Abstract
Despite the recognition of the functional role of Hymenoptera (ants, bees and wasps) and Isoptera (termites) in tropical ecosystems, their detailed feeding habits are not well known. To examine the feeding habits of these groups, we measured nitrogen (N) and carbon (C) stable isotope ratios (δ15N and δ13C) of hymenopterans (12 families, ≥16 genera and ≥32 species) and isopterans (one family and 10 species) collected in a tropical rain forest, Sarawak, Malaysia. We compared the isotopic signatures of these insects to those previously reported for other consumers collected in the same forest. The δ15N and δ13C values of these insects overlapped with those of the other consumers, indicating that they have access to diverse C and N sources in the forest. The δ15N values of ants and termites indicated that their feeding habits range along a continuum from herbivory (i.e. dependent on honeydew and nectar) to predation and from wood-feeders to soil-feeders, respectively. In addition, the δ15N values of wasps varied greatly from −0.1‰ (Braconidae sp.) to 8.6‰ (Bembix sp.), suggesting that their feeding habits also range from omnivory to predation. The ant species Camponotus gigas had δ13C values similar to those of invertebrate detritivores and omnivores rather than to those of invertebrate herbivores, although the diet of this species consists mostly of honeydew. This discrepancy suggests that the ant uses carbohydrates as an energy source, the isotopic signatures of which are not well retained in the body tissues. Values of both δ15N and δ13C of the predatory army ant Leptogenys diminuta and the soil-feeding termite Dicuspiditermes nemorosus did not differ significantly, indicating that both trophic level and the humification of feeding substrates can increase the isotopic signatures of terrestrial consumers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Hymenoptera and Isoptera are highly abundant and diverse insect groups within tropical ecosystems (Brian, 1978; Roubik, 1989; Hölldobler and Wilson, 1990; Abe et al., 2000). In an Amazonian tropical forest, ants and termites comprise one-third of total animal biomass, and together with wasps and bees, they constitute 75% of overall insect abundance (Fittkau and Klinge, 1973). These animals have long been recognised to play major roles in tropical rain forest ecosystems. For example, ants function as scavengers and predators (Hölldobler and Wilson, 1990), and through associations with aphids and homopterans, some ant species can access plant-derived food sources and thus function as herbivores (Blüthgen et al., 2003; Davidson et al., 2003). With the aid of microbial symbionts, termites decompose substantial amounts of organic matter at different stages of decomposition, from fresh plant material to soil organic matter (Breznak and Brune, 1994; Bignell and Eggleton, 2000; Watanabe and Tokuda, 2010). Wasps can function as predators or parasitoids (Richter, 2000), and bees serve as pollinators in tropical rain forests, where many plant species are dependent on bees for pollen transfer (Roubik, 1989; Momose et al., 1998).
Despite their importance in ecosystems, the precise feeding habits of these insects are not well understood. One reason for the lack of information is that the feeding behaviours of some species are difficult to directly observe due to their cryptic lifestyle (e.g. nocturnal foraging or living in canopy layers or below ground). Another reason is that the food sources of these animals consist of several food items, making quantifying the relative contribution of each food source difficult. For example, some ant and wasp species feed on both nectar and animal prey (Hölldobler and Wilson, 1990; Gadagkar, 1991; Matsuura, 1991). In addition, soil organic matter, which is the food source of soil-feeding termites, is highly complex and composed of minerals, decayed plant materials and micro-organisms (Bignell and Eggleton, 2000). Most importantly, estimating the food sources assimilated by insects is extremely difficult using field observations.
