Abstract
Quercetin, 3,3’,4’,5,7-pentahydroxyflavone, is one of the most abundant naturally occurring polyphenolic compounds. Evidences suggest that quercetin has biological properties that may play an important role in prevention of human diseases, such as cancer, cardiovascular diseases, diabetes, and allergies. Many of the biological actions of this flavonoid have been attributed to its antioxidant properties. In the present study, we have determined the protection of protein carbonyl formation by quercetin in erythrocytes subjected to oxidative stress. In vitro oxidative stress in human erythrocytes was induced by incubating with 10−5 M tert-butylhydroperoxide. This resulted in a significantly increased level of carbonyl content in erythrocyte membrane. Treatment with quercetin caused a decrease in the carbonyl content. The effect of quercetin was concentration and time-dependent. The protection of carbonyl formation in proteins substantiates the strong biological antioxidant property of quercetin.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Quercetin, 3,3’,4’,5,7-pentahydroxyflavone, is one of the most abundant naturally occurring polyphenolic compounds, widely distributed as secondary metabolites in the plants (Fig. 1). Belonging to the flavonoid group of polyphenols, quercetin is ubiquitously present in foods, including vegetables, especially onions (Allium cepa), fruits, tea, and wine (Boots et al., 2008).
Reactive oxygen species (ROS) are continuously produced during cell metabolism. Under normal conditions, they are scavenged and converted to nonreactive species by different intracellular, enzymatic, and nonenzymatic antioxidant systems (Hyman et al., 2005). Overproduction or an ineffective elimination of ROS may induce oxidative stress and cause damage to all types of biomolecules, such as proteins, lipids, and nucleic acids (Droge, 2002). A certain amount of oxidative damage takes place even under normal conditions; however, the rate of this damage increases during aging and other pathological events, as the efficiency of antioxidative and repair mechanisms decreases, leading to the condition of oxidative stress (Gil et al., 2006).
The attack by ROS against proteins modifies amino acid (lysine, arginine, proline, and histidine) residues generating carbonyl moieties, which has been identified as an early marker for protein oxidation and is used as a measure of protein damage (Levine et al., 1990).
There is overwhelming evidence to suggest that nutritional sources of antioxidants, such as fruits, vegetables, tea, or wine would attenuate tissue damage caused by oxidative challenges (Cao et al., 1998). Polyphenolic compounds abundantly present in these nutritional sources could play a major role in enhancing the antioxidant system (Hollman et al., 1996; Rizvi et al., 2005).
Several in vivo and in vitro studies have been conducted to evaluate the biological effects of quercetin. Evidence suggests that quercetin has biological properties that may play an important role in the prevention of human diseases, such as cancer, cardiovascular diseases, diabetes, and allergies (Liu et al., 2008; Reutrakul et al., 2007). Many of the biological actions of this flavonoid have been attributed to its antioxidant properties (Perez-Vizcaino et al., 2006; Reutrakul et al., 2007). We report the protective effect of quercetin on carbonyl formation in erythrocytes subjected to oxidative stress by incubating with tert-butylhydroperoxide (t-BHP).
Materials and methods
Erythrocyte ghost preparation
Human venous blood from different healthy volunteers was obtained by venipuncture in heparin. The blood was centrifuged at 1,800 g for 10 minutes at 4°C. After the removal of plasma, buffy coat, and upper 15% of the packed red blood cells (RBCs), the RBCs were washed twice with cold PBS (0.9% NaCl, 10 mM Na2HPO4, pH 7.4). Erythrocyte ghosts from leucocyte-free RBCs were prepared by osmotic shock procedure by the methods of Marchesi and Palade (1967). The protocol of study was in conformity with the guidelines of the Institutional Ethical Committee.
Determination of membrane protein carbonyls
Erythrocyte membrane protein carbonyls were measured according to procedure of Levine et al. (1990): 0.2 ml of erythrocyte membrane samples in PBS were taken in two tubes as test and control, 4.0 ml of 10 mM 2,4-dinitrophenylhydrazine (DNPH) prepared in 2 M HCl was added to the test sample, and 4.0 ml of 2 M HCl alone was added to the control sample. The contents were mixed thoroughly and incubated for 1 hour in the dark at 37°C. The tubes were shaken intermittently every 10 minutes to facilitate the reactions with proteins. After that, 20% TCA (w/v) was added to both tubes and the mixture left in ice for 10 minutes. The tubes were then centrifuged at 3,500 rpm for 20 minutes to obtain the protein pellets. The supernatant was carefully aspirated and discarded. The protein pellets were washed three times with ethanol: ethyl acetate (1:1, v/v) solution to remove unreacted DNPH and lipid remnants. Finally protein pellets were dissolved in 6 M guanidine hydrochloride and incubated for 10 minutes at 37°C. The insoluble materials were removed by centrifugation. Carbonyl content was determined by taking the spectra of the supernatant at 370 nm. Each sample was read against the control. The carbonyl content was calculated by using an absorption coefficient (e) of 22,000 M−1 cm−1, and data were expressed in nmol/mg protein. The erythrocyte membrane protein content was determined by the method of Lowry et al. (1951), using BSA as standard.
Induction of oxidative stress
Oxidative stress was induced in vitro by incubating washed erythrocyte ghosts with 10−5 M tert-butylhydroperoxide (t-BHP) with or without quercetin. The concentration of t-BHP used in the present study to induce oxidative stress in erythrocytes was in the range of concentrations used in other previously published reports (Luqman and Rizvi, 2006).
In vitro experiments with quercetin
Erythrocyte ghosts (0.8–1.5 mg of protein) were incubated with the quercetin (2-(3,4-dihydroxyphenyl)-3,5,7-trihydroxy-4H-chromen-4-one) at different doses in PBS (pH 7.4) for 1 hour at 37°C before the estimation of protein carbonyls formation. Parallel control experiments also were performed in which quercetin was replaced with an equal amount of solvent.
Statistical analyses were performed using GraphPad Prism version 4.00 for Windows (GraphPad Software, San Diego, CA, USA). Results showing p < 0.05 were assumed to be significant.
Results and discussion
Epidemiological studies, using data from consumption of quercetin-rich fruits and vegetables, advocate the beneficial health effects of quercetin. These effects include reduced rate of coronary heart diseases, cancer, incident of asthma, infections, and inflammation (Reutrakul et al., 2007). The possible mechanism by which quercetin exerts these beneficial effects is thought to be its antioxidant activity (Liu et al., 2008).
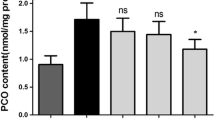
Subjecting erythrocytes to oxidative stress (in vitro) by incubating them with t-BHP (10−5 M) caused an approximately 250% increase in the protein carbonyl group content above the basal value. Incubation of erythrocytes with quercetin showed a significant protection against the t-BHP-induced oxidative stress as evidenced by the decrease in the protein carbonyl group content. We observed that the effect of quercetin is dose/concentration-dependent; the protective effect increases with an increase in the concentration of quercetin from 0.1 μM to 100 μM (Fig. 2).
Dose-dependent effect of quercetin on protein carbonyl content in oxidatively stressed human erythrocytes. *Incubation with t-BHP caused increase in protein carbonyl group level (p < 0.001) compared with control. **Treatment with quercetin shows significant protection against t-BHP induced stress at different concentration at 100 μM (p < 0.001), 10 μM (p < 0.01), and 1 μM (p < 0.05). Effect at concentration 0.1 μM was not significant. Carbonyl content is expressed as nmol/mg protein
Erythrocytes are highly susceptible to the oxidative damage due to the high cellular concentration of oxygen and hemoglobin—a potentially powerful promoter for the oxidative processes (Bryszewska et al., 1995). Oxidative modification of proteins may be selective and specific. Use of protein carbonyls as index of oxidative stress has some advantages compared with the measurement of other oxidation products because of the relative early formation and the relative stability of carbonylated proteins (Dalle-Donne et al., 2003). Accumulation of protein carbonyls is associated with a number of diseases, including amyotrophic lateral sclerosis, Alzheimer’s disease, respiratory distress syndrome, muscular dystrophy, and rheumatoid arthritis (Berlett and Stadtman, 1997).
Our results show that quercetin can protect erythrocytes from oxidative stress under in vitro conditions. The same conditions are thought to occur in vivo, and we hypothesize that quercetin may provide protection against oxidation induced damage to membrane proteins under conditions that challenge the body’s redox status. Protection of carbonyl formation in t-BHP induced oxidative stressed erythrocytes by quercetin in micromolar concentrations assumes significance because it has been reported that plasma quercetin levels after the intake of a quercetin-rich diet are usually in micromolar range ≈ 1–2 μM (Conquer et al., 1998; Wiczkowski et al., 2008). It has been reported that quercetin is significantly absorbed and its peak plasma concentration was achieved after 2–2.7 hours of administration (McAnlis et al., 1999).
The dose-dependent effect of quercetin can be explained by previously reported observations in which it has been documented that the repeated quercetin supplementation could attain a considerable plasma level, sometimes reaching up to 4 μmol; a higher plasma concentration is associated with enhanced activity (Manach et al., 2005; Wiczkowski et al., 2008).
Quercetin is considered the most potent scavenger of ROS among the other members of the flavonoid family. The antioxidative capability of quercetin is attributed to the presence of two antioxidant pharmacophores within the molecule that have the optimal configuration for free radical scavenging (Fig. 1) (Boots et al., 2008). It also is suggested that the quercetin substantially empowers the endogenous antioxidant shield due to its contribution to the total plasma antioxidant capacity, which is six times higher than trolox, an antioxidant generally used as a reference (Arts et al., 2004).
In our experiments, we also evaluated the time-dependent effect of quercetin. A significant protective effect against protein oxidation was observed after only 15 minutes of incubation with quercetin. The protective effect increases gradually up to 120 minutes, after which a slight reduction is observed (Fig. 3).
In view of reports of the protective effect of quercetin on oxidation induced alterations in erythrocyte malondialdehyde, reduced glutathione, and membrane–SH groups (Coskun et al., 2005; Rizvi and Mishra, 2009), our present findings on the protection of carbonyl formation in proteins substantiate the strong biological antioxidant property of quercetin. We hypothesize that quercetin-rich diet may reduce the damage of biomolecules in several degenerating diseases, including cardiovascular, cancer, and diabetes.
References
Arts MJTJ, Dallinga JS, Voss HP, Haenen GRMM, Bast A (2004) A new approach to assess the total antioxidant capacity using the TEAC assay. Food Chem 88:567–570. doi:10.1016/j.foodchem.2004.02.008
Berlett BS, Stadtman ER (1997) Protein oxidation in aging, disease, and oxidative stress. J Biol Chem 272:20313–20316. doi:10.1074/jbc.272.33.20313
Boots AW, Haenen GR, Bast A (2008) Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol 585:325–337. doi:10.1016/j.ejphar.2008.03.008
Bryszewska M, Zavodnik IB, Niekurzak A, Szosland K (1995) Oxidative processes in red blood cells from normal and diabetic individuals. Biochem Mol Biol Int 37:345–354
Cao G, Booth SL, Sadowski JA, Prior RL (1998) Increases in human plasma antioxidant capacity after consumption of controlled diets high in fruit and vegetables. Am J Clin Nutr 68:1081–1087
Conquer JA, Maiani G, Azzini E, Raguzzini A, Holub BJ (1998) Supplementation with quercetin markedly increases plasma quercetin concentration without effect on selected risk factors for heart disease in healthy subjects. J Nutr 128:593–597
