Abstract
Differences in the dispersal flight patterns among termite families are correlated with the difference between the two life history characteristics exhibited by this group: “separate-piece nesters” versus “single-piece nesters.” However, information remains limited on the phenology and the life history characteristics of single-piece nesters, impeding our understanding of this topic. We report the flight phenology of an Asian single-piece nester termite Neotermes koshunensis on Okinawa Island, Japan. In 1983–1984, a light-trap survey showed that N. koshunensis exhibited an extended dispersal flight period from late April to early November, peaking in June, with a female-biased sex ratio. Between 1983 and 2012, the collection of 134 whole colonies of N. koshunensis from the surrounding area confirmed the presence of alates and pre-alate nymphs within the colonies over 7 months, reflecting the extended flight season of this termite species, probably in association with the extended dispersal flight season. However, in some cases, alates and pre-alate nymphs were also retained in the colonies after the dispersal flight season (i.e., winter, from December to February). The daily number of trapped alates in 1983 was positively correlated with temperature and relative humidity; however, alate production inside the colony was also positively correlated with temperature, relative humidity, and precipitation. Thus, these environmental factors might promote the flight activity of this termite by enhancing alate production inside the colony. Furthermore, temperature also had a significantly positive effect in the model incorporating the density of alates in the colony, along with environmental factors; thus, temperature might facilitate the release of alate from colonies. The accumulation of information on the phenology and life history characteristics of alate advances our understanding of the different dispersal strategies used by termites, providing insights into how the different families have evolved.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The most common strategy used by termites to establish new colonies is through the production and flight of alates (winged imagos), with species-specific flight patterns occurring (reviewed by Nutting 1969). The dispersal pattern of termite alates is associated with their “life type,” rather than their phylogenetic status. For example, the flight pattern of the most primitive termite, Mastotermes darwiniensis (Mastotermitidae), is similar to that of the more derived termites belonging to the families of Rhinotermitidae and Termitidae (Nutting 1969; Neoh and Lee 2009a, b; Nalepa et al. 2001; Sangamma and Chimkod 2012). These termites are termed “separate-piece nesters” (sensu Abe 1987; Eggleton and Tayasu 2001). The alates of termites from this group exhibit a very concentrated flight period (in some cases, it is just a few days) that is distinct from other periods over the course of the dispersal flight season, which lasts several months (Medeiros et al. 1999; Nalepa et al. 2001; Martius 2003; Bourguignon 2009; Neoh and Lee 2009a, b).
Compared to separate-piece nesters, the families of Archotermopsidae and Kalotermitidae have the longer dispersal flight season, with a lower peak in flight activity, followed by a gentle increase (and subsequent decrease) in flight activity, because these termites continuously release small numbers of alates (Jones et al. 1988; Martius et al. 1996; Medeiros et al. 1999; Martius 2003; Huang et al. 2004a, b; Huang et al. 2007; Bourguignon 2009; Howell et al. 2009). These termites are called “single-piece nesters” (sensu Abe 1987; Eggleton and Tayasu 2001). The dispersal flight pattern of alates from these colonies is poorly understood because studies on the phenology of these termites in relation to their life history characteristics remain limited. Thus, information on the evolution and diversity of the dispersal strategies of alates in relation to the life cycle remains limited.
Here, we report the seasonality of disperser production and the phenology of dispersal flight in the field colonies of a single-wood nester termite Neotermes koshunensis (Kalotermitidae). We also investigate whether meteorological factors influence the dispersal flight patterns of this termite species.
Materials and methods
Study species
Neotermes koshunensis is distributed in China (Fujian, Guangdong, Guangxi, Hainan, Yunnan, Zhejiang), Taiwan, and Japan (Ryukyu Islands, Okinawa Islands) (Ikehara 1966, Krishna et al. 2013). Its colonies inhabit the dead branches of living and dead trees (Fig. S1). As a result, we were able to collect entire colonies. This species is classified as a dry-wood termite; however, they cannot eat hard dried wood. Usually, their nests are found in “green” (or living) trees (Fig. S1). This species has a linear caste development pathway, whereby all castes differentiate from pseudergates (older larvae, functional worker caste) after molting (Roisin 2000; Katoh et al. 2007).
