Abstract
In the present study, we screened newly synthesized antiviral aminopyrazoloquinoline derivatives for their cytotoxic and genotoxic potential in human normal and breast cancer cell lines using apoptosis and DNA adducts biomarkers. The compounds, along with the well-known antiviral drug acyclovir, were incubated with the normal (MCF-10A, MCF-12A) and cancer (MCF-7, MDA-MB-231) cell lines at 10, 50, and 100 μM for 72 h at 37°C. The most potent antiviral methoxy derivative (compound 3) was found to be more cytotoxic in the normal breast epithelial cell lines (MCF-10A and MCF-12A) and MDA-MB-231 cell lines at 50 μM. MCF-7 cells were found to be almost completely resistant to all these compounds while MDA-MB-231 cell lines were significantly killed by apoptosis. Acyclovir was ineffective in all these cell lines. We further tested these compounds using modulation of benzo[a]pyrene (BP)-DNA adduct formation in these cell lines. An inverse correlation was found between the degree of apoptosis and BP-DNA adduct levels for most of these compounds, although this seems to be the case only with the cancer cell lines. Our results suggest that the newly synthesized antiviral compounds have an associated risk of cytotoxicity and/or genotoxicity compared to acyclovir.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Acyclovir had been the drug of choice for herpes simplex virus (HSV) for several decades; however, the emergence of acyclovir-resistant isolates in immunocompromised patients and organ and bone marrow recipients has motivated the discovery of new antiviral compounds (Englund et al., 1990; Ljungman et al., 1990; Darville et al., 1998; Morfin and Thouvenot, 2003; Danve-Szatanek et al., 2004). Bearing this in mind, we synthesized several pharmacologically active aminopyrazoloquinoline derivatives (Fig. 1) with and without sugar moieties, which have been shown to possess different degree of antiviral activities against HSV type 1 (HSV-1) (Bekhit et al., 2004; Bekhit et al., 2005). The aldehydo-sugar derivatives of pyrazoloquinoline (compounds 4 and 5) inhibited HSV-1 replication (Bekhit et al., 2005). However, the precursors of these aldehydo-pentose derivatives exhibited more or less comparable anti-HSV-1 activity to the widely used drug acyclovir (Bekhit et al., 2005). 3-amino-7-methoxy-1H-pyrazolo[3,4-b]quinoline (compound 3) was found to be an equally effective antiviral compound as acyclovir on an equimolar basis (Bekhit et al., 2005).
Chemical structures of aminopyrazoloquinoline derivatives. Compound 1: 3-amino-1H-pyrazolo[3,4-b]quinoline; compound 2: 3-amino-7- methyl-1H-pyrazolo[3,4-b]quinoline; compound 3 : 3-amino-7-methoxy-1H-pyrazolo[3,4-b]quinoline; compound 4: aldehydo-D-arabinose{7-methyl-1H-pyrazolo[3,4-b]quinoline-3-yl}imine; compound 5: aldehydo-D-xylose{7-methoxy-1H-pyrazolo[3,4-b]quinoline-3-yl}imine; compound 6: acyclovir
The design of new drugs with the least possible genotoxic and/or clastogenic effects in patients is of utmost importance to limit or minimize serious side-effects. In the majority of cases, the cytotoxic and genotoxic potential are determined by the biomarkers of the cell cycle (e.g., apoptosis and viability) or effect-based biomarkers (e.g., sister chromatid exchanges, chromosomal aberrations, and strand breaks). Little attention has been given in the past to the biomarkers of exposure (DNA damage) to assess the genotoxic potential of the promising new drug candidates.
In the present study, we evaluated the cytotoxic and genotoxic potential of the selected antiviral aminopyrazoloquinoline derivatives using both apoptosis and carcinogen-DNA adduct modulation as biomarkers in various human normal and breast carcinoma cell lines. An association between degree of apoptosis and BP-DNA adduct level in different cell lines is discussed.
Materials and methods
Chemicals and cell lines
The aminopyrazoloquinoline derivatives were synthesized as described earlier (Bekhit et al., 2004; Bekhit et al., 2005). Human breast carcinoma cell lines (MCF-7, MDA-MB-231) and normal human breast epithelial cell lines (MCF-10A, MCF-12A) were purchased from the American Type Culture Collection (Rockville, MD, USA). All the cell culture reagents and media were obtained from the Sigma Chemical Company (St. Louis, MO, USA) except cholera toxin, which was purchased from Miller and Miller (Chemical) Ltd. (London, UK). A Vybrant apoptosis assay kit 2 was purchased from Molecular Probes Inc. (OR, USA). [γ-32P]ATP was purchased from ICN Pharmaceuticals (Costa Mesa, CA, USA). Other chemicals used for the 32P-postlabeling have been described elsewhere (Gupta 1996).
Cell culture, treatments, and analyses
MCF-7 and MDA-MB-231 cells were cultured in RPMI1640 media using 10% calf serum to its confluence. The MCF-10A and MCF-12A were cultured in Dulbecco’s modified Eagle’s medium/Ham’s nutrient mixture F12 (1:1, v/v) supplemented with cholera toxin (100 ng/ml), epidermal growth factor (20 ng/ml), insulin (10 μg/ml), hydrocortisone (0.5 μg/ml), and 5% calf serum. For the benzo[a]pyrene (BP)-DNA adducts modulation studies, cells were subcultured in 3–5 replicates using 60 mm dishes and treated with 100 μM of the test compounds dissolved in dimethyl sulfoxide (DMSO) (approximately 1%) for 24 h. With the change of fresh medium, BP (0.5 μM) was added to the treated cells for a further 24 h of incubation in a CO2 incubator at 37°C. The cells were harvested by trypsinization and kept frozen at −80°C until DNA isolation. BP-DNA adducts were analyzed by the nuclease P1-mediated 32P-postlabeling technique as described earlier (Gupta 1996; Arif et al., 2004a; Arif et al., 2004b).
For apoptosis and cell viability assays, the cells were treated with the test compounds (10, 50, and/or 100 μM) in triplicate for 72 h followed by analysis by flow cytometry using the annexin V kit as described earlier (Arif et al., 2006).
Statistical analysis
Values are presented as mean ± standard deviation (SD) (n = 3–5). The statistical significance was determined by Student’s t-test and a p value less than 0.05 was considered significant.
Results and discussion
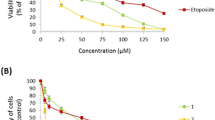
In the present study, we evaluated aminopyrazoloquinoline derivatives for cytotoxic potential using cell cycle progression markers. At relatively lower concentration (10 μM), all of these compounds showed no appreciable cell death, apoptosis or cell cycle arrest in the normal (MCF-12A) or breast cancer (MCF-7, MDA-MB-231) cells (data not shown). However, when increasing the concentration to 50 μM, both compound 1 and its methylated derivative (compound 2) were slightly effective in the normal and cancer cell lines (Fig. 2). At a concentration of 50 μM, compound 2 caused insignificant apoptosis in the normal MCF-10A and MCF-12A cell lines while no effect was observed in either of the cancer cells. No appreciable cell arrest was observed in the G2/M phase. Further increasing the concentration to 100 μM did not significantly change the scenario for compounds 1 and 2. However, the methoxy derivative of aminopyrazoloquinoline (compound 3), the most active anti-HSV-1 agent (Bekhit et al., 2005), showed the maximum toxicity. This compound at 50 μM dose caused 20–30% cell death in the normal cells while 30–55% of the cancer cells were killed (Fig. 2). The fraction of cells arrested in the G2/M phase was 20–35% in the case of normal cells, with approximately 30% apoptosis in MCF-12A; MCF-10A showed only about 5% apoptosis. However, in the MDA-MB-231 cell lines, about 35% of the cells were arrested in the G2/M phase with more than 45% apoptotic cells. Interestingly, at the 50 μM concentration, MCF-7 cells were the least affected with less than 10% apoptosis. Furthermore, at the 100 μM concentration, this compound killed more than 90% of the normal (MCF-10A, MCF-12A) and MDA-MB-231 cells with up to 80% apoptosis; however, only 50% cytotoxicity was observed among the MCF-7 cells with just 15% apoptosis. Accordingly, both ER cells (MCF-10A and MDA- MB-231) showed a maximum 80% apoptosis. The ER+ normal MCF-12A cells showed about 60% apoptosis. However, interestingly other ER+ malignant MCF-7 cells tolerated this compound very well, with less than 20% apoptosis.
Percentage of viable, apoptotic, and G2/M arrested cells following treatment with the test compounds at various concentrations. The data was normalized against the control (100% for viability or 0% for apoptosis). The empty bars represent 50 μM while solid bars show 100 μM concentrations of the compounds. The values are represented as mean ± SD (n = 3)
Furthermore, the aldehydo-sugar derivatives showed differential responses (Fig. 2). The arabinose derivative (compound 4) of the relatively ineffective compound 2 showed similar responses in MCF-10A, MCF-12A, and MCF-7 cells as was observed with the parent compound 2 except significant numbers (20–30%) of MCF-12A cells were arrested in the G2/M phase. However, the addition of arabinose sugar significantly affected the MDA-MB-231 cells, killing 40–60% of cells at both 50 and 100 μM concentrations with 20–50% apoptosis. Interestingly 20–30% of cells were arrested in the G2/M phase, similar to the MCF-12A cells. In contrast, the cytotoxic action of the most toxic methoxy derivative (compound 3) of aminopyrazoloquinoline was drastically inhibited by the addition of xylose sugar (compound 5). Surprisingly this coincided with their respective anti-HSV-1 activity (Bekhit et al., 2005) where the addition of xylose sugar drastically reduced the anti-HSV-1 activity of compound 3. At the 50 μM concentration, both normal and cancer cells showed no sign of cytotoxicity or apoptosis. However, increasing the dose to 100 μM killed about 50% of the normal cells (MCF-10A and MCF-12A) and caused 40% apoptosis. Surprisingly, both MCF-7 and MDA-MB-231 were almost completely resistant to the cytotoxic action of this compound, even at the 100 μM concentration. However, the widely used antiviral drug acyclovir did not cause any significant apoptosis, cytotoxicity or cell arrest in either of the cell lines at either of the 50 or 100 μM concentrations.
The resistance of the MCF-7 cell lines to any of these compounds compared to other cell lines regardless of their nonmalignant or malignant nature suggests the involvement of caspase-3 as one of the causes of apoptosis by these compounds. It is one of the executioners of the apoptosis and MCF-7 cells are deficient of caspase-3 (Liang et al., 2001). In contrast, the widely used anti-HSV-1 drug acyclovir did not cause cytotoxicity, apoptosis or cell cycle arrest in any of the cell lines. These new antiviral aminopyrazoloquinoline derivatives have great and comparable potential of competing with the acyclovir for the treatment of HSV-1 infection in resistant immunocompromised patients and bone and organ transplant recipients. However, these new antiviral compounds have an associated risk of side-effects in terms of cytotoxicity in other organs.
We further tested the genotoxic potential of the antiviral aminopyrazoloquinoline derivatives using BP-derived DNA adduct formation in various normal and malignant human breast epithelial cell lines. These compounds did not result in any type of adducts per se but instead modulated the BP-derived DNA adducts. The DNA adduct marked with an arrow in the panels A (Fig. 3) has been previously characterized as BP-7,8-dihydrodiol-9,10-epoxide-deoxyguanine (Arif et al., 2004a; Arif et al., 2004b). Differential modulation of BP-DNA adducts by all these compounds was noticed in various cell lines. In general, BP-DNA adducts formation in normal breast epithelial cells (MCF-10A, MCF-12A) were not affected by these compounds, except compound 3 which showed more than 40% reduction in the BP-DNA adduct levels in the MCF-12A cells. All other compounds, including acyclovir, were ineffective (Figs. 3 and 4). On the other hand, the scenario in the breast cancer cell lines, especially MCF-7, was quite different as they exhibited a 3.5-fold induction of BP-DNA adducts by compound 2. Compounds 3 and 4 also modulated the BP-DNA adduct levels by 2–2.5 times in the MCF-7 cells (Fig. 4). The remaining compounds, including acyclovir, were ineffective. However, the MDA-MB-231 cells showed an increase in the BP-DNA adduct level due to compound 1. The methoxy derivatives (compounds 3 and 5) significantly reduced BP-DNA adduct formation by more than 60% in the MDA-MB-231 cells. In agreement with other cell lines, acyclovir did not show any modulation of BP-DNA adducts in these cancer cell lines.
Representative 32P-adduct maps of DNA from various cells treated with benzo[a]pyrene (BP) (0.5 μM) in the absence (A) or in the presence of the indicated test compounds (B–G). DNA (10 μg) was digested, enriched and the adducts were analyzed by nuclease P1-mediated 32P-postlabeling assay as described in the text. The autoradiographs were exposed using the storage phosphor screens and scanned in a Typhoon 8600 imager. The arrow indicates that the adduct spot was derived from BP-7,8-dihydrodiol-9,10-epoxide-deoxyguanine (Arif et al., 2004a; Arif et al., 2004b)
Furthermore, acyclovir neither induced apoptosis nor modulated BP-DNA adduct levels in cancer or normal cells indicating that it is noncytotoxic and nongenotoxic at these concentrations.
Nevertheless, our results emphasize the idea of using more than one cell line along with multiple biomarkers in the screening of new compounds to reach a reliable conclusion on their cytotoxic, genotoxic, and/or anticancer potentials prior to proceed with preclinical and clinical studies.
References
Arif JM, Kunhi M, Siddiqui YM, El- Sayed KA, Orabi KY, Al-Hazzani AA, Al-Ahdal MN, Al-Khodairy FM (2004a) Role of intermediary biomarkers in determining the anticancer efficacy of marine compounds. Med Chem Res 13: 553–562
Arif JM, Kunhi M, Siddiqui YM, El- Sayed KA, Orabi KY, Al-Hazzani AA, Al- Ahdal MN, Al- Khodairy FM (2004b) Differential modulation of benzo[a]pyrene-derived DNA adducts in MCF-7 cells by marine compounds. Int J Cancer Prev 1: 259–268
Arif JM, Kunhi M, Bekhit AA, Subramanian MP, Al-Hussein KA, Aboul-Enein HY, Al-Khodairy FM (2006) Evaluation of apoptosis-induction by newly synthesized phthalazine derivatives in breast cancer cell lines. Asian Pacif J Cancer Prev 7:249–252
Bekhit AA, El-Sayed OA, Aboul-Enein HY, Siddiqui YM, Al-Ahdal MN (2004) Synthesis of aldehydo-sugar derivatives of pyrazoloquinoline as inhibitors of herpes simplex virus type 1 replication. J Enzyme Inhib Med Chem 19:33–38
Bekhit AA, El- Sayed OA, Aboul-Enein HY, Siddiqui YM, Al-Ahdal MN (2005) Evaluation of some pyrazoloquinolines as inhibitors of herpes simplex virus type 1 replication. Arch Pharm (Weinheim) 338:74–77
Danve-Szatanek C, Aymard M, Thouvenot D, Morfin F, Agius G, Bertin I, Billaudel S, Chanzy B, Coste-Burel M, Finkielsztejn L, Fleury H, Hadou T, Henquell C, Lafeuille H, Lafon ME, Le Faou A, Legrand MC, Maille L, Mengelle C, Morand P, Morinet F, Nicand E, Omar S, Picard B, Pozzetto B, Puel J, Raoult D, Scieux C, Segondy M, Seigneurin JM, Teyssou T, Zandotti C (2004) Surveillance network for herpes simplex virus resistance to antiviral drugs: 3-year follow-up. J Clin Microbiol 42:242–249
Darville JM, Ley BE, Roome AP, Foot AB (1998) Acyclovir-resistant herpes simplex virus infections in a bone marrow transplant population. Bone Marrow Transpl 22:587–589
Englund JA, Zimmerman ME, Swierkosz EM, Goodman JL, Scholl DR, Balfour HH (1990) Herpes simplex virus resistant to acyclovir. A study in a tertiary care center. Ann Intern Med 112:416–422
Gupta RC (1996) In: Technologies for Detection of DNA Damage and Mutations, Pfeifer GP (ed), Plenum, New York, pp. 45–61
Liang Y, Yan C, Schor NF (2001) Apoptosis in the absence of caspase 3. Oncogene 20:6570–6578
Ljungman P, Ellis MN, Hackman RC, Shepp DH, Meyers JD (1990) Acyclovir-resistant herpes simplex virus causing pneumonia after marrow transplantation. J Infect Dis 62:244–248
Morfin F, Thouvenot D (2003) Herpes simplex virus resistance to antiviral drugs. J Clin Virol 26:29–37
Acknowledgements
The authors would like to thank the Typhoon core facility for providing help in scanning of the radioactive maps. The study was approved by the Research Advisory Council (RAC no. 2030041). The majority of the work was carried out at Biological and Medical Research, King Faisal Specialist Hospital & Research Center, Riyadh, Saudi Arabia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arif, J.M., Kunhi, M., Subramanian, M.P. et al. Cytotoxic and genotoxic potentials of newly synthesized antiviral aminopyrazoloquinoline derivatives. Med Chem Res 17, 297–304 (2008). https://doi.org/10.1007/s00044-007-9065-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00044-007-9065-0