Stable isotope techniques have been widely used to determine the feeding habits and trophic positions of consumers within ecosystems (Post, 2002; Fry, 2006). This technique is based on the assumptions that the nitrogen (N) isotope ratios (δ15N) of animals are approximately 3.4‰ higher than those of their diets, whereas the carbon (C) isotope ratios (δ13C) of animals are similar (<1‰) to those of their diets (DeNiro and Epstein, 1978; Minagawa and Wada, 1984). Considering the energy transfer efficiency through the food chain (Lindeman, 1942) and resource partitioning, which could allow species coexistence (Tilman, 1982), the great biomass and diversity of the hymenopterans and isopterans predict that many species are dependent on the most abundant food sources in the ecosystem, such as primary production or dead organic matter, and that they consume a broad spectrum of food resources. Indeed, these predictions have been partly confirmed by the stable isotope technique. For example, the high abundance of ants in canopy layers was once considered a paradox due to the scarcity of potential prey that could support such large ant populations. Nitrogen isotopic studies revealed that most canopy ants are dependent on honeydew and nectar via aphids and homopterans (Blüthgen et al., 2003; Davidson et al., 2003). In termites, the C and N isotopic signatures correspond well to their known feeding habits and vary greatly, indicating the diversification of food resource utilization (Higashi and Abe, 1996; Tayasu et al., 1997; Bourguignon et al., 2009). However, the C and N isotopic signatures of many species of tropical ants and termites, and especially of wasps, are still unexplored.
Here, we measured the δ15N and δ13C values of ants, bees, wasps and termites collected from a tropical rain forest in Sarawak, Malaysia. We then compared their C and N isotopic signatures to those previously reported for other consumers of four trophic groups (detritivores, herbivores, omnivores and predators) in the same forest (Hyodo et al., 2010b). We hypothesised that the isotopic signatures of these insects would indicate diverse feeding habits ranging from herbivory, omnivory (and detritivory for termites) to predatory, which could overlap with the other consumers.
Materials and methods
Study site and sample collection
Sampling was conducted in Lambir National Park (4°02′ N, 113°05′ E, 20–150 m asl), Sarawak, Malaysia, from November 2004 to June 2005. Mean annual rainfall is approximately 2,700 mm, with no distinct dry season. The hymenopterans (12 families, ≥16 genera and ≥32 species) and isopterans (one family and 10 species) used in this study and their known feeding habits are listed in Table 1. These insects were collected randomly from the forest floor or from soil and deadwood. Some individuals were also collected from canopy layers using a walkway and tower crane system (Yumoto and Nakashizuka, 2005). Collected insects were kept in a freezer for 24 h to terminate their activity and were then dried in a drying oven at 60°C for 24 h. The whole bodies of the insects were ground into powder using a mortar and pestle prior to analyses. We also compared the isotopic signatures of the hymenopterans and isopterans to those previously reported for detritivores, herbivores, omnivores and predators (Hyodo et al., 2010b). The data of the fungus-growing termite, Macrotermes malaccensis and the soil-feeding termite, Dicuspiditermes nemorosus have already been reported (Hyodo et al., 2010b).
Stable N and C isotope analyses
For stable N and C isotope analyses, the samples were placed in tin capsules, and isotope ratios were then measured using a mass spectrometer (Deltaplus XP, Germany) coupled with an elemental analyser. The precision of the online procedure was better than ±0.2‰ for both isotope ratios. The natural abundances of 15N and 13C are expressed in per mil (‰) deviation from international standards: δ15N or δ13C = (R sample/R standard – 1) × 1,000, where R in δ15N or δ13C is 15N/14N or 13C/12C, respectively. Atmospheric N and Pee Dee belemnite were used as the international standards for N and C, respectively.
Statistical analyses
To examine whether δ15N or δ13C values differed among species within each group (i.e. ants, wasps and termites), we used one-way analysis of variance (ANOVA) for samples with more than three replicates of individuals (wasps) or colonies (ants and termites). For comparisons of bee species (Apis spp. and Trigona spp.), we used t tests. Some samples of the same genera were treated as one group due to the small sample size, assuming that congeners exhibited similar feeding habits. These species included two species of Apis (A. dorsata and A. koschevnikovi), four species of Trigona (T. itama, T. melanocephala, T. fimbriata and one unidentified species) and two species of Ropalidia (R. flavopicta and an unidentified species). Tukey–Kramer honestly significant difference (HSD) tests were applied to examine differences among species.
We also examined differences in δ15N and δ13C values among all species with more than three replicates using one-way ANOVA. Tukey–Kramer HSD post hoc tests were also applied to test for differences among species. These statistical analyses were performed using JMP statistical software (version 8.0.2 for Macintosh; SAS Institute, Cary, NC, USA).
