having different concentrations were examined by inhibiting human LDL oxidation in the presence of CuSO4 as an oxidation inhibitor with different time intervals (2, 4 and 6 h, respectively). The % inhibition of human LDL oxidation is shown in the Table 2. From the graph, initially compound (2) showed less inhibition on LDL oxidation but all its amino acids derivatives showed enhanced in the inhibition of LDL oxidation. All the synthesized compounds showed inhibition of LDL in dose-dependent manner. Among the analogues at 10 µM, compounds 2h, 2j, 2i and 2d showed 78.69, 75.76, 73.45 and 72.54% at the end of 6 h, whereas, at 25 µM inhibited 83.54, 77.14, 75.23 and 75.85%, respectively, showing significant activity. As oxidation modified LDL may play a role in the pathogenesis of atherosclerosis, the prevention of LDL oxidation may reduces the risk of this disease. In this study, it had been demonstrated that the polyunsaturated fatty acids in LDL could be protected from oxidation by the 3-chloro-1-(5H-dibenz[b,f]azepine-5-yl)propan-1-one derivatives. The presence of phenolic –OH, aromatic N–H and S–H groups present in the respective amino acids might account for the prevention of LDL oxidation.
Conclusion
In conclusion, we have reported a convenient protocol for the synthesis of novel 3-chloro-1-(5H-dibenz[b,f]azepine-5-yl)propan-1-one derivatives by base condensation reaction. The antioxidant property was evaluated using different in vitro models. Initially, compound 2 showed negligible activity. Whereas, coupling of different amino acids showed enhance in the antioxidant activity. Among the derivatives, compounds 2d, 2g, 2h, 2i, 2j and 2k were identified as potent antioxidants. It is conceivable from these studies that coupling of amino acid to 3-chloro-1-(5H-dibenz[b,f]azepine-5-yl)propan-1-one can exhibit interesting antioxidant properties. These effects may be useful in the treatment of pathologies in which free-radical oxidation plays a fundamental role.
Experimental
All the reagents were used as purchased from commercial suppliers without further purification. Melting points were determined by an open capillary method and are uncorrected. Thin layer chromatography (TLC) was performed with aluminium sheets—Silica gel 60 F254 purchased from Merck. The compounds were purified using column chromatography with silica gel (60–120 mesh), using chloroform: methanol: acetic acid, 65:30:05 as eluent. IR: Nicolet 5700 FT-IR spectrophotometer; 1H NMR: Bruker 250 MHz spectrometer; mass spectra were obtained by the Waters–Q–TOF ultima spectrometer. Micro analytical data were obtained by Elemental–Vario EL–III.
Antioxidant evaluation
Inhibition of lipid peroxidation by β-carotene and linoleic acid assay
Each compound at final concentrations of 10 and 25 µM was incorporated into β-carotene–linoleic acid model system independently, and the activity was monitored spectrophotometrically at 470 nm (Miller, 1971).
Preparation of the suspension
The substrate suspension was prepared by addition of β-carotene (4 mg dissolved in 5 ml chloroform) into a covered round bottomed flask containing Tween-40 (600 mg) followed by the addition of linoleic acid (60 µl). The chloroform was removed completely under vacuum using rotavapour at 40°C. The resulting solution was diluted with triple distilled water (30 ml), and the emulsion was mixed well and diluted with oxygenated water (120 ml).The aliquots (4 ml) were transferred to different stopper test tubes containing compound (50 and 100 µM/ml) in distilled ethanol. Control was prepared with distilled ethanol (1 ml) and emulsion (4 ml). BHA and ascorbic acid solution as internal standards of the same concentration were also analyzed for comparison. Zero adjustment was done using distilled water. Absorbance of the samples was measured at a wavelength of 470 nm, immediately (t = 0) and subsequently after every 30 min for 3 h (t = 180). The tubes were placed in a water bath at 50°C between the readings. Antioxidant activity of each compound was evaluated in triplicates in terms of photooxidation of β-carotene using the following formula:
where A o is the initial absorbance of the sample, A t absorbance of the sample after time ‘t’, \( A_{\text{o}}^{0} \) initial absorbance of the control, \( A_{\text{o}}^{t} \) absorbance of control after time ‘t’.