Study site and trapping methods
Alates were collected using a light trap for 452 days from April 23, 1983 to July 17, 1984 in the subtropical laurel forest of the Ryukyu limestone area in Nishihara (N 26.24785°, E 127.76114°), Okinawa, Japan. Of note, our colony-sampling points (explained in a later paragraph) were at least 1 km (this was based on their estimated flight distance, Abe 1989) away from the light trap point of 1983 to 1984; thus, the results of our light trap surveys were not affected by our collection of colonies. The light trap was prepared by installing a 20 W fluorescent light and a 20 W black light over a plastic vessel (40 × 30 × 6 cm) containing water. The light trap was lit every day from 18:00 to 06:00 the next morning during the survey period, except for September 24–26, 1983, when a typhoon struck Okinawa Island. Trapped termites were collected every morning. The males and females were counted separately (Miyaguni et al. 2012). To ascertain the period during which dispersal flight occurred, we conducted two fixed-time surveys (on May 29 and June 4, 1983), during which we checked the trap every hour for 24 h, collecting alates from 08:00 in the morning until 08:00 the next morning. We assume that the number of alates collected by the light trap reflected dispersal flight activity in the field.
Pattern of disperser production in the field
To demonstrate seasonal changes in the disperser production of the N. koshunensis colony, we investigated seasonal changes in the ratio of each caste in the field colonies. A total of 134 wild colonies were collected during 1983–2012 around Nakagusuku and Nishihara, and were transferred to our laboratory. Colony members were removed from each wild colony by cutting the wood into small pieces. The number of caste members was counted, including small larvae, pseudergates, early nymphs, pre-alate nymphs, alates, pre-soldiers, soldiers, queens, kings, and male neotenics (Roisin 2000; Katoh et al. 2007; Miyaguni et al. 2013). In the analyses, the number of individuals from all colonies was pooled by collection month, without considering the year of collection. Then, the ratio of pre-alate nymphs and alates to monthly termite members was calculated. Because alates differentiate from pseudergates via early nymphs and pre-alate nymphs, it is important to calculate the number of pre-alate nymphs and alates in relation to the sum of the four castes when estimating the timing of disperser production.
Data analysis of the factors affecting flight activity
Previous studies have reported the dispersal flight behavior of termites by analyzing how the number of trapped alates is correlated with meteorological factors, based on the assumption that meteorological factors trigger termite flight and flight activity (Nutting 1969; Neoh and Lee 2009a, b). However, the number of trapped alates is limited by the number of alates in field colonies. The number of trapped alates might increase because the increase in the production of alates is independent of a weather-based trigger. Moreover, the production of dispersers might be related to long-term environmental conditions. We conducted our data analysis at three different stages to study when alates are released by the colonies.
In the first stage, the relationship between the production of dispersers (alates and pre-alate nymphs) inside the colony and long-term environmental conditions was investigated. The monthly means of air temperature, atmospheric pressure, relative humidity, precipitation and wind velocity during 1983–2012 at the Naha Meteorological Observation Point (located 10 km southwest of the study location) were obtained from the database of the Japan Meteorological Agency (http://www.jma.go.jp/jma/index.html). The relationship between the ratio of alates inside the colony and each environmental condition each month was analyzed by Pearson’s product moment correlation coefficient. The relationship between the ratio of pre-alate nymphs and each environmental condition each month was also analyzed using the same method.
In the second stage, the relationship between the release of alates from the colony and environmental conditions was investigated by generalized linear models (GLM) with a negative binominal error distribution. The number of trapped alates from April 25 to November 4 in 1983 was used as the response variable. The daily means of the air temperature, atmospheric pressure, relative humidity, precipitation, and wind velocity during same period at the Naha Meteorological Observation Point were used as the explanatory variables.
In the third stage, we used GLM with a Poisson error distribution and log-link function to explain the number of trapped alates. For explanatory variables, we included the monthly ratio of alates and meteorological factors. The number of alates caught in the light trap during the survey period in 1983 was re-counted to obtain the numbers of alates that were trapped each month, which were used as a response variable. The monthly means of the air temperature, relative humidity, atmospheric pressure, wind velocity, and total precipitation from April 1983 to March 1984 at the Naha Meteorological Observation Point were used.
All analyses were performed using R software, version R 3.2.2 (R Development Core Team 2015).
Results
Flight pattern and sex ratio
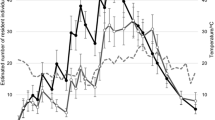
In 1983, dispersal flight started in late April and ended in early November (Fig. 1). The number of alates increased gradually until numbers peaked on June 29 (209 alates, 6.6% of all alates in this year), after which numbers declined gradually until the end of the season (November). From May 27 to August 8 (74 days), dispersal flight occurred daily. The two fixed-time surveys showed that dispersal flight started after 20:00, and ended at 02:00 or 04:00 the next morning (Fig. 2). The largest hourly catch was trapped between 20:00 and 21:00. We observed that the alates of N. koshunensis flew near the trap, even though tandem behavior (when males maintained almost constant contact with the posterior of the female’s abdomen using his antennae until finding a suitable nesting site; Nutting 1969; Stuart 1969) was not observed. In total, 3169 alates (1764 females and 1405 males) were collected. The (male) sex ratio was 0.443; thus, it was significantly skewed towards females (binomial test, P < 0.0001) (Fig. 1).
In 1984, dispersal flight began on May 6 and peaked in June, as observed in 1983 (Fig. 1). This survey was discontinued on July 17, 1984. In this year, equal numbers of male and female alates (757 females and 757 males) were collected. Of note, the biased sex ratio recorded in 1983 started after dispersal flight peaked in June (Fig. 1).
Pattern of disperser production in field colonies
Seasonal changes in the ratio of pre-alate nymphs and alates to monthly numbers of termite members in the field are shown in Fig. 3 (see also Table S1). The ratio of alates increased rapidly from May, peaked in June, and decreased gradually until October. During this period, a high percentage of colonies contained alates. Some colonies (1, 1, and 5 colonies in December, January, and February, respectively) retained 1–173 alates, even in winter (December–February). The ratio of pre-alate nymphs increased rapidly and peaked in May, decreasing rapidly after June. The ratio of alates exceeded that of pre-alate nymphs in August and September. In the colonies where alates were retained, the number of alates exceeded pre-alate nymphs in zero out of 11 colonies in May, six out of 23 colonies in June, three out of seven colonies in July, nine out of 10 colonies in August, and five out of eight colonies in September. The ratio of pre-alate nymphs also exhibited a small peak in October. In the colonies that retained alates, the number of pre-alate nymphs exceeded alates in five out of six colonies in October. Furthermore, some colonies (two, one, and eight colonies in December, January, and February, respectively) retained one to 552 pre-alate nymphs, even in the winter (December–February), reflecting our observations of alates.
Seasonal change in the ratio of dispersers in field colonies. Solid lines show the ratio of dispersers (black represents alates, while gray represents pre-alate nymphs on the left side of the lower vertical axis). The broken line shows the ratio of colonies with alates (on the right side of the upper vertical axis). Numbers in parentheses denote sample size, i.e., the numbers of colonies collected each month (on the right side of the upper vertical axis). Gray bar shows the monthly number of dispersal flight alates collected by the light trap in 1983 (on the right side of the lower vertical axis)
Relationship between disperser production in field colonies and environmental factors
The rate of alate production inside the colonies was significantly correlated with the monthly means of air temperature, atmospheric pressure, relative humidity, and precipitation; however, no significant correlation was found between the rate of alate production and monthly mean wind velocity (Fig. 4). The increase in the rate of alate production was correlated with increasing temperature, relative humidity, and precipitation (Fig. 4). Atmospheric pressure was correlated with a decrease in the rate of alate production (Fig. 4). No significant correlations were found between the rate of pre-alate nymph production and any of the environmental factors.
Relationship between disperser production of the colony and monthly environmental conditions: a seasonal change in the ratio of dispersers (solid black lines = alates, solid gray lines = pre-alate nymph) in the colony and environmental factors (broken lines); b correlations between the ratio of alates in the field colonies and the environmental factors
Relationship between daily flight activity and environmental factors
Multivariate GLM with negative binominal distributions showed that daily flight activity was significantly correlated with air temperature, atmospheric pressure, relative humidity, and precipitation (Table 1). The increase in daily flight activity by alates was correlated with increasing air temperature and relative humidity (Table 1). In comparison, the decline in daily flight activity was correlated with increasing atmospheric pressure and precipitation (Table 1). Wind velocity had no significant relationship with daily flight activity.
Data analysis of the factors affecting monthly flight activity
Multivariate GLM with Poisson distributions showed that monthly flight activity was significantly correlated with the density of alates in the field colonies and air temperature. However, the estimated coefficient of temperature was much smaller than that of the density of dispersing alates in field colonies (Table 2).