Results
δ15N of ants, bees, wasps and termites
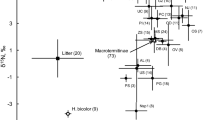
δ15N values varied significantly among ant species (F 5,36 = 34.7, P < 0.0001; Fig. 1; Table 1; Appendix). Crematogaster borneensis, which depends on food bodies produced by the myrmetic host plants (Macaranga trachyphylla), had lower δ15N than the other species, whereas the predatory army ants Leptogenys diminuta had the highest values of δ15N. The δ15N of honey bees (Apis spp.) was significantly lower than that of stingless bees (Trigona spp.) (t = 4.80, P = 0.00006). The other bees showed low δ15N values. For wasps, δ15N values varied widely among species (F 4,14 = 20.3, P < 0.0001). The paper wasp Ropalidia spp. and the nocturnal wasp Provespa anomala were significantly depleted in 15N compared to the sand wasp Bembix sp., and the parasitoid wasp Ichneumonidae sp. (Bembecinus sp.) exhibited δ15N values intermediate between these two groups. The spider-hunting wasps Pompilidae sp. and Chalybion bengalense had high values of δ15N, which were similar to those of Bembix sp., although the results for these species were not statistically tested due to the lack of replication. The δ15N of termites also varied widely (F 6,44 = 197.0, P < 0.0001). The soil-feeding termite D. nemorosus had similar δ15N values to those of the soil/wood interface-feeding termite Prohamitermes mirabilis, both of which were significantly higher than the other termite species. In addition, the δ15N of Homallotermes foraminifera, which was classified as a soil/wood interface feeder, was significantly lower than that of the soil-feeding termite, D. nemorosus, but was similar to that of the litter-feeding termite Longipeditermes longipes. The lichen-feeding termite Hospitalitermes hospitalis had significantly lower δ15N values compared to the other termite species. The wood-feeding termite, Microcerotermes sabahensis and the fungus-growing termite (M. malaccensis and the other two fungus-growing species) had intermediate δ15N values.
δ15N and δ13C values (means ± SE, circles) of ants, bees, wasps, and termites, together with average values (from Hyodo et al., 2010b) for other consumers (triangles) and plant material and soil (squares) in Lambir National Park. The numbers on each value correspond to the species listed in Table 1. IH invertebrate herbivores, IO invertebrate omnivores, DT detritivores, IP invertebrate predators, VH vertebrate herbivores, VO vertebrate omnivores, VP vertebrate predators, UL understorey leaves, CL canopy leaves, LL leaf litter, WL woody litter, Soil soil (0–5 cm)
Values of δ15N significantly differed among all the species examined (F 19,93 = 64.4, P < 0.0001). Bembix sp. was the most enriched in 15N compared to the other insects, with the exceptions of L. diminuta and D. nemorosus. Hospitalitermes hospitalis was significantly more depleted in 15N than the other species, except for Apis spp. and the fungus-growing termite M. malaccensis. Note that the values of 15N for the termites, D. nemorosus, P. mirabilis and Termes rostratus did not significantly differ from those of the predatory wasps, Pompilidae sp., C. bengalense and Bembix sp., the parasitoid wasp, Ichneumonidae sp., or the army ant L. diminuta.
δ13C of ants, bees, wasps and termites
Values of δ13C significantly differed among ant species (F 5,36 = 69.8, P < 0.0001); the δ13C of C. borneensis was significantly lower than values for the other ant species. For bees, Apis spp. were more enriched in 13C than Trigona spp. (t = 4.795, P = 0.0006). The δ13C values of wasp species did not differ significantly (F 4,14 = 2.796, P = 0.0698) and the other bees had variable δ13C values. Values of δ13C for termites differed significantly among species (F 6,44 = 13.2, P < 0.0001). Termite species feeding on decomposed organic matter (i.e. D. nemorosus, P. mirabilis, H. foraminifera and M. malaccensis) had significantly higher δ13C than other termites feeding on relatively fresh organic matter (i.e. M. sabahensis, H. hospitalis and L. longipes).
δ13C values significantly differed across all species examined (F 19,93 = 24.4927, P < 0.0001). Bembix sp. and Apis spp. had significantly higher δ13C than the termite species, except for D. nemorosus and M. malaccensis, as well as the arboreal honeydew- and nectar-feeding ant Tetraponera attenuata and the plant-ant C. borneensis. Of all the species, C. borneensis was most depleted in 13C. As observed for δ15N, values of 13C for the soil-feeding termites, such as D. nemorosus, were indistinguishable from those of the predatory wasps (e.g. Bembix sp.) and army ants (L. diminuta).
Comparison of the isotopic values of hymenopterans and isopterans to other consumers in a tropical rain forest
To characterize the isotopic signatures of the hymenopterans and isopterans, we compared their isotopic signatures to those previously reported for other tropical consumers, plants and soil organic matter (Hyodo et al., 2010b). The consumers include detritivores (e.g., cockroach, fungus beetle and pygmy grasshopper), invertebrate herbivores (e.g., leaf beetle, weevil and lepidopteran), vertebrate herbivores (e.g., fruit bat and dove), invertebrate omnivores (e.g., katydid, cricket and darkling beetle), vertebrate omnivores (e.g., squirrel and rat), invertebrate predators (e.g., tiger beetle, praying mantis and spiders) and vertebrate predators (e.g., vampire bat, tree shrew and black-throated babbler). The δ15N and δ13C of hymenopterans and isopterans overlapped with those of the other consumers. Among all the consumers examined in the forest, Bembix sp. had the highest mean δ15N value and H. hospitalis had the lowest mean δ15N value, whereas C. borneensis had the lowest mean value of δ13C.
The comparison of the isotopic signatures of the specialist predator wasps with those of their prey allows for the estimation of trophic enrichment within this forest ecosystem. The spider-hunting wasps Pompilidae sp. were more enriched in both 15N and 13C (by 3.8 and 1.6‰, respectively) relative to the average for all spiders (δ15N: 3.6 ± 0.3‰; δ13C: −27.0 ± 0.2‰; mean ± SE, n = 38). The spider-hunting wasp, C. bengalense was enriched in 15N by 5‰ but depleted in 13C by 1.4‰ relative to the average for all spiders. In addition, values of δ15N and δ13C of the homopteran-hunting wasp Bembecinus sp. were 1.9 and 1.3‰ higher, respectively, than values for all homopterans (Homoptera, δ15N: 1.7 ± 0.6‰ and δ13C: −27.8 ± 0.6‰, n = 16).
Discussion
We demonstrated that δ15N and δ13C values for hymenopterans and isopterans varied greatly and overlapped among the four groups of insects. Furthermore, as hypothesised, the isotopic signatures of hymenopterans and isopterans overlapped with those of the other consumers belonging to the four trophic groups (i.e., detritivores, herbivores, omnivores and predators). Considering that C and N isotopic signatures are indicative of the flows of energy and material in food webs, our results suggest that these insects use diverse C and N sources, which likely explain their great abundance as well as diversity in tropical ecosystems (Brian, 1978; Hölldobler and Wilson, 1990; Abe et al., 2000).
The observed isotopic patterns of ants and termites were generally consistent with the results of earlier studies. The δ15N values of ants ranged along a continuum from species associated with canopy plants, such as T. attenuata, to those foraging on the ground, such as L. diminuta (Blüthgen et al., 2003; Davidson et al., 2003). Termites exhibited higher δ15N values as their feeding substrates become more humified from wood to soil organic matter (Tayasu et al., 1997). The lichen-feeding termite H. hospitalis was the most depleted in 15N among all of the samples. These low values can be explained by the dependence of H. hospitalis on “food ball”, which is made from lichens (Jones and Gathorne-Hardy, 1995). The food balls have been reported to have low δ15N values (−3.4 ± 1.59, mean ± SD, n = 11: −5.2 ± 0.72, n = 11) for the congeners H. birmanicus and H. bicolor, respectively (Tayasu et al., 2000). The low δ15N values of lichens likely reflect that N of lichens originated from atmospheric N deposition, which is depleted in 15N (−10 to 0‰; Nadelhoffer and Fry, 1994). The δ13C values of termites were consistent with earlier studies demonstrating enrichment in 13C when feeding substrates were more humified or included fungal tissues (Tayasu, 1998; Hyodo et al., 2003).
Our results confirmed the previously known feeding habits of C. borneensis. This ant species inhabits the hollow stems of its host plant (Macaranga trachyphylla) and feeds primarily on food bodies provided by the plant (Fiala et al., 1989; Itino et al., 2001). The low δ13C values of C. borneensis indicate its dependence on the food bodies, which also exhibit low δ13C values (average δ13C = −33.7‰, n = 2; F. Hyodo, unpubl. data). The depletion in 13C of the food bodies indicates that this food source is rich in lipids (Heil et al., 1998), which are generally depleted in 13C relative to bulk tissue (DeNiro and Epstein, 1977; Hobbie and Werner, 2004).
In contrast to C. borneensis, we observed a discrepancy between the previously known feeding habit of C. gigas and that inferred from the isotopic signatures. Pfeiffer and Linsenmair (2000) demonstrated that although C. gigas feeds on other arthropods and bird droppings, its diet consists mostly (90%) of nectar and honeydew on a by-weight basis. However, the δ13C values of this ant species were more similar to the average values for invertebrate omnivores than to those for invertebrate herbivores. Note that C. gigas exhibited the relatively high δ13C values, even though the ant sample used in this study included the abdomen, which is known to be depleted in 13C compared to the rest of the body, likely due to the storage of nectar, honeydew and lipid (Tillberg et al., 2006; Feldhaar et al., 2010). It was also reported that the bullet ant Paraponera clavata exhibits δ13C values more similar to those of prey than to those of nectar, even though nectar dominates its diet (Tillberg and Breed, 2004). Given our results and those of previous studies, the nectar and honeydew consumed by omnivorous ant species are likely used as energy sources, and the isotopic composition is not well retained in the body tissues. This is consistent with previous findings that the C isotopic composition of the body protein of omnivores reflects only the protein in its diet (Ambrose and Norr, 1993; Gannes et al., 1997). Therefore, the C isotopic signatures may underestimate the relative importance of carbohydrate sources, such as nectar, in omnivorous hymenopteran diets. Further feeding experiments are required to examine the fate of dietary carbohydrate sources in hymenopteran tissues.
The observed isotopic signatures provide insight into the feeding habits of several species. For example, the feeding habit of Crematogaster difformis, an arboreal ant that nests in myrmecophytic epiphytic ferns (Inui et al., 2009), likely differs from those of other arboreal ants. Arboreal ants tend to depend largely on extrafloral nectaries provided by plants and on honeydew excreted by homopterans (Blüthgen et al., 2003; Davidson et al., 2003). In contrast, our isotopic data suggest that C. difformis exhibits a more omnivorous feeding habit similar to that of the weaver ant Oecophylla smaragdina, which consumes honeydew and nectar sources as well as other arthropods and often dominates the ant community (Blüthgen and Fiedler, 2002). The unique feeding habit of C. difformis among arboreal ants may be consistent with the recent finding that this ant species dominates the canopy ant fauna and regulates the density of herbivores within the canopy layers (Tanaka et al., 2009). Moreover, the relatively high δ15N of C. difformis can be explained by their consumption of the cockroach Pseudoanaplectinia yumoto, which also inhabits epiphytic ferns (Inui et al., 2009). The average δ15N of the cockroach (−1.0‰, n = 5) was approximately 3‰ lower than that of C. difformis, whereas the δ13C signatures of the two species were similar (δ13C for P. yumoto: −25.8‰, n = 5).
The feeding habits of termites have been inferred using field observations of feeding substrates and sites as well as gut content analysis (Bignell and Eggleton, 2000). The feeding habits of P. mirabilis, which inhabits the soil surface rich in organic matter (Eggleton et al., 1999; Jones and Praestyo, 2002; Jones et al., 2010), and of H. foraminifera, which feeds on very decayed wood (Jeeva et al., 1999; Jones and Praestyo, 2002; Jones et al., 2010), remain unclear. Our results indicated that P. mirabilis does not differ from the soil-feeding termite D. nemorosus in terms of C and N isotopic composition, strongly suggesting that P. mirabilis can be classified as a soil feeder. Furthermore, H. foraminifera is unlikely to be a soil feeder but can instead be considered a wood feeder or a wood–soil interface feeder.
In general, predatory wasps consume arthropod prey or scavenge animal protein to develop brood while consuming carbohydrates, such as nectar and sap, as energy sources (Matsuura, 1991; Martin, 1995; Richter, 2000). The spider-hunting wasps Pompilidae sp. and C. bengalense and the homopteran-hunting wasp Bembecinus sp. were indeed enriched by about 3‰ in 15N relative to invertebrate prey. This trophic enrichment in 15N is consistent with previous results (3.4 ± 1.1‰, mean ± SD) from food-web studies (Minagawa and Wada, 1984; Post, 2002). Bembix sp., which hunts flies (Bohart and Menke, 1976) and was frequently observed hunting scavenger flies at the study site, had high δ15N values similar to the spider-hunting wasps Pompilidae sp. and C. bengalense. This result suggests that the prey flies may depend on dead body or faeces of consumers at higher trophic level or on soil organic matters, both of which could exhibit high values of δ15N (Hyodo et al., 2010a).
Values of δ15N of wasps varied greatly from −0.1‰ for the parasitoid wasp (Braconidae sp.) to 8.6‰ for the fly-hunting wasp (Bembix sp.). Parasitoid wasps oviposit on or in arthropod prey, which then become the food source for wasp larvae (Wäckers, 2004). The large difference in δ15N between the two groups of parasitoid wasps (Ichneumonidae sp. and Braconidae sp.) may reflect the difference in their hosts. Likewise, the wasps P. anomala and Ropalidia spp. were more depleted in 15N compared to the predatory wasp Vespa affinis and to the other general invertebrate predators at the study site. Based on their isotopic signatures, P. anomala and Ropalidia spp. probably use food sources similar to those of omnivorous ants such as O. smaragdina and C. gigas. These results suggest that the feeding habits of the wasps range from omnivory and some degree of dependence on nectar and honeydew, which also contains some amino acids (Baker and Baker, 1986; Blüthgen et al., 2004), to predation and dependence mostly on animal prey, as has been shown for ants (Blüthgen et al., 2003; Davidson et al., 2003). Meanwhile, honeybees, stingless bees and other bees exhibited low δ15N values, which were indicative of their use of plant materials. The significant differences in δ15N and δ13C between Apis spp. and Trigona spp. may indicate food resource partitioning (Roubik, 1989). The reasons for the relatively variable δ13C in these species remain unclear. Future studies should examine the isotopic signatures of their food sources, i.e. nectar and pollen.
Overall, the present results provide insight into the feeding habits of tropical hymenopterans and isopterans. Note that similar isotopic signatures of consumers do not necessarily mean similar feeding habits, because the signatures do not provide information about whether the isotope ratios are brought about by feeding on single or several food items, and because the trophic enrichment in 13C and 15N can vary between different taxa (McCutchan et al., 2003; Vanderklift and Ponsard, 2003). This should be taken into account especially when the isotopic signatures of soil-feeding invertebrates are compared, because the soil organic matter on which they feed is a mixture of organic substrates, which can have different isotopic signatures (Pollierer et al., 2009). In particular, the soil-feeding termite, D. nemorosus exhibited values of δ15N and δ13C that were indistinguishable from those of the army ant L. diminuta and the fly-hunting wasp Bembix sp., despite clear differences in the feeding habits of these species. This result indicates that values of δ15N and δ13C can increase not only from trophic enrichment, but also from the humification of feeding substrates in terrestrial food webs (Hyodo et al., 2008). Therefore, this study highlights that stable isotope techniques should be coupled with knowledge of the natural history and physiology of focal organisms to achieve a more precise interpretation of the isotopic signatures.
References
Abe T. 1979. Studies on the distribution and ecological role of termites in a lowland rain forest of West Malaysia (2) Food and feeding habits of termites in Pasoh Forest Reserve. Jap. J. Ecol. 29: 121-135
Abe T., Bignell D.E. and Higashi M. 2000. Termites: Evolution, Sociality, Symbiosis, Ecology. Kluwer Academic Publishers, Dordrecht. 488 pp
Ambrose S.H. and Norr L. 1993. Carbon isotopic evidence for routing of dietary protein to bone collagen, and whole diet to bone apatite carbonate: purified diet growth experiments. In: Molecular Archaeology of Prehistoric Human Bone (Lambert J. and Grupe G., Eds). Springer-Verlag, Berlin, Germany. pp 1-37
Baker H. and Baker I. 1986. The occurrence and significance of amino acids in floral nectar. Plant Syst. Evol. 151: 175-186
Bignell D.E. and Eggleton P. 2000. Termites in ecosystems. In: Termites: Evolution, Sociality, Symbiosis, Ecology (Abe T., Bignell D.E. and Higashi M., Eds). Kluwer Academic Publishers, Dordrecht. pp 363-387
Blüthgen N. and Fiedler K. 2002. Interactions between weaver ants Oecophylla smaragdina, homopterans, trees and lianas in an Australian rain forest canopy. J. Anim. Ecol. 71: 793-801
Blüthgen N., Gebauer G. and Fiedler K. 2003. Disentangling a rainforest food web using stable isotopes: dietary diversity in a species-rich ant community. Oecologia 137: 426-435
Blüthgen N., Gottsberger G. and Fiedler K. 2004. Sugar and amino acid composition of ant-attended nectar and honeydew sources from an Australian rainforest. Austral Ecol. 29: 418-429
Bohart R.M. and Menke A.S. 1976. Sphecid Wasps of the World. University of California Press, Berkeley. 600 pp
Bourguignon T., Šobotník J., Lepoint G., Martin J.M. and Roisin Y. 2009. Niche differentiation among neotropical soldierless soil-feeding termites revealed by stable isotope ratios. Soil Biol. Biochem. 41: 2038-2043
Breznak J.A. and Brune A. 1994. Role of microorganisms in the digestion of lignocellulose by termites. Annu. Rev. Entomol. 39: 453-487
Brian M.V. 1978. Production Ecology of Ants and Termites. Cambridge University Press, Cambridge. 409 pp
Buschinger A., Klein R.W. and Maschwitz U. 1994. Colony structure of a bamboo-dwelling Tetraponera sp. (Hymenoptera: Formicidae: Pseudomyrmecinae) from Malaysia. Insect. Soc. 41: 29-41
Davidson D.W., Cook S.C., Snelling R.R. and Chua T.H. 2003. Explaining the abundance of ants in lowland tropical rainforest canopies. Science 300: 969-972
DeNiro M.J. and Epstein S. 1977. Mechanism of carbon isotope fractionation associated with lipid-synthesis. Science 197: 261-263
DeNiro M.J. and Epstein S. 1978. Influence of diet on the distribution of carbon isotopes in animals. Geochim. Cosmochim. Acta 42: 495-506
Eggleton P., Homathevi R., Jones D.T., MacDonald J.A., Jeeva D., Bignell D.E., Davies R.G. and Maryati M. 1999. Termite assemblages, forest disturbance and greenhouse gas fluxes in Sabah, East Malaysia. Phil. Trans. R. Soc. B 354: 1791-1802
Feldhaar H., Gebauer G. and Blüthgen N. 2010. Stable isotopes: past and future in exposing secrets of ant nutrition (Hymenoptera: Formicidae). Myrmecol. News 13: 3-13
Fiala B., Maschwitz U., Tho Y.P. and Helbig A.J. 1989. Studies of a South East Asian ant-plant association - protection of Macaranga trees by Crematogaster borneensis. Oecologia 79: 463-470
Fittkau E.J. and Klinge H. 1973. On biomass and trophic structure of the central Amazonian rain forest ecosystem. Biotropica 5: 2-14
Fry B. 2006. Stable Isotope Ecology. Springer, New York. 308 pp
Gadagkar R. 1991. Belonogaster, Mischocyttarus, Parapolybia, and independent-founding Ropalidia. In: The Social Biology of Wasps (Ross K.G. and Matthews R.W., Eds). Cornell University Press, Ithaca and London. pp 149-190
Gannes L.Z., O’Brien D.M. and Martínez del Rio C. 1997. Stable isotopes in animal ecology: assumptions, caveats, and a call for more laboratory experiments. Ecology 78: 1271-1276
Grimaldi D. and Engel M.S. 2005. Evolution of the Insects. Cambridge University Press, Cambridge, U.K. 755 pp
Heil M., Fiala B., Kaiser W. and Linsenmair K.E. 1998. Chemical contents of Macaranga food bodies: adaptations to their role in ant attraction and nutrition. Funct. Ecol. 12: 117-122
Higashi M. and Abe T. 1996. Global diversification of termites driven by the evolution of symbiosis and sociality. In: Biodiversity: An Ecological Perspective (Abe T., Levin S.A. and Higashi M., Eds). Springer-Verlag, New York. pp 83-112
Hobbie E.A. and Werner R.A. 2004. Intramolecular, compound-specific, and bulk carbon isotope patterns in C3 and C4 plants: a review and synthesis. New Phytol. 161: 371-385
Hölldobler B. and Wilson E.O. 1990. The Ants. Belknap Press of Harvard University Press, Cambridge. 732 pp
Hyodo F., Kohzu A. and Tayasu I. 2010a. Linking aboveground and belowground food webs through carbon and nitrogen stable isotope analyses. Ecol. Res. 25: 745-756
Hyodo F., Matsumoto T., Takematsu Y., Kamoi T., Fukuda D., Nakagawa M. and Itioka T. 2010b. The structure of a food web in a tropical rain forest in Malaysia based on carbon and nitrogen stable isotope ratios. J. Trop. Ecol. 26: 205-214
Hyodo F., Tayasu I., Inoue T., Azuma J.-I. and Kudo T. 2003. Differential role of symbiotic fungi in lignin degradation and food provision for fungus-growing termites (Macrotermitinae: Isoptera). Funct. Ecol. 17: 186-193
Hyodo F., Tayasu I., Konate S., Tondoh J.E., Lavelle P. and Wada E. 2008. Gradual enrichment of 15N with humification of diets in a below-ground food web: relationship between 15N and diet age determined using 14C. Funct. Ecol. 22: 516-522
Inui Y., Tanaka H.O., Hyodo F. and Itioka T. 2009. Within-nest abundance of a tropical cockroach Pseudoanaplectinia yumotoi associated with Crematogaster ants inhabiting epiphytic fern domatia in a Bornean dipterocarp forest. J. Nat. Hist. 43: 1139-1145
Itino T., Davies S.J., Tada H., Hieda O., Inoguchi M., Itioka T., Yamane S. and Inoue T. 2001. Cospeciation of ants and plants. Ecol. Res. 16: 787-793
Jeeva D., Bignell D.E., Eggleton P. and Maryati M. 1999. Respiratory gas exchanges of termites from the Sabah (Borneo) assemblage. Physiol. Entomol. 24: 11-17
Jones D.T. and Gathorne-Hardy F.J. 1995. Foraging activity of the processional termite Hospitalitermes hospitalis (Termitidae, Nasutitermitinae) in the rain-forest of Brunei, North-West Borneo. Insect. Soc. 42: 359-369
Jones D.T. and Praestyo A.H. 2002. A survey of the termites (Insecta: Isoptera) of Tabalong District, south Kalimantan, Indonesia. Raffles Bull. Zool. 50: 117-128
Jones D.T., Rahman H., Bignell D.E. and Prasetyo A.H. 2010. Forests on ultramafic-derived soils in Borneo have very depauperate termite assemblages. J. Trop. Ecol. 26: 103-114
Klein A.M., Steffan-Dewenter I. and Tscharntke T. 2004. Foraging trip duration and density of megachilid bees, eumenid wasps and pompilid wasps in tropical agroforestry systems. J. Anim. Ecol. 73: 517-525
Lindeman R.L. 1942. The trophic-dynamic aspect of ecology. Ecology 23: 399-417
Martin S.J. 1995. Hornets (Hymenoptera: Vespinae) of Malaysia. Malay. Nat. J. 49: 71-82
Maschwitz U. and Mühlenberg M. 1975. Strategy of predation in some oriental Leptogenys species (Formicidae-Ponerinae). Oecologia 20: 65-83
Matsuura K. 1991. Vespa and Provespa. In: The Social Biology of Wasps (Ross K.G. and Matthews R.W., Eds). Cornell University Press, Ithaca. pp 232-262
McCutchan J.H., Lewis W.M., Kendall C. and McGrath C.C. 2003. Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. Oikos 102: 378-390
Michener C.D. 2000. The Bees of the World. The Johns Hopkins University Press, Baltimore, Md. 913 pp
Minagawa M. and Wada E. 1984. Stepwise enrichment of 15N along food chains: further evidence and the relation between δ15N and animal age. Geochim. Cosmochim. Acta. 48: 1135-1140
Momose K., Yumoto T., Nagamitsu T., Kato M., Nagamasu H., Sakai S., Harrison R.D., Itioka T., Hