Abstract
Herbal medicine has formed the basis of health care throughout the world since the dawn of civilization. Each plant is a unique chemical factory capable of synthesizing unlimited numbers of highly complex and unusual chemical substances whose structures could otherwise escape the imagination forever. Phytomedicines have been used as a treatment for many diseases, ranging from skin disease to cancer. Most of the anticancer drugs that we use today are derived from plants. The anticancer effect of plants is due to specific phytochemicals or the complex synergistic interactions among their various constituents. The aim of the present study was to evaluate the cytotoxic properties of an extract of the Curucuma zedoariae rhizome, a plant belonging to the family Zingeberaceae, and to determine its IC50 value. Various organic extracts were isolated using Soxhlet apparatus in order of increasing solvent polarity, namely petroleum ether, ethyl acetate, acetone, and methanol. Colorimetric [3-(4,5-Dimethylthiazol-2-yl)-2,5 diphenyltetrazolium bromide] assay (MTT assay) was done to determine the cytotoxicity against human cervical carcinoma cells (He La). Acridine orange–ethidium dromide dual staining and DNA fragmentation assays were done to detect apoptotic features. Among the various extracts studied, the petroleum ether extract was found to exhibit maximum cytotoxicity against He La cells. Our results suggest that the petroleum ether extract of the Curucuma zedoariae rhizome may have potential as an anticancer agent. Further steps have to be done to purify the compound and to elucidate the antitumor activity of the extract.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
According to the definition of the International Union against Cancer, ‘Cancer is a disturbance of growth characterised primarily by an excessive proliferation of cells without apparent relationship to the physiological demands of the organ involved’. Cancer is still a dreaded disease which accounts for 9% deaths throughout the world. Cancer can occur in any organ of the body and there are many subtypes. It is therefore, necessary to discover separate drugs for the various types of cancers (Suffness et al., 1991).
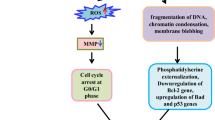
In recent times, plants from jungles have been turned to as rich sources of plant species (Singhal, 2004). There is a need to evaluate the potential of plant-based medicine as an adjuvant to counteract the side-effects of modern therapy and reduce costs (Dahanukar et al., 1997). According to the World Health Organization (WHO), “a medicinal plant is any plant which, in one or more of its organs, contain substances that can be used for therapeutic purposes, or which are precursors for chemopharmaceutical semisynthesis’. Plants have been very useful sources of clinically relevant antitumor compounds. The anticancer effect of plants is due to specific phytochemicals or the complex synergistic interactions among the various constituents of the plant. Herbal drug technology is used to convert botanical materials into medicine, where standardization and quality control with the proper integration of modern scientific techniques and traditional knowledge is important. Apoptosis is an important mode of action for many antitumor agents (Radford et al., 1994). Apoptosis is a protective measure that prevents malignant transformation; in fact effective tumor therapy may involve the induction of apoptosis (Cohen, 1991).
Curcuma zedoariae is a medicinal tuber belonging to the family Zingiberaceae. The rhizomes possess antibacterial, antiulcer properties and are also a gastrointestinal stimulant. It is a constituent of a wide variety of Ayurvedic preparations such as Dasamularishtam, Valiya Rasnadi Kashayam, etc. The plant rhizome is known as ezhu in Chinese and is used extensively in traditional Chinese medicine to treat various ovarian and cervical cancers. The anti-inflammatory (Jang et al., 2001), antifungal (Gupta et al., 1976), antiulcer (Watanabe et al., 1986) and hepatoprotective activity (Rana et al., 1992) of this plant rhizome have been reported. Some novel compounds such as curuminoids have been isolated from the Curcuma zedoariae plant rhizome and have demonstrated inhibitory activity against ovarian cancer cell lines (OVCAR- 3) (Syu et al., 1998). Also elemene, isolated from the zedoariae rhizome, has been found to exhibit substantial antitumor activity against promyelocytic leukemic HL-60 cells (Lien et al., 1985). Curcumin and curcumenol are reported to inhibit the growth of S-180 sarcoma cells and mouse cervical U-14 cells (Lai et al., 2005).
In the present study, we analyzed the cell death and percentage cytotoxicity using a cervical cancer cell line (He La). Morphological features of apoptosis were also studied using acridine orange–ethidium bromide dual staining.
Bioactivity directed fractionation and identification by gas chromatography mass spectrometry (GC-MS) showed the presence of isocurcumenol (26%), methylsterolate (25%), elemene (4%), and isolongifolene (10%) in the active petroleum ether fraction of the Curcuma zedoariae extract.