Inhibition of human low-density lipoproteins (LDL) oxidation assay
Antioxidant effects of newly synthesized compounds were examined by incubating human LDL in the presence of CuSO4 as an oxidation initiator (Princen et al., 1992).
Fresh blood was obtained from fasting adult human volunteers, and plasma was immediately separated by centrifugation at 1500 rpm for 10 min at 4°C. LDL (0.1 mg LDL protein/ml) was isolated from freshly separated plasma by preparative ultra centrifugation using a Beckman L8-55 ultra centrifuge. The LDL was prepared from the plasma, the isolated LDL was extensively dialyzed against phosphate buffered saline (PBS) pH 7.4 and sterilized by filtration (0.2 µm Millipore membrane system, USA) and stored at 4°C under nitrogen. 1 ml of various concentrations (10 and 25 µM) of compounds was taken in test tubes, 40 µl of copper sulphate (2 mM) was added and the volume was made up to 1.5 ml with phosphate buffer (50 mM, pH 7.4). A tube without copper sulphate with compound served as a positive control. All of the tubes were incubated at 37°C for 45 min. To the aliquots of 0.5 ml drawn at 2, 4 and 6 h intervals from each tube, 0.25 ml of thiobarbutaric acid (TBA, 1% in 50 mM NaOH) and 0.25 ml of trichloro acetic acid (TCA, 2.8%) were added. The tubes were incubated again at 95°C for 45 min and cooled to room temperature and centrifuged at 2500 rpm for 15 min. A pink chromogen was extracted after the mixture was cooled to room temperature by further centrifugation at 2000 rpm for 10 min. Thiobarbituric acid reactive species in the pink chromogen were detected at 532 nm by a spectrophotometer against an appropriate blank. Data were expressed in terms of malondialdehyde (MDA) equivalent, estimated by comparison with standard graph drawn for 1,1,3,3-tetramethoxy-propane (which was used as standard) which give the amount of oxidation and the results were expressed as protection per unit of protein concentration (0.1 mg LDL protein/ml). Using the amount of MDA, the percentage protection was calculated using the formula:
Synthesis of 3-chloro-1-(5H-dibenz[b,f]azepine-5yl)propan-1-one (2)
To the well-stirred solution of 5H-dibenz[b,f]azepine (2 mM) and triethylamine (2.2 mM) in 50 ml benzene, 3-chloro propionylchloride (2.2 mM) in 25 ml benzene was added drop by drop for about 30 min. Then, the reaction mixture is stirred at room temperature for about 6 h. Progress of the reaction was monitored by TLC using 9:1 hexane:ethyl acetate mixture as mobile phase. After the completion of reaction, the reaction mass was quenched in ice cold water and extracted in diethyl ether. The ether layer was washed twice with 5% NaHCO3 followed by distilled water. Finally, the ether layer is dried with anhydrous Na2SO4. The light yellow solid product was obtained by desolventation through rotary evaporator at 35°C.
Light yellow solid, Yield (85%), M.p. 109–110°C. IR (KBr) νmax (cm−1): 3067.9–2700 (Ar C–H), 1675.3 (C=O), 2971.0–3026.1 (CH2); 1H NMR (250 mHz) (CDCl3) δ (ppm): δ 2.8 (d, 2H, CH2–C=O), 3.7 (d, 2H, CH2Cl), 7.5–7.6 (m, 8H, Ar–H), 6.9 (d, 2H, Ar–H of seven-membered ring); Mass (m/z %): M+ 284.56; Anal. Calcd. for C17H14ClNO: C, 71.96; H, 4.97; Cl, 12.49; N, 4.94; O, 5.64%; Found: C, 71.97; H, 4.97; Cl, 12.48; N, 4.95; O, 5.65%.
