Abstract
Slave-making ants exploit the worker force of host colonies permanently and have to make recurrent raids in order to replenish the slave’s stock. Some of these parasite species exploit different host species and few studies so far have been devoted to host species recognition mechanisms. Here, we tried to determine if opportunist slave-making ants using different host species rely on innate or experience-induced preferences to discriminate host from non-host species. We show that Myrmoxenus ravouxi slave-making workers are not only more aggressive toward heterocolonial host and potential host species workers when compared with non-host species workers, but also toward heterocolonial host workers than toward heterocolonial conspecifics. Moreover, M. ravouxi workers display more antennations and contacts toward the heterocolonial host species when compared with the non-host species. We also show that they do not discriminate between homocolonial and heterocolonial conspecifics. Together, our results suggest that this opportunistic slave-making ant species may have a complex social recognition template based on both innate and experience-based mechanisms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Slave-making ants invade and periodically raid neighboring colonies of their host species to pillage their brood (Buschinger, 1986, 2009), creating “chimeric” societies of two or more species. With a lifespan of sometimes more than 10 years, these colonies may comprise individuals from different colonies and/or species with no overt conflict. The slave brood is brought back from raided host colonies, following a typical behavioral sequence (Buschinger et al., 1980).
For example, in the slave-making ant Myrmoxenus ravouxi, around the end of spring, parasite workers prospect around the nest until they find a host colony. Then, when a scout is successful, it returns to its nest and stimulates her parasite nestmates. This “group recruitment” will launch the raid itself (Winter, 1979). After almost all defenders have fled or have been killed by the parasite workers, the members of the parasite colony (both parasites and slaves) take the brood and bring it back to their nest, where it will emerge. M. ravouxi is an opportunistic slave-maker parasitizing more than five Temnothorax species (Buschinger and Winter, 1983; Buschinger, 1989, 1997; Seifert, 2007). Such a strategy could represent an adaptive advantage for the parasite, thus accounting for the wider repartition area of M. ravouxi within its genus (Buschinger, 1997; Seifert, 2007). Yet, it is still crucial for a slave-making worker to recognize its different host from the non-host species during raids. Indeed, workers from non-host species, more distant phylogenetically (Emery, 1909; Beibl et al., 2005), may not be suitable nurses for the parasite brood. This could increase the mortality rate and impact the parasitized colonies’ fitness. Moreover, non-host defenses may be more difficult to overcome for parasite workers, as social parasites often exhibit specific adaptations to their host species (Lenoir et al., 2001; Foitzik et al., 2001, 2003; Brandt and Foitzik, 2004). Therefore, discrimination abilities in parasite species between potential hosts and other species are likely to evolve to be more efficient to discriminate suitable host species.
Members of a colony use a neural template of a common colonial label, which is learned as early as the pre-imaginal development phase in ants (Isingrini et al., 1985), to discriminate nestmates from aliens (Lenoir et al., 1999). Inside the colony, individual chemical cues are blended together in the common label via allogrooming, passive contacts and trophallaxis (Soroker et al., 1994, 2003). This mechanism has been termed the “Gestalt” model by Crozier and Dix (1979).
Experiments with artificial mixed-species colonies of ants have shown that workers were less agressive toward non-nestmates when their colonial odor was more complex (Errard, 1994; Errard et al., 2006). This has been attributed to the fact that a richer colonial label leads ants to form a broader recognition template that increases their tolerance threshold, leading to more frequent acceptation errors (Reeve, 1989; Errard et al., 2006). Slave workers raise and feed parasite brood until their emergence and forage for the entire colony, while slave-making workers are most of the time inactive inside the nest (Buschinger, 1986). Emerging parasite workers may thus learn the colonial label essentially from the interactions with their slave workers. They may develop also a larger colonial template which reduces their ability to discriminate their host from their own species (Errard, 1994; Errard et al., 2006).
Myrmoxenus ravouxi often lives in areas where different host species are present. Nonetheless, parasitized colonies with more than one enslaved species are rare and we do not know if this is because the pillaged brood from a different host species is destroyed by slaves or if parasite workers exclusively raid neighboring colonies of the imprinted host species. Indeed, we know that the species of the actual slave workers may influence slave-maker workers behavior, since expressed preferences for a more common host in a population may yield to adaptive plasticity, particularly during raids (Schumann and Buschinger, 1995). Nonetheless, the authors of this study also demonstrated that workers from the slave-making ant Chalepoxenus muellerianus, enslaving two different host species, displayed an innate preference toward their main host species, irrespective of the species they were reared with.