Discussion
The phenology of dispersal flight in N. koshunensis reflects that described for the dispersal of alates of other single-wood nesters in previous studies (Medeiros et al. 1999; Martius 2003; Huang et al. 2004b; Cabrera and Scheffrahn 2005; Huang et al. 2007; Bourguignon 2009). In our study, the dispersal flight period of N. koshunensis lasted several months (i.e., from May to October). A gradual increase and a gradual decrease in flight activity were observed through the season (Fig. 1), with just 6.6% of all alates being captured on the days when most individuals were trapped. In comparison, separate-piece nesters exhibit major flight days across a given season, implying the synchronized release of alates by local colonies, which might enhance outbreeding (Jones 1981). Furthermore, in the current study, flight continued for as long as 6–8 h each day (Fig. 2). Thus, the synchronous release of similar volumes of alates, as recorded for multiple-wood nesters, was not likely. We did not assess the flight activity of individual colonies; thus, we cannot state whether the observed continuous flight arises due to differences in the timing of the intensive release of alates by each colony or whether each colony releases only a few alates continuously. Further studies are required to examine the pattern of alate release by each colony.
Previous studies investigating sexual differences in the development of termites did not report protogyny (the emergence of adult females earlier than males), whereas protandry (the emergence of adult males earlier than females) has been reported for some termite species (Luykx 1986; Matsuura 2006; Vargo and Husseneder 2011). Our light trapping study in 1983 showed that more females emerged before males (Fig. 1); thus, protogyny might exist in this taxon. However, further evidence is required, because the sexual differences in phototactic responses might have influenced our results (Cheng et al. 2016).
We also measured how the disperser production phase in N. koshunensis shifted over time by analyzing the ratio of pre-alate nymphs to alates in colonies in the field (Fig. 3). The observed trend in the flight activity of N. koshunensis based on light traps reflected our direct counts of dispersers in the field-collected colonies (Figs. 1, 3). Furthermore, our results were consistent with those recalculated from colony data in Appendix of Maki and Abe (1986) (Table S2).
Our study supported previous studies (reviewed by Nutting 1969) in that certain termite species appear to produce dispersers in relation to the annual biological clock or meteorological cues, which is reflected by the seasonal production and flight of alates (possibly during the optimal period for successful reproduction by the alates of a given species). However, a noticeable number of N. koshunensis colonies also retained alates and pre-alate nymphs in winter, which is outside of the dispersal flight period (Fig. 1). A previous study also recorded the presence of alates in four out of 10 colonies during January and February (Maki and Abe 1987). This phenomenon might arise because some alates and pre-alate nymphs produced during the summer might remain in the colony after the dispersal flight season for use in the subsequent season (i.e., the following year). As another possible explanation, the environmental conditions preceding flight might enhance the development of alates inside colonies; however, flight did not occur by the lack of daily environmental conditions that trigger the release alates. Consequently, these alates were kept in the colony until the next swarming season. Alternatively, the production of alates in winter might represent an alternative strategy of the colony to potential adverse conditions, different to the typical seasonal disperser production by the colony. For instance, a decline in food resources led to the production of alates in Cryptotermes secundus (Kalotermitidae) (Korb and Lenz 2004). The frequency of colonies with a decline in food resources in N. koshunensis was unknown; however, this process occurs in all of the colonies of single-piece nesters because their colonies never move to other trees. Thus, a similar mechanism might explain the off-season disperser production of N. koshunensis. Future studies should investigate the status of colonies in relation to the production of disperser in this species.
Our study suggested that alate production inside the colony is positively correlated with temperature, relative humidity, and precipitation (Fig. 4). This result was supported by previous studies, which showed that the development of termite individuals is enhanced at higher temperatures and relative humidity (Nutting 1969; Mensa-Bonsu 1976). The negative relationship between alate production and atmospheric pressure might be pseudo-correlated, because atmospheric pressure was negatively correlated with temperature and relative humidity (Fig. 4). The daily number of trapped alates in 1983 was positively correlated with temperature and relative humidity (Table 1); however, the main effect of these environmental factors might enhance the flight activity of this termite by enhancing alate production inside the colony (not a direct trigger of the colony’s release of alate) (Figs. 3, 4). Alternatively, air temperature had a significantly positive effect, even in the model incorporating the density of alates in the colony along with environmental factors (Table 2). Thus, air temperature might increase the dispersal flight of alates from colonies. Moreover, precipitation might also be associated with the onset of flight by alates present in the colonies, because it has the opposite effect on alate production inside the colony with flight activity in the field (Fig. 4; Table 1). The negative relationship between the daily number of trapped alates and atmospheric pressure seemed to be pseudo-correlated. The environmental factors that enhance or lessen alate release from colonies need to be quantified through controlled experiments under laboratory conditions.
This single-wood nester exhibits less intensive flight behavior than multiple-wood nesters. The selective advantage of releasing alates at a low intensity over a long period remains unclear. A previous study predicted that the flight season of Kalotermitidae termites would reflect their tolerance to high temperatures and preference for dry wood, because the number of catches and frequency of individuals in this family was higher at the end of the dry season (Medeiros et al. 1999). However, because the flight peak of N. koshunensis matched the peak period of precipitation in Okinawa, this prediction was not supported, at least for this termite species. The ability of termites to adjust to changes in resource availability to optimize colony founding might be beneficial. For instance, typhoons approach Okinawa Island from May to December (Japan Meteorological Agency, http://www.jma.go.jp/jma/index.html), which is the same period of N. koshunensis dispersal flight activity. Typhoons cause parts of living trees to break off, making them optimal resources for alates to establish colonies. In contrast, alates that disperse and establish colonies before a typhoon might be at a disadvantage. Thus, the strategy of releasing small numbers of alates over an extended period might be important for “hedge betting” the timing of investment in colonies in environments where it is difficult to predict the future climate. In conclusion, further quantification of this strategy is required to determine the adaptive capacity of termites.
References
Abe T (1987) Evolution of life types in termites. In: Kawano S, Connell JH, Hidaka T (eds) Evolution and coadaptation in biotic communities. University of Tokyo Press, Tokyo, pp 125–148
Abe T (1989) Ecology of termites—introduction to tropical ecology. University of Tokyo Press, Tokyo (in Japanese)
Bourguignon T, Leponce M, Roisin Y (2009) Insights into the termite assemblage of a neotropical rainforest from the spatio-temporal distribution of flying alates. Insect Conserv Divers 2(3):153–162
Cabrera BJ, Scheffrahn RH (2005) Western Drywood termite, Incisitermes minor (Hagen) (Insecta: Isoptera: Kalotermitidae). Entomol. and Nematol. Dept., Florida Coop. Ext. Serv., IFAS, Univ. Florida Publ. EENY-248
Cheng WJ, Zheng XL, Wang P, Zhou LL, Si SY, Wang XP (2016) Male-biased capture in light traps in Spodoptera exigua (Lepidoptera: Noctuidae): Results from the studies of reproductive activities. J Insect Behav 29:368–378
Eggleton P, Tayasu I (2001) Feeding groups, lifetypes and the global ecology of termites. Ecol Res 16:941–960
Howell HN Jr, Austin JW, Gold RE (2009) Swarming Dates and Distribution of Zootermopsis laticeps Banks (Isoptera: Termopsidae) Alates in El Paso County, Texas. J Agric Urban Entomol 26:11–21
Huang ZY, Dai ZR, Zhong JH, Qian X, Liu BR, Xia CG, Huang HT, Xia F, Yang RH, Zhang RL (2004a) Swarm periods of primary reproductives Cryptotermes domesticus. Entomol Knowl 41:236–238
Huang ZY, Dai ZR, Zhong JH, Qian X, Liu BR, Xia CG, Huang HT, Xia F, Yang RH, Zhang RL (2004b) Studies on influence of temperature, relative humidity and atmosphere to swarming of primary reproductives in Cryptotermes domesticus (Haviland) (Isopterra: Kalotermitidae). Nat Enemies Insects 26:126–131
Huang ZY, Qian X, Zhong JH, Xia CG, Hu J (2007) Progress of biological studies on primary reproductives in Cryptotermes domesticus (Isopterra: Kalotermitidae). Sociobiology 50:599–605
Ikehara S (1966) Distribution of termites in Ryukyu Archipelago. Bulletin of Arts and Science Division, University of the Ryukyus. Math Nat Sci 9:49–178
Japan Meteorological Agency (2017) http://www.jma.go.jp/jma/index.html. Accessed 03 Feb 2017
Jones SC (1981) Studies of dispersal, colony caste and sexual composition, and incipient colony development of Pterotermes occidentis (Walker) (Isoptera: Kalotermitidae). Sociobiology 6:221–242
Jones SC, La Fage JP, Howard RW (1988) Isopteran sex ratios: phylogenetic trends. Sociobiology 14:89–156