General procedure for the synthesis of 3-chloro-1-(5H-dibenz[b,f]azepine-5yl)propan-1-one derivatives (2a–k)
Amino acid (1.2 mM) in methanol (25 ml) was treated with K2CO3 (600 mg) under N2 atmosphere. Later the solution of 3-chloro-1-(5H-dibenz[b,f]azepine-5-yl)propan-1-one (1 mM) in methanol (50 ml) was added drop by drop for 30 min. The reaction mixture was refluxed for 6–8 h. The progress of the reaction mixture was monitored by TLC. The reaction mixture was then desolventized in rotary evaporator, and the compound is extracted in ethyl acetate. The ethyl acetate layer was washed with water and dried over anhydrous Na2SO4. The light yellow solid was obtained by further desolventation in rotary evaporator at 50°C.
The products were separated and purified by column chromatography, using mixture of chloroform:methanol:acetic acid = 85:12:3.
Compound 2a: 2-(3-(5H-dibenz[b,f]azepine-5-yl)-3-oxopropylamino)acetic acid [Glycine analogues]
Light yellow solid, Yield (77%), M.p. 79–81°C, IR (KBr) νmax (cm−1): 1669.7 (C=O), 3317.6 (N–H), 3428.5–2713.2 (OH–carboxylic acid), 3052.6–2748.1(Ar–H), 3022.2 (CH2) cm−1; 1H NMR (250 MHz) (DMSO–d6) δ (ppm): 7.3–7.7 (m, 8H, Ar–H), 7.0 (s, 2H, Ar–H of seven-membered ring), 3.5 (d, 2H, CH2 of glycine), 3.0 (t, 2H, CH2–NH), 2.5 (t, 2H, CH2–C=O), 1.9 (s, 1H, NH), 12.8 (s, 1H, OH of COOH). Mass (m/z %): M+ 322; Anal. Calcd. for C19H18N2O3: C, 70.79; H, 5.63; N, 8.69; O, 14.89%. Found: C, 70.76; H, 5.66; N, 8.68; O, 14.91%.
Compound 2b: 2-(3-(5H-dibenz[b,f]azepine-5-yl)-3-oxopropylamino)propanoic acid [ l -Alanine analogues]
Light yellow solid, Yield (81%), M.p. 83–85°C, IR (KBr) νmax (cm−1): 1669.3 (C=O), 3315.5 (N–H), 3489.1–2761.2 (OH–carboxylic acid), 3052.1–2748.0 (Ar–H), 3022.2 (CH2); 1H NMR (250 MHz) (DMSO–d6) δ (ppm): 7.3–7.7 (m, 8H, Ar–H), 7.0 (s, 2H, Ar–H of seven-membered ring), 3.0 (t, 2H, CH2C=O), 2.4 (t, 2H, CH2–NH), 1.2 (d, 3H, CH3 of alanine), 3.4 (q, 1H, CH of alanine), 2.0 (s, 1H, NH of alanine), 12.0 (s, 1H, OH of COOH of alanine). Mass (m/z %): M+ 336.15; Anal. Calcd. for C20H20N2O3: C, 71.41; H, 5.99; N, 8.33; O, 14.27%. Found: C, 71.44; H, 5.98; N, 8.34; O, 14.29%.