In this experiment, we chose to test if a highly opportunist slave-making parasite was able to discriminate between non-host, potential host, host and parasite workers (homo or heterocolonial for the last two) to assess if these social parasites rely on innate or experience-induced preferences to discriminate their host species and raid neighboring colonies.
We studied whether M. ravouxi could discriminate (1) between a familiar (actual) and non-familiar host species and (2) between a potential (unfamiliar) host species and a non-host species. We monitored slave-making workers’ aggressiveness and affiliative behaviors, such as antennations and contacts, to test for host species discrimination when different social stimuli were presented. We also tested whether M. ravouxi workers exhibited a different behavior toward their own species. Indeed, the Gestalt model predicts that the colonial odor will be homogenized in a mixed-species colony, making species discrimination difficult (Errard, 1994; Errard and Hefetz, 1997; Errard et al., 2006). However, the behavioral repertoire and the role of slaves and slave makers in parasitized colonies are very different (Buschinger and Winter, 1983; Buschinger, 1986) and species recognition in this context could allow better productivity of parasitized colonies, which could for example increase raid efficiency.
Methods
Species
We chose M. ravouxi as our focal species. This species is a slave-making social parasite with a wide distribution in Europe ranging from France to Greece (Buschinger, 1997). It is known to parasitize several species of the diversified genus Temnothorax (Buschinger and Winter, 1983, Buschinger, 1989, Buschinger, 1997), among which are Temnothorax unifasciatus and T. rabaudi. We used the main host (T. unifasciatus) and a potential host species (T. rabaudi) from an M. ravouxi parasitized population to assess if the social parasite was able to discriminate between its host, a potential host and a non-host species (T. nylanderi).
We collected the parasite (N = 12), parasite-free host (N = 14), potential host (N = 9) and non-host (N = 7) colonies in Vaison-la-Romaine (44°14′N, 5°04′E) in August 2006. Colonies were reared at the laboratory in small plastic boxes (15 × 10 × 5 cm) with a plaster soil as foraging area, in a nest made from two microscope slides superimposed with a 1-mm free space between them. All colonies were fed once a week with honey and fruit flies. The experiments took place in the late spring, from May to June 2007, after a 3-month wintering period at 8 °C from December to March. The photoperiod was 12/12 h with a night temperature of 15 °C and a day temperature of 22 °C at the time of the experiment. The humidity was kept around 60 %.
Experimental design
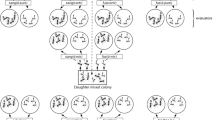
We used dyadic presentations to test the discrimination abilities of M. ravouxi workers. These comparisons could be categorized in “nestmates versus non-nestmates” and “host versus non-host” recognition tasks (Table 1).
Our experimental device was a circular arena (3 cm diameter) where two workers (used as stimuli) were tied at opposite sides with a thin nylon filament between their head and thorax. To limit moves and reciprocal interactions, these workers were also anesthetized beforehand with carbon dioxide for 15 s. Then, we introduced a slave-making worker in the arena and covered the device with a thin glass slide. No ant was used for more than one test, as experimental focus or as social stimuli.
Behavioral observations
Our tests lasted for 10 min. We recorded mean antennation and close-contact durations, which we assume are representative, respectively, of the interest for the congener and affiliative behaviors. The intensity of the agonistic response of M. ravouxi workers during the tests was assessed using the mean duration of bites. Stinging was also observed but less often than bites, maybe because parasite workers preferentially display this behavior during raids. We discarded the tests were no contact occurred within 2 min.
Comparisons were performed using permutation tests for paired samples. All statistical tests were implemented with StatXact (Cytel Studio, version 8.0.0, 2007).
Results
Antennations
Myrmoxenus ravouxi workers antennated heterocolonial workers from the host species T. unifasciatus longer than workers from the non-host species T. nylanderi (Fig. 1; Permutation test; t = −2.848, P = 4.8.10−4). Other comparisons were not statistically different (P > 0.368).