Katoh H, Matsumoto T, Miura T (2007) Alate differentiation and compound-eye development in the dry-wood termite Neotermes koshunensis (Isoptera, Kalotermitidae). Insect Sociaux 54:11–19
Krishna K, Grimaldi DA, Krishna V, Engel MS (2013) Treatise on the Isoptera of the world: vol 2, Basal families. Bull Am Mus Nat Hist 377:201–621
Korb J, Lenz M (2004) Reproductive decision-making in the termite, Cryptotermes secundus (Kalotermitidae), under variable food conditions. Behav Ecol 15:390–395
Luykx P (1986) Termite colony dynamics as revealed by the sex-and caste-ratios of whole colonies of Incisitermes schwarzi Banks (Isoptera: Kalotermitidae). Insectes Soc 33(3):221–248
Maki K, Abe T (1986) Proportion of soldiers in the colonies of a dry wood termite, Neotermes koshunensis (Kalotermitidae). Physiol Ecol Japan 23:109–117
Martius C (2003) Rainfall and air humidity: non-linear relationships with termite swarming in Amazonia. Amazoniana 17:387–397
Martius C, Bandeira AG, da Silva Medeiros LG (1996) Variation in termite alate swarming in rain forests of central Amazonia. Ecotropica 2:1–11
Matsuura K (2006) Early Emergence of males in the termite Reticulitermes speratus (Isoptera: Rhinotermitidae): protandry as a side effect of sexual size dimorphism. Ann Entomol Soc Am 99:625–628
Mensa-Bonsu A (1976) The biology and development of Porotermes adamsoni (Froggatt) (Isoptera, Hodotermitidae). Insectes Soc 23:155–166
Gomes da Silva Medeiros L, Bandeira AG, Martius C (1999) Termite swarming in the northeastern Atlantic rain forest of Brazil. Stud Neotrop Fauna Environ 34:76–87
Miyaguni Y, Sugio K, Tsuji K (2012) Refinement of methods for sexing instars and caste members in Neotermes koshunensis (Isoptera, Kalotermitidae). Sociobiology 59:65–68
Miyaguni Y, Sugio K, Tsuji K (2013) The unusual neotenic system of the Asian Dry Wood Termite, Neotermes koshunensis (Isoptera: Kalotermitidae). Sociobiology 60:1217–1222
Nalepa CA, Miller LR, Lenz M (2001) Flight characteristics of Mastotermes darwiniensis (Isoptera, Mastotermitidae). Insect Soc 48:144–148
Neoh KB, Lee CY (2009a) Flight activity and flight phenology of the Asian subterranean termite, Coptotermes gestroi (Blattodea: Rhinotermitidae). Sociobiology 54:521–530
Neoh KB, Lee CY (2009b) Flight activity of two sympatric termite species, Macrotermes gilvus and Macrotermes carbonarius (Termitidae: Macrotermitinae). Environ Entomol 38:1697–1706
Nutting WL (1969) Flight and colony foundation. In: Krishna K, Weesner FM (eds) Biology of Termites vol 1. Academic Press, New York, pp 233–282
R Development Core Team (2015) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
Roisin Y (2000) Diversity and evolution of caste patterns. In: Abe T, Bignell DE, Higashi M (eds) Termites: evolution, sociality, symbioses, ecology. Kluwer Academic Publishers, Dordrecht, pp 95–119
Sangamma I, Chimkod VB (2012) Swarming behavior of the termites, Odontotermes brunneus and Odontotermes wallonensis. World J Sci Technol 2:1–4
Stuart AM (1969) Social behavior and communication. In: Krishna K, Weesner (eds) Biology of Termites vol 1. FM, Academic Press, New York, pp 193–232
Vargo EL, Husseneder (2011) Genetic structure of termite colonies and populations. In: Bignell DE, Roisin Y, Lo N (eds) Biology of termites: a modern synthesis. Springer, pp 247–321
Acknowledgements
The authors thank late Prof. Takuya Abe for providing suggestions and encouragement on fieldwork. We also thank Dr. Shigeto Dobata and Dr. Hirotaka Tanaka for providing advice on the data analysis, and the anonymous referees for their constructive advice.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Sugio, K., Miyaguni, Y. & Tayasu, I. Characteristics of dispersal flight and disperser production in an Asian dry-wood termite, Neotermes koshunensis (Isoptera, Kalotermitidae). Insect. Soc. 65, 323–330 (2018). https://doi.org/10.1007/s00040-018-0616-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00040-018-0616-9