Compound 2c: 2-(3-(5H-dibenz[b,f]azepine-yl)-3-oxopropyl amino)-3-hydroxy butanoic acid [ l -Threonine analogue]
Light yellow solid, Yield (78%), M.p. 91–93°C, IR (KBr) νmax (cm−1): 2617.6–3316.8 (OH–carboxylic acid), 3022.2–2796.5 (Ar–H), 3361.4 (N–H), 1667.3 (C=O), 3022.2 (CH2); 1H NMR (250 mHz) (CDCl3) δ (ppm): δ 2.12 (d, 2H, CH2CO), 2.6 (d, 2H, CH2N), 2.0 (s, 1H, NH), 3.5 (d, 1H, CH–N), 3.7 (t, 1H, CH–OH), 5.0 (s, 1H, OH), 1.25 (d, 3H, CH3), 11.5 (s, 1H, COOH), 7.35–7.81 (m, 8H, Ar–H), 7.0 (s, 2H, Ar–H of seven-membered ring); Mass (m/z %): M+ 366.; Anal. Calcd. for C21H22N2O4: C, 68.84; H, 6.05; N, 7.65; O, 17.47%; Found: C, 68.84; H, 6.05; N, 7.65; O, 17.46%.
Compound 2d: 2-(3-(5H-dibenz[b,f]azepine-5-yl)-3-oxopropylamino)-3-mercapto propanoic acid [ l -Cysteine analogue]
Light yellow solid, Yield (79%), M.p. 77–79°C, IR (KBr) νmax (cm−1): 1669.6 (C=O), 3317.7 (N–H), 3457.4–2661 (OH–carboxylic acid), 3052.6–2748.7 (Ar–H), 3022.2 (CH2); 1H NMR (250 MHz) (DMS–d6) δ (ppm): 7.1–7.8 (m, 8H, Ar–H), 7.0 (s, 2H, Ar–H of seven-membered ring), 1.6 (s, 1H, SH), 2.0 (s, 1H, NH), 2.6 (t, 2H, CH2C=O), 3.2 (m, 2H, CH2–NH), 3.6 (m, 2H, CH2 of cysteine), 3.8 (t, 1H, CH of cysteine), 12.5(s, 1H, OH of COOH of cysteine). Mass (m/z %): M+ 368.45; Anal. Calcd. for C20H20N2O3S: C, 65.20; H, 5.47; N, 7.60; O, 13.03; S, 8.70%. Found: C, 65.21; H, 5.49; N, 7.63; O, 13.07; S, 8.70%.
Compound 2e: 2-(3-(5H-dibenzo[b,f]azepin-5-yl)-3-oxopropylamino)-4-(methylthio) butanoic acid [ l -Methionine analogue]
Light yellow solid, Yield (73%), M.p. 79–81°C, IR (KBr) νmax (cm−1): 1668.2 (C=O), 3320.5 (N–H), 3433.2–2513.2 (OH–carboxylic acid), 3052.7–2747.6 (Ar–H), 3022.2 (CH2); 1H NMR (250 MHz) (DMSO–d6) δ (ppm): 7.1–7.8 (m, 8H, Ar–H), 7.0 (s, 2H, Ar–H of seven-membered ring), 2.0(s, 1H, NH), 1.9 (t, 2H, CH2 of methionine), 2.4 (s, 3H, CH3 of methionine), 2.5 (t, 2H, CH2C=O), 2.6 (t, 2H, CH2 of methionine), 3.0 (t, 2H, CH2–NH), 3.4 (t, 1H, CH of methionine), 12.2 (s, 1H, OH of COOH of methionine). Mass (m/z %): M+ 396.50; Anal. Calcd. for C22H24N2O3S: C, 66.64; H, 6.10; N, 7.07; O, 12.11; S, 8.09%. Found: C, 66.66; H, 6.12; N, 7.05; O, 12.13; S, 8.08%.
Compound 2f: 1-(3-(5H-dibenz[b,f]azepine-5-yl)-3-oxopropyl)pyrrolidine-2-carboxylic acid [ l -Proline analogue]
Light yellow solid, Yield (79%), M.p. 75–77°C, IR (KBr) νmax (cm−1): 1669.2 (C=O), 3484.8 (OH–carboxylic acid), 3052.1–3748.2 (Ar–H), 3022.2 (CH2); 1H NMR (250 MHz) (DMSO–d6) δ (ppm): 7.3–7.7 (m, 8H, Ar–H), 7.0 (s, 2H, Ar–H of seven-membered ring), 3.4 (t, 1H, CH of pyrolidine group of proline), 3.3 (t, 2H, CH2–NH), 2.4 (m, 2H, CH2–C=O), 2.6 (d, 1H, CHN of pyrolidine ring), 1.8 (t, 1H, CH2, C4a of pyrolidine ring), 1.6 (m, 3H, CH2, 2H of C3 and 1H of C4 of pyrolidine ring), 12.1 (s, 1H, OH of COOH of pyrolidine). Mass (m/z %): M+ 362.42; Anal. Calcd. for C22H22N2O3: C, 72.91; H, 6.12; N, 7.73; O, 13.24%. Found: C, 72.94; H, 6.11; N, 7.76; O, 13.26%.
Compound 2g: 2-(3-(5H-dibenz[b,f]azepine-5-yl)-3-oxopropylamino)-3-phenyl propanoicacid [ l -Phenyl alanine analogue]
Light yellow solid, Yield (73%), M.p. 84–86°C, IR (KBr) νmax (cm−1): 2572.6–3191.5 (OH–carboxylic acid), 3021.9–2800.1 (Ar–H), 3317.8 (N–H), 1678.9 (C=O), 2884.7–2918.0 (CH2); 1H NMR (250 mHz) (CDCl3) δ (ppm): δ 2.12 (d, 2H, CH2–CO), 2.58 (d, 2H, CH2–N), 3.2–3.5 (m, 2H, benzylic CH2 & t, 1H, CH–N), 7.0 (s, 2H, Ar–H of seven-membered ring), 7.35–7.45 (m, 8H, Ar–H), 7.5 (m, 5H, Ar–H of phenyl alanine), 11.5 (s, 1H, carboxylic OH), 2.0 (s, 1H, NH); Mass (m/z %): M+ 409.14; Anal. Calcd. for C26H24N2O3: C, 75.71; H, 5.86; N, 6.79; O, 11.64%; Found: C, 75.74; H, 5.88; N, 6.78; O, 11.65%.
Compound 2h: Synthesis of 3-(4-hydroxyphenyl)-2-(3-(5H-dibenz[b,f] azepine-5-yl)-3-oxopropylamino)propanoic acid [ l -Tyrosine analogue]
Light yellow solid, Yield (72%), M.p. 91–93°C, IR (KBr) νmax (cm−1): 2467.0–3390.2 (OH–carboxylic acid), 3023.1–2896.0 (Ar–H), 3316.5 (N–H), 1679.0 (C=O), 2812.0–2836.0 (CH2); 1H NMR (250 mHz) (CDCl3) δ (ppm): δ 2.12 (d, 2H, CH2–CO), 2.58 (d, 2H, CH2–N), 3.2–3.5 (m, 2H, benzylic CH2 & t, 1H, CH–N), 6.8 (s, 2H, Ar–H, ortho to OH of tyrosine), 7.0 (s, 2H, Ar–H of seven-membered ring), 7.1 (s, 2H, Ar–H, para to OH of tyrosine), 7.35–7.54 (m, 8H, Ar–H), 9.5 (s, 1H, OH of tyrosine), 11.5 (s, 1H, carboxylic OH), 2.0 (s, 1H, NH); Mass (m/z %): M+ 427.84. Anal. Calcd. for C26H24N2O4: C, 72.88; H, 5.65; N, 6.54; O, 14.94%; Found: C, 72.88; H, 5.64; N, 6.55; O, 14.93%.