Materials and methods
Collection of plant material
The rhizomes of Curcuma zedoariae were collected from the Central Tuber Crops Research Institute (CTCRI), Thiruvananthapuram and the plant was identified by the taxonomists of the Department of Botany, University of Kerala, Thiruvananthapuram.
Preparation of the extract
The rhizomes were shade dried and pulverized. Extraction was done using methanol in a Soxhlet apparatus. The crude methanolic extract exhibited cytotoxicity against cervical cancer cells. Hence various organic extracts were isolated using Soxhlet apparatus in order of increasing solvent polarity, namely petroleum ether, ethyl acetate, acetone, and methanol. The extracts were concentrated in a rotary evaporator. The extracts were dissolved in dimethyl sulfoxide (DMSO) and used for the study.
Cell viability assay by MTT
Five thousand cells were plated in 100 μl of the medium Dulbecco’s Modified Eagle’s Medium (DMEM) with 10% Foetal Bovine Serum (FBS) in 96-well Corning plates in the presence or absence of various concentrations of the extracts for 48 and 72 h.
Media-treated cells were taken as a negative control. At the end of the incubation, 25 μl of MTT solution (5 mg/mL in Phosphate Buffered Saline (PBS)) was added to each well. After a 2-h incubation at 37°C, 100 μl of the extraction buffer was added (20% sodium dodecylsulfate) in 50% dimethylformamide. After a 4-h incubation, optical densities at 570 nm were taken using a multiwell plate reader with the extraction buffer as a blank (Scudiero et al., 1988). The minimum concentration required for 50% cytotoxicity and the inhibitory concentration required for 50% cytotoxicity (IC 50) were calculated.
Acridine orange–ethidium bromide dual staining
Cells were cultured in 24-well titre plates. One million He La cells were incubated in DMEM medium with 10% FCS containing various concentrations of the drug in a CO2 incubator at 37°C for 18 and 24 h (Volders et al., 1997). Acridine orange and ethidium bromide staining of DNA allowed visualization of the condensed chromatin of dead apoptotic cells. The medium was removed, cells were trypsinized, pelleted gently, and 1 μl of acridine orange (100 μg/mL) + ethidium bromide (100 μg/mL) in 1 mL PBS was added and immediately washed once with PBS and resuspended in 10 μl 10% glycerol in PBS; the slides were analyzed by fluorescence microscopy (Nikon Diaphot, UV 410). The number of cells showing features of apoptosis was counted as a function of the total number of cells present in the field.
DNA fragmentation assay
A DNA fragmentation assay (Anto et al., 2000) was carried out to assess the characteristic features of apoptosis with the extract. He La cells were seeded in culture flasks at a seeding density of 4 × 105 cells/flask and treated with various concentrations of the fraction containing the compounds. One was kept as a negative control. Cells treated with tetradecanoyl phorbol acetate were taken as a positive control. The cells were harvested and washed with PBS. Oligonucleosomal DNA fragments were analyzed in the gels under ultraviolet light after staining with ethidium bromide.
Gas chromatography mass spectroscopy
Bioactivity-directed fractionation of the extract was done by GC-MS for the identification of the compounds present in the extract (GC-MS Shimadzu QP 2010) with a DB-5 column, an injection temperature of 200°C, an interphase temperature of 200°C, and an ion source temperature of 200°C. The column temperatures were programmed from 50°C to 280°C at a rate of 10°C/min.
Results and discussion
The petroleum ether fraction exhibited cytotoxicity against He La cells at 48 and 72 h. There was an increase in the percentage of cytotoxicity with increasing time and concentration. The inhibitory concentration required for 50% cytotoxicity (IC50) was 257.3 μg/mL and 144 μg/mL at 48 and 72 h, respectively (Figs. 1 and 2). The percentage of cytotoxicity at 48 and 72 h was also compared (Fig. 3).
Control cells appeared green in color whereas treated cells appeared orange on acridine orange–ethidium bromide dual staining at 500 μg/mL concentrations (Figs. 4 and 5). Acridine orange is a cationic dye that enters only live cells and stains DNA green, hence the green cells in the control. On the contrary ethidium bromide stains DNA orange, but is excluded by the live cells. The cells treated with the petroleum ether fraction containing the compounds appeared orange due to the entry of ethidium bromide into the cell due to the lack of membrane integrity.
A very weak DNA ladder was obtained on analysis on a gel documentation system.
Bioactivity-directed fractionation of the extract led to the identification of four major
Compounds by GC-MS (Table 1): isocurcumenol (26%), methylsterolate (25%), elemene (4%), and isolongifolene (10%). Mass spectra of these compounds are given in Figs. 6, 7, 8, and 9). The structure of isocurcumenol is given in Fig. 10.
Conclusion
The evaluation of ancient herbal medicines may indicate novel strategies for the treatment of cancer, which remains the leading cause of death worldwide. Pharmacologically safe compounds that can inhibit proliferation of tumor cells have potential as anticancer agents. Phytochemicals show promise in this area due to their potential chemopreventive or chemotherapeutic actions. Of 121 prescription drugs in use for cancer treatment, 90 are derived from plant species; 74% of these drugs were discovered by investigating a folklore chain.
The present study showed a dose- and time-dependent increase in the percentage of cytotoxicity towards He La cervical cancer cells due to the fraction containing the active compounds. The results obtained from acridine orange–ethidium bromide dual staining suggest apoptosis induction
The GC-MS profile of the petroleum ether extract identified the presence of the major compounds isocurcumenol (26%), methylsterolate (25%), elemene (4%), and isolongifolene (10%) as active chemical constituents.
This study reveals the importance of Curcuma zedoariae as a good candidate for anticancer drug development. Further studies have to be carried out in this plant to analyze the mechanism of action of the compounds present on apoptosis and cancer.
References
Anto RJ, Maliekal TT, Karunagaran DL (2000) 929 cells harbouring ectopically expressed Rel A resist curcumin induced apoptosis. J Biol Chem 275:15601–15604
Cohen JJ (1991) Programmed cell death in the immune system. Adv Immunol 50:55–85
Dahanukar SA, Thatte UM (1997) Current status of Ayurveda in phytomedicine. Phytomedicine 4:359–368
Gupta SK, Banerjee AB, Achari B (1976). Isolation of ethyl-P-methoxy cinnamate, the major antifungal principle of Curcuma zedoariae. Lloydia 39:218–222
Jang MK, Sohn DH, Ryu JH (2001). A curcuminoid and sesquiterpenes as inhibitors of macrophage TNF- α release from Curcuma zedoariae. Planta medica 67:550–552
Kirsh Volders M, Elhajouji A, Cundari E, Van Hummelen P (1997) The in vitro micronuclear test: A multi-endpoint assay to detect simultaneously mitotic delay, apoptosis, chromosome breakage and non-disjunction. Mutat Res 436:69–97
Lai EY, Chyau CC, Mau JL, Chen CC, Lai YJ, Shih CF, Lin LL (2004) Antimicrobial activity and cytotoxicity of the essential oil of Curcuma zedoariae. Am J Chin Med 32:281–290
Lien EJ, Wy Li (1985). Advances in Chinese medical materials research. 433–452
Radford IR, Murphy TK, Radley JM, Ellis SL (1994) Radiation response of mouse lymphoid and myeloid cell lines Part 2. Int J Radiat Biol 65(2):217–227
Rana AC, Avadhoot Y (1992) .Experimental evaluation of hepatoprotective activity of Gymnema sylvestre and Curcuma zedoariae. Fitoterapia 63:60–67. Scientific publishing 433–452
Scudiero DA, Shoemaker RH, Paull KD, Monks A, Tierney S, Nofziger H, Currens MJ, Seniff D, Boyd MR (1988) Evaluation of a soluble tetrazolium formasan assay for cell growth and drug sensitivity in culture using human and other tumour cell lines. Cancer Res 48:4827–4833
Singhal MK (2004) Jungles: Rich source of medicinal plants. Natural Product Radiance 3:203
Suffness M, Pezzuto JM (1991) Assays related to cancer Drug discovery In: Hostettman K (Ed) Methods in Plant Biochemistry Assays for bioactivity, Academic, London, New York. 6:71–133
Syu WJ, Shen CC, Don MJ, Ou JC, Lee GH, Sun CM (1998) Cytotoxicity of curcuminoids and some novel compounds from Curcuma zedoariae. J Nat Prod 61:1531–1534
Watanabe KM, Shibata M, Yano S, Cai Y, Shibuya H, Kitagawa I (1986) Antiulcer activity of extracts and isolated compounds From Zedoariae (Gajutsu) cultivated in Yakushima (Japan). Yakugaku zasshi 106:1137–1142
Acknowledgements
The first author, Lakshmi. S, would like to thank the Regional Cancer Centre, the Central Tuber Crops Research Institute, and the Regional Research Laboratory for the facilities to carry out the work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lakshmi, S., Dhanya, G.S., Joy, B. et al. Inhibitory effect of an extract of Curcuma zedoariae on human cervical carcinoma cells. Med Chem Res 17, 335–344 (2008). https://doi.org/10.1007/s00044-007-9069-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-007-9069-9