Mean duration of antennation in seconds (±SE) of M. ravouxi workers during test choices with homocolonial and heterocolonial M. ravouxi parasites (Homoc. parasite and Heteroc. parasite), homocolonial and heterocolonial T. unifasciatus slaves (Homoc. and Heteroc. slaves), unparasitized T. unifasciatus hosts (Unpar. host), potential T. rabaudi hosts (Pot. host) and T. nylanderi non-hosts (Non-host). ***P < 0.001
Contacts
Myrmoxenus ravouxi workers stayed more in contact with their parasite sisters than with their slaves (Fig. 2; Permutation test; t = 2.384, P = 0.0134). They also remained for more time in contact with heterocolonial free-living host workers from the host species T. unifasciatus than with workers from the non-host species T. nylanderi (t = −2.169, P = 9.8.10−4). Other comparisons were not significant (P > 0.127).
Mean duration of contact in seconds (±SE) during test choices with homocolonial and heterocolonial M. ravouxi parasites (Homoc. parasite and Heteroc. parasite), homocolonial and heterocolonial T. unifasciatus slaves (Homoc. and Heteroc. slaves), unparasitized T. unifasciatus hosts (Unpar. host), potential T. rabaudi hosts (Pot. host) and T. nylanderi non-hosts (Non-host). *P < 0.05, ***P < 0.001
Bites
Myrmoxenus ravouxi workers were more aggressive toward T. unifasciatus heterocolonial host workers than toward M. ravouxi heterocolonial parasite workers (Fig. 3; permutation test; t = −1.831, P = 0.0312). They also bit more T. unifasciatus heterocolonial host workers than T. nylanderi non-host workers (t = −1.96, P = 0.0156). Finally, they aggressed more workers from the potential host species T. rabaudi than workers from the non-host species T. nylanderi (t = −2.198, P = 9.8.10−3). Other comparisons were not statistically different (P > 0.125).
Mean duration (logarithmic scale) of bites in seconds (±SE) during test choices with homocolonial and heterocolonial M. ravouxi parasites (Homoc. parasite and Heteroc. parasite), homocolonial and heterocolonial T. unifasciatus slaves (Homoc. and Heteroc. slaves), unparasitized T. unifasciatus hosts (Unpar. host), potential T. rabaudi hosts (Pot. host) and T. nylanderi non-hosts (Non-host). *P < 0.05, **P < 0.01
Table 1 summarizes our different results.
Discussion
In heterospecific encounters, slave-making workers displayed more aggressive behaviors against workers from host and potential host species than against non-host workers. Moreover, parasite workers did not bite more workers from the host than from the potential host species. Taken together, these results suggest that M. ravouxi workers discriminate host species from non-host species, but not their host species from a potential host species. Our colonies were kept isolated in the laboratory from autumn to spring, so that no familiarization effect to any species could affect the behavioral response of our workers during the tests (Heinze et al., 1996; Knaden and Wehner, 2003; Sanada-Morimura et al., 2003). Therefore, M. ravouxi workers’ discrimination between host—familiar or not—and non-host species is at least partially independent of previous experience.
Because slave-making ants depend on their host species during the entire colony’s life, raids are necessary to replenish the worker force as the slave stock decreases with time. Thus, the selection pressure is strong on parasite societies to be able to find and pillage host colonies and to discriminate suitable host from non-host species (Foitzik et al., 2001, 2003; Hare and Alloway, 2001; Blatrix and Herbers, 2003; Brandt and Foitzik, 2004; Fischer and Foitzik, 2004; Brandt et al., 2005; Fischer-Blass et al., 2006). Therefore, a genetically based recognition template of potential hosts appears all the more adaptive than it can allow host shifting or raiding on different species according to the most commonly available host species. Indeed, M. ravouxi exploits at least six different hosts (Buschinger, 1989; Seifert, 2007). Phylogenetic data suggest that these host species are closely related to each other, and that the Myrmoxenus genus has already a long independent evolutionary history from the Temnothorax genus (Beibl et al., 2005). We can thus hypothesize that this slave-making species derived from and/or parasitized a common ancestor to its group of host species (Emery, 1909; Baur et al., 1995; Parker and Rissing, 2002; Sumner et al., 2004). These species may share some common cuticular compounds, which may be used by the slave maker to recognize them as potential hosts. Moreover, M. ravouxi olfactory system may have been selected for better discrimination of host species, as a consequence of a coevolutionary process (Thompson, 1994) between this slave-making ant and its host species. However, the fact that we recorded no difference in aggressiveness of M. ravouxi workers for the host-potential host comparisons could also be interpreted as a consequence of a standardized environment, as we kept in isolation our colonies for 6 months prior to the tests. Some studies indeed demonstrated that diet and environment could influence the colony odor (Crosland, 1989; Liang and Silverman, 2000; van Zweden et al., 2009b; see Sturgis and Gordon, 2012). Still, van Zweden et al. (2009a) also showed that a homogenized environment will not affect the discrimination ability of ants, suggesting that heritable cues play the most important part in nestmate recognition (van Zweden et al., 2010).
Parasite workers antennated and stayed more in contact with their host species workers than with the non-host workers, displaying non-agonistic and affiliative behaviors toward them. This may be related to the fact that the recognition template of M. ravouxi workers comprises some of the familiar host species-specific chemical cues. In the study of Schumann and Buschinger (1995), C. muellerianus workers imprinted on the potential host label inspected potential host species nest entrances more often than workers imprinted on the main host species, so that an effect of experience could not be entirely rejected. In our experiment, M. ravouxi workers were certainly more familiar with the species-specific chemical cues of T. unifasciatus, and their recognition template is likely to include them, at least partially. This could explain here the more frequent affiliative behaviors displayed toward the host species. Heterocolonial workers from their host species may elicit affiliative or aggressive behaviors depending on whether they are perceived under or over the tolerance threshold of parasite workers (Reeve, 1989). Nonetheless, the experimental design used here offer a choice between different social stimuli at the same time. This forced the contrast between stimuli and may have influenced the behavioral decisions because of these stressful conditions, but gives us an interesting insight into the discrimination abilities of M. ravouxi workers.
We did not record any difference in aggressiveness of parasite workers toward their slaves and their sisters. Within parasitized colonies, parasite and slaves are likely to share common cuticular compounds and to display the same colonial label (Franks et al., 1990; Kaib et al., 1993; Bonavita-Cougourdan et al., 1996, 1997; d’Ettorre et al., 2002; Brandt et al., 2005; reviewed in Lenoir et al., 2001). Homocolonial slaves are perceived as nestmates by M. ravouxi workers (see Lenoir et al., 1999). Nonetheless, it has been demonstrated that in experimental mixed-species colonies, different species can keep distinct profiles (Errard et al., 2006). Here, M. ravouxi workers displayed an affiliative preference for their nestmate sisters, as they stayed more in contact with them than with the slaves of their colony. Consequently, this test clearly showed that M. ravouxi workers discriminated their sisters from their slaves in these experimental conditions.
Parasite workers did not behave differently toward conspecific heterocolonial workers than toward their sisters, but, in the presence of a parasite and a slave of another colony, they were also more aggressive toward slaves. It reinforces the idea that parasite workers’ discrimination between species inside a parasitized colony could be the consequence of them having a colonial template where species-specific cues play a major role, thus allowing the discrimination of host slaves, which display a slightly different chemical signature than the parasite workers. The high weight of parasite species-specific cues in the matching process between the perceived odor and the template, combined to the permissive tolerance threshold imposed by the mixed colony condition, could then explain the high tolerance for homospecific congeners, be they from another colony or not. This ability could even be beneficial, since relying on specific cues may for instance enable M. ravouxi workers to avoid agonistic interactions when encountering heterocolonial homospecific workers during raiding periods. Although Schumann (1992) observed occasional intraspecific raids in C. muellerianus in a field study, since then very few studies have been devoted to intraspecific slavery in slave-making ants (Le Moli et al., 1993; Kronauer et al., 2003). Moreover, it seems difficult to disentangle the mechanisms of such a behavior without repeated field observations coupled with population studies. Territorial conflicts could lead to attacks of neighboring colonies, and the brood could then be opportunely brought back into the nest (Wilson, 1975; Alloway, 1980; Stuart and Alloway, 1982; Pollock and Rissing, 1989; Foitzik and Heinze, 1998; see Buschinger, 2009). But Le Moli et al. (1993) recorded almost no aggressive behavior between residents and invaders in Polyergus rufescens, and pillages did not last. This could be interpreted as an “error” in the raiding attempt, with invaders not completing the entire cycle of their raids. Indeed, during the raiding period, one may hypothesize that scouts could trail territorial marks of a colony to find a suitable target (Franks et al., 2007a, b; Cao and Dornhaus, 2012). In parasitized colonies, because foragers are enslaved host workers only (Buschinger, 1986), these marks may contain species-specific host chemical cues that could be mistakenly lead parasite workers to initiate a raid, as observed by Schumann (1992) and Le Moli et al. (1993), on a parasite colony. Because parasitized colonies’ chemical label includes both species-specific cues of host and parasite species, it thus support the idea that host species-specific cues may also have a heavier relative weight either in the perceptual component or in the decision process of slave-making ants, which increases the efficiency of raids by facilitating the recognition of host species.