Coskun O, Kanter M, Korkmaz A, Oter S (2005) Quercetin, a flavonoid antioxidant, prevents and protects streptozotocin-induced oxidative stress and beta-cell damage in rat pancreas. Pharmacol Res 51:117–123. doi:10.1016/j.phrs.2004.06.002
Dalle-Donne I, Rossi R, Giustarini D, Milzani A, Colombo R (2003) Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta 329:23–38. doi:10.1016/S0009-8981(03)00003-2
Droge W (2002) Free radicals in the physiological control of cell function. Physiol Rev 82:47–95
Gil L, Siems W, Mazurek B, Gross J, Schroeder P, Voss P, Grune T (2006) Age-associated analysis of oxidative stress parameters in human plasma and erythrocytes. Free Radic Res 40:495–505. doi:10.1080/10715760600592962
Hollman PC, vd Gaag M, Mengelers MJ, van Trijp JM, de Vries JH, Katan MB (1996) Absorption and disposition kinetics of the dietary antioxidant quercetin in man. Free Radic Biol Med 21:703–707. doi:10.1016/0891-5849(96)00129-3
Hyman M, Pizzorno J, Weil A (2005) A rational approach to antioxidant therapy and vitamin E. Altern Ther Health Med 11:14–17
Levine RL, Garland D, Oliver CN, Amici A, Climent I, Lenz AG, Ahn BW, Shaltiel S, Stadtman ER (1990) Determination of carbonyl content in oxidatively modified proteins. Methods Enzymol 186:464–478. doi:10.1016/0076-6879(90)86141-H
Liu J-L, Du J, Fan L-L, Liu XY, Gu L, Ge Y-B (2008) Effects of quercetin on hyper-proliferation of gastric mucosal cells in rats treated with chronic oral ethanol through the reactive oxygen species-nitric oxide pathway. World J Gastroenterol 14:3242–3248. doi:10.3748/wjg.14.3242
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193:265–275
Luqman S, Rizvi SI (2006) Protection of lipid peroxidation and carbonyl formation in proteins by capsaicin in human erythrocytes subjected to oxidative stress. Phytother Res 20:303–306. doi:10.1002/ptr.1861
Manach C, Williamson G, Morand C, Scalbert A, Rémésy C (2005) Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am J Clin Nutr 81:230S–242S
Marchesi VT, Palade GE (1967) The localization of Mg-Na-K-activated adenosine triphosphatase on red cell ghost membranes. J Cell Biol 35:385–404. doi:10.1083/jcb.35.2.385
McAnlis GT, McEneny J, Pearce J, Young IS (1999) Absorption and antioxidant effects of quercetin from onions, in man. Eur J Clin Nutr 53:92–96. doi:10.1038/sj.ejcn.1600682
Perez-Vizcaino F, Bishop-Bailley D, Lodi F, Duarte J, Cogolludo A, Moreno L, Bosca L, Mitchell JA, Warner TD (2006) The flavonoid quercetin induces apoptosis and inhibits JNK activation in intimal vascular smooth muscle cells. Biochem Biophys Res Commun 346:919–925. doi:10.1016/j.bbrc.2006.05.198
Reutrakul V, Ningnuek N, Pohmakotr M, Yoosook C, Napaswad C, Kasisit J, Santisuk T, Tuchinda P (2007) Anti HIV-1 flavonoid glycosides from Ochna integerrima. Planta Med 73:683–688. doi:10.1055/s-2007-981538
Rizvi SI, Mishra N (2009) Anti-oxidant effect of quercetin on type2 diabetic erythrocytes. J Food Biochem (in press)
Rizvi SI, Zaid MA, Anis R, Mishra N (2005) Protective role of tea catechins against oxidation-induced damage of type 2 diabetic erythrocytes. Clin Exp Pharmacol Physiol 32:70–75. doi:10.1111/j.1440-1681.2005.04160.x
Wiczkowski W, Romaszko J, Bucinski A, Szawara-Nowak D, Honke J, Zielinski H, Piskula MK (2008) Quercetin from shallots (Allium cepa L. var. aggregatum) is more bioavailable than its glucosides. J Nutr 138:885–888
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Pandey, K.B., Rizvi, S.I. Protection of protein carbonyl formation by quercetin in erythrocytes subjected to oxidative stress. Med Chem Res 19, 186–192 (2010). https://doi.org/10.1007/s00044-009-9183-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-009-9183-y