Compound 2i: 2-(3-(5H-dibenz[b,f]azepine-5-yl)-3-oxopropylamino)-3-(1H-indol-3-yl)propanoic acid [ l -Tryptophan analogue]
Light yellow solid, Yield (73%), M.p. 96–98°C, IR (KBr) νmax (cm−1): 1668.3 (C=O), 3317.2 (N–H), 3487.7–2716.2 (OH–carboxylic acid), 3095.6–2747.3 (Ar–H), 3022.2 (CH2); 1H NMR (250 MHz) (DMSO–d6) δ (ppm): 7.2–7.7 (m, 8H, Ar–H), 7.0 (s, 2H, Ar–H of seven-membered ring), 2.0 (s, 1H, NH, CH2NH), 2.4 (t, 2H, CH2C=O), 3.0 (t, 2H, CH2–NH), 3.2 (t, 2H, CH2 of tryptophan), 3.7 (t, 1H, CH of tryptophan), 10.2 (s, 1H, NH of indole ring), 7.3 (m, 1H, CH–NH of indole ring of tryptophan), 7.4–7.7 (m, 4H, Ar–H of tryptophan), 12.1 (s, 1H, OH of COOH of tryptophan); Mass (m/z %): M+ 451.19; Anal. Calcd. for C28H25N3O3: C, 74.48; H, 5.58; N, 9.31; O, 10.63%. Found: C, 74.47; H, 5.59; N, 9.33; O, 10.65%.
Compound 2j: 1-(3-(5H-dibenz[b,f]azepine-5-yl)-3-oxopropyl)-3-hydroxy Pyrolidine-2-carboxylic acid [ l -Hydroxy proline analogue]
Light yellow solid, Yield (74%), M.p. 82–84°C, IR (KBr) νmax (cm−1): 2572.0–3261.6 (OH–carboxylic acid), 3021.3–2769.0 (Ar–H), 1669.9 (C=O), 2895.9(CH2); 1H NMR (250 mHz) (CDCl3) δ (ppm): δ 2.0–2.5 (t, 2H, CH2CO, and m, 2H, CH2NH), 2.58 (d, 2H, CH2–N of pyrolidine), 1.7 (d, 2H, CH2 of pyrolidine), 4.80 (s, 1H, OH of pyrolidine), 3.8 (m, 1H, CH-OH), 3.1 (d, 1H, CH to carboxylic acid), 11.5 (s, 1H, carboxylic OH), 7.35–7.54 (m, 8H, Ar–H), 7.0 (s, 2H, Ar–H of seven-membered ring); Mass (m/z %): M+ 378.23; Anal. Calcd. for C22H22N2O4: C, 69.83; H, 5.86; N, 7.40; O, 16.91%; Found: C, 69.83; H, 5.86; N, 7.38; O, 16.89%.
Compound 2k: 2-(3-(5H-dibenz[b,f]azepine-5-yl)-3-oxopropylamino)-3-(1H-imidazole-4-yl)propanoic acid [ l -Histidine analogue]
Light yellow solid, Yield (74%), M.p. 78–80°C, IR (KBr) νmax (cm−1): 1667.6 (C=O), 3322.8 (N–H), 3510.0–2480 (OH–carboxylic acid), 3052.2–2747.6 (Ar–H), 3022.2 (CH2); 1H NMR (250 MHz) (DMSO–d6) δ (ppm): 7.1–7.8 (m, 8H, Ar–H), 6.9 (s, 2H, Ar–H of seven-membered ring), 1.95 (s, 1H, NH, CH2NH), 2.5 (t, 2H, CH2C=O), 3.2 (d, 2H, CH2 of histidine), 3.3 (t, 2H, CH2–NH), 3.9 (t, 1H, CH–NH of histidine), 7.3 (m, 1H, CH of imidazole ring of histidine), 8.6 (m, 1H, Ar–CH, N=CHNH of histidine), 12.3 (s, 1H, OH of COOH of tryptophan), 13.5 (s, 1H,NH of imidazole ring). Mass (m/z %): M+ 402.45; Anal. Calcd. for C23H22N4O3: C, 68.64; H, 5.51; N, 13.92; O, 11.93%. Found: C, 68.66; H, 5.52; N, 13.91; O, 11.95%.
References
Beckman KB, Ames BN (1998) The free radical theory of ageing. Phys Rev 78:447–453
Block G (1992) A role for antioxidants in reducing cancer risk. Nutr Rev 50:207–213
Chirtian Beh L, Moosmann B (2000) Antioxidant neuroprotection in Alzheimer’s disease as preventive and therapeutic approach. Free Rad Biol Med 33:182–191
Fouche J, Leger A (1971) German Patent, 2,031,236; Chem. Abstr. 74:76346r
Frankel EN, Kanner J (1993) Inhibition of oxidation of human low-density lipoprotein by phenolic substances in red wine. Lancet 341:454–457
Halliwel B, Gutteridge JMC (1989) Free radical in biology and medicine. Clarendon Press, Oxford, pp 416–494
Hempel A, Camerman N, Camerman A, Mastropaolo D (2005) Oxcarbazepine: structure and anticonvulsant activity. Acta Cryst E 61:o1313–o1315
Jialal I, Devaraj S (1996) Low-density lipoprotein oxidation, antioxidants, and atherosclerosis: a clinical biochemistry perspective. Clin Chem 42:498–506
Krichka LJ, Ledwith A (1974) Dibenz[b,f]azepines and related ring systems. Chem Rev 74:101–123
Lucarini M, Pedrielli P, Pedulli GF, Valgimigli L, Gigmes D, Toroda P (1999) Bond dissociation energies of the N−H bond and rate constants for the reaction with alkyl, alkoxyl, and peroxyl radicals of phenothiazines and related compounds. J Am Chem Soc 121:11546–11553
Miller HE (1971) A simplified method for the evaluation of antioxidants. J Am Oil Chem Soc 48:91
Moskovitz J, Yim MB, Chock PB (2000) Free radicals and disease. Arch Biochem Biophys 397:354–359
Princen HM, Poppel GV, Vogelezang C, Buytenhek R, Kok FJ (1992) Supplementation with vitamin E but not beta-carotene in vivo protects low density lipoprotein from lipid peroxidation in vitro. Effect of cigarette smoking. Arter Thromb Vasc Biol 12:554–562
Rice-Evans AC, Miller NJ, Paganga G (1996) Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Rad Biol Med 20:933–956
Steinberg D, Parthasarathy S, Carew TE, Khoo JC, Witztum JL (1989) Beyond cholesterol. Modifications of low-density lipoprotein that increase its atherogenicity. New Engl J Med 320:915–924
Teissedre PL, Waterhouse AL (2000) Inhibition of oxidation of human low-density lipoproteins by phenolic substances in different essential oils varieties. J Agric Food Chem 48:3801–3805
Vijay Kumar H, Gnanendra CR, Channe Gowda D, Naik N (2008) In vitro antioxidant activity of dibenz[b,f]azepine and its analogues. E-J Chem 5(S2):1123–1132
Vinson JA, Dabbagh YA, Serry MM, Jang J (1995) Plant flavonoids, especially tea flavonols, are powerful antioxidants using an in vitro oxidation model for heart disease. J Agric Food Chem 43:2800–2802
Yen GC, Chen HY (1995) Antioxidant activity of various tea extracts in relation to their antimutagenicity. J Agric Food Chem 43:27–32
You-Zhi T, Zai-Qun L (2007) Free-radical-scavenging effect of carbazole derivatives on AAPH-induced hemolysis of human erythrocytes. Bioorg Med Chem 15:1903–1913
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kumar, H.V., Kumar, C.K. & Naik, N. Synthesis of novel 3-chloro-1-(5H-dibenz[b,f]azepine-5yl)propan-1-one derivatives with antioxidant activity. Med Chem Res 20, 101–108 (2011). https://doi.org/10.1007/s00044-009-9292-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-009-9292-7