References
Alloway T.M. 1980. The origins of slavery in leptothoracine ants (Hymenoptera: Formicidae). Am. Nat. 115: 247-261
Baur A., Chalwatzis N., Buschinger A. and Zimmermann F.K. 1995. Mitochondrial DNA sequences reveal close relationships between social parasitic ants and their host species. Curr. Genet. 28: 242-247
Beibl J., Stuart R.J., Heinze J. and Foitzik S. 2005. Six origins of slavery in formicoxenine ants. Insect. Soc. 52: 291-297
Blatrix R. and Herbers J.M. 2003. Coevolution between slave-making ants and their hosts: host specificity and geographical variation. Mol. Ecol. 12: 2809-2816
Bonavita-Cougourdan A., Rivière G., Provost E., Bagnères A.G., Roux M., Dusticier G. and Clément J.L. 1996. Selective adapation of the cuticular hydrocarbon profiles of the slave-making ants Polyergus rufescens Latr. and their Formica rufibarbis Fab. and F. cunicularia Latr. slaves. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 113: 313-329
Bonavita-Cougourdan A., Bagnères A.G., Provost E., Dusticier G. and Clément J.L. 1997. Plasticity of the cuticular hydrocarbon profile of the slave-making ant Polyergus rufescens depending on the social environment. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 116: 287-302
Brandt M. and Foitzik S. 2004. Community context and specialization influence coevolution between a slavemaking ant and its hosts. Ecology 85: 2997-3009
Brandt M., Heinze J., Schmitt T. and Foitzik S. 2005. A chemical level in the coevolutionary arms race between an ant social parasite and its hosts. J. Evol. Biol. 18: 576-586
Buschinger A., Ehrhardt W. and Winter U. 1980. The organization of slave raids in dulotic ants - a comparative study (Hymenoptera; Formicidae). Z. Tierpsychol. 53: 245-264
Buschinger A. and Winter U. 1983. Population studies of the dulotic ant, Epimyrma ravouxi, and the degenerate slavemaker, E. kraussei (Hymenoptera: Formicidae). Entomol. Gen. 8: 251-266
Buschinger A. 1986. Evolution of social parasitism in ants. Trends Ecol. Evol. 1: 155-160
Buschinger A. 1989. Evolution, speciation, and inbreeding in the parasitic ant genus Epimyrma (Hymenoptera, Formicidae). J. Evol. Biol. 2: 265-283
Buschinger A. 1997. Socially parasitic formicoxenine ants from Western Europe—a review (Hymenoptera, Formicidae). In: Proc. Int. Coll. Social Insects (Kipyatkov V.E., Ed), vol 3-4. Russian Language Section of the IUSSI, Socium, St. Petersburg, pp 1-9
Buschinger A. 2009. Social parasitism among ants: a review (Hymenoptera: Formicidae). Myrmecol. News 12: 219-235
Cao T.T. and Dornhaus A. 2012. Ants use pheromone markings in emigrations to move closer to food-rich areas. Insect. Soc. 59: 87-92
Crosland M.W.J. 1989. Kin recognition in the ant Rhytidoponera confusa. I. Environmental odour. Anim. Behav. 37: 912-919
Crozier R.H. and Dix M.W. 1979. Analysis of two genetic models for the innate components of colony odor in social Hymenoptera. Behav. Ecol. Sociobiol. 4: 217-224
d’Ettorre P., Mondy N., Lenoir A. and Errard C. 2002. Blending in with the crowd: social parasites integrate into their host colonies using a flexible chemical signature. Proc. R. Soc. London B Biol. Sci. 269: 1911-1918
Emery C. 1909. Über den Ursprung der dulotischen, parasitischen und myrmekophilen Ameisen. Biol. Centralbl. (Berlin) 29: 352-362
Errard C. 1994. Development of interspecific recognition behavior in the ants Manica rubida and Formica selysi (Hymenoptera: Formicidae) reared in mixed-species groups. J. Insect Behav. 7: 83-99
Errard C. and Hefetz A. 1997. Label familiarity and discriminatory ability of ants reared in mixed groups. Insect. Soc. 44: 189-198
Errard C., Hefetz A. and Jaisson P. 2006. Social discrimination tuning in ants: template formation and chemical similarity. Behav. Ecol. Sociobiol. 59: 353-363
Fischer B. and Foitzik S. 2004. Local co-adaptation leading to a geographical mosaic of coevolution in a social parasite system. J. Evol. Biol. 17: 1026-1034
Fischer-Blass B., Heinze J. and Foitzik S. 2006. Microsatellite analysis reveals strong but differential impact of a social parasite on its two host species. Mol. Ecol. 15: 863-872
Foitzik S. and Heinze J. 1998. Nest site limitation and colony take over in the ant, Leptothorax nylanderi. Behav. Ecol. 9: 367-375
Foitzik S., Deheer C.J., Hunjan D.N. and Herbers J.M. 2001. Coevolution in host-parasite systems: behavioural strategies of slave-making ants and their hosts. Proc. R. Soc. London B Biol. Sci. 268: 1139-1146
Foitzik S., Fischer B. and Heinze J. 2003. Arms-races between social parasites and their hosts: Geographic patterns of manipulation and resistance. Behav. Ecol. 14: 80-88
Franks N., Blum M., Smith R.K. and Allies A.B. 1990. Behavior and chemical disguise of cuckoo ant Leptothorax kutteri in relation to its host Leptothorax acervorum. J. Chem. Ecol. 16: 1431-1444
Franks N.R, Dornhaus A., Hitchcock G., Guillem R., Hooper J. and Webb C. 2007a. Avoidance of conspecific colonies during nest choice by ants. Anim. Behav. 73: 525-534
Franks N.R, Hooper J.W, Dornhaus A., Aukett P.J, Hayward A.L. and Berghoff S.M. 2007b. Reconnaissance and latent learning in ants. Proc. R. Soc. London B Biol. Sci. 274: 1505-1509
Hare J.F. and Alloway T.M. 2001. Prudent Protomognathus and despotic Leptothorax duloticus: differential costs of ant slavery. Proc. Natl Acad. Sci. U.S.A. 98: 12093-12096
Heinze J., Foitzik S., Hippert A. and Hölldobler B. 1996. Apparent dear-enemy phenomenon and environment-based recognition cues in the ant Leptothorax nylanderi. Ethology 102: 510-522
Isingrini M., Lenoir A. and Jaisson P. 1985. Preimaginal learning as a basis of colony-brood recognition in the ant Cataglyphis cursor. Proc. Natl Acad. Sci. U.S.A. 82: 8545-8547
Kaib M., Heinze J. and Ortius D. 1993. Cuticular hydrocarbons profiles in the slave-making ant Harpogoxenus sublaevis and its hosts. Naturwissenschaften 80: 281-285
Knaden M. and Wehner R. 2003. Nest defense and conspecific enemy recognition in the desert ant Cataglyphis fortis. J. Insect Behav. 16: 717-730
Kronauer D.J.C., Gadau J. and Hölldobler B. 2003. Genetic evidence for intra- and interspecific slavery in honey ants (genus Myrmecocystus). Proc. R. Soc. London B Biol. Sci. 270: 805-810
Le Moli F., Grasso D.A, d’Ettorre P. and Mori A. 1993. Intraspecific slavery in Polyergus rufescens Latr. (Hymenoptera, Formicidae): field and laboratory observations. Insect. Soc. 40: 433-437
Lenoir A., Fresneau D., Errard C. and Hefetz A. 1999. Individuality and colonial identity in ants: the emergence of the social representation concept. In: Information Processing in Social Insects (Detrain C., Deneubourg J.L. and Pasteels J., Eds), Birkhäuser Verlag, Basel. pp 219-237
Lenoir A., D’Ettorre P., Errard C. and Hefetz A. 2001. Chemical ecology and social parasitism in ants. Annu. Rev. Entomol. 46: 573-599
Liang D. and Silverman J. 2000. “You are what you eat”: Diet modifies cuticular hydrocarbons and nestmate recognition in the Argentine ant, Linepithema humile. Naturwissenschaften 87: 412-416
Parker J.D. and Rissing S.W. 2002. Molecular evidence for the origin of workerless social parasites in the ant genus Pogonomyrmex. Evolution 56: 2017-2028
Pollock G.B. and Rissing S. W. 1989. Intraspecific brood raiding, territoriality, and slavery in ants. Am. Nat. 133: 61-70
Reeve H.K. 1989. The evolution of conspecific acceptance thresholds. Am. Nat. 133: 407-435
Sanada-Morimura S., Minai M., Yokoyama M., Hirota T., Satoh T. and Obara Y. 2003. Encounter-induced hostility to neighbors in the ant Pristomyrmex pungens. Behav. Ecol. 14: 713-718
Schumann R.D. 1992: Raiding behavior of the dulotic ant Chalepoxenus muellerianus (Finzi) in the field (Hymenoptera: Formicidae, Myrmicinae). Insect. Soc. 39: 325-333
Schumann R.D. and Buschinger A. 1995. Imprinting effects on host-selection behavior of slave-raiding Chalepoxenus muellerianus (Finzi) workers (Hymenoptera: Formicidae). Ethology 99: 243-251
Seifert B. 2007. Die Ameisen Mittel- und Nordeuropas. Lutra Verlags- und Vertriebsgesellschaft, Tauer
Soroker V., Vienne C., Hefetz A. and Nowbahari E. 1994. The postpharyngeal gland as a “Gestalt” organ for nestmate recognition in the ant Cataglyphis niger. Naturwissenschaften 81: 510-513
Soroker V., Lucas C., Simon T., Fresneau D., Durand J.L. and Hefetz A. 2003. Hydrocarbon distribution and colony odour homogenisation in Pachycondyla apicalis. Insect. Soc. 50: 212-217
Stuart R.J. and Alloway T.M. 1982. Territoriality and the origin of slave raiding in leptothoracine ants. Science 215: 1262-1263
Sturgis S.J. and Gordon D.M. 2012. Nestmate recognition in ants (Hymenoptera: Formicidae): a review. Myrmecol. News 16: 101-110
Sumner S., Aanen D.K., Delabie J. and Boomsma J.J. 2004. The evolution of social parasitism in Acromyrmex leaf-cutting ants: a test of Emery’s rule. Insect. Soc. 51: 37-42
Thompson J.N. 1994. The Coevolutionary Process. The University of Chicago Press, Chicago
van Zweden J.S., Dreier S. and d’Ettorre P. 2009a. Disentangling environmental and heritable nestmate recognition cues in a carpenter ant. J. Insect Physiol. 55: 158-163
van Zweden J.S., Heinze J., Boomsma J.J. and d'Ettorre P. 2009b. Ant queen egg-marking signals: matching deceptive laboratory simplicity with natural complexity. PLoS ONE 4: e4718
van Zweden J.S. and d’Ettorre P. 2010. Nestmate recognition in social insects and the role of hydrocarbons. In: Insect Hydrocarbons: Biology, Biochemistry and Chemical Ecology (Blomquist G.J. and Bagnères A.G., Eds). Cambridge University Press, Cambridge. pp 222-243
Wilson E.O. 1975. Slavery in ants. Sci. Am. 232: 32-36
Winter U. 1979. Epimyrma goesswaldi Menozzi, eine sklavenhaltende Ameise. Naturwissenschaften 66: 581-582
Acknowledgments
Olivier Delattre was supported by the French Ministry of Research. Nicolas Châline and Stéphane Chameron were supported by the ANR project SEUILS ANR-09-JCJC-0031.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Delattre, O., Châline, N., Chameron, S. et al. Opportunist slave-making ants Myrmoxenus ravouxi discriminate different host species from a non-host species. Insect. Soc. 60, 7–13 (2013). https://doi.org/10.1007/s00040-012-0257-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00040-012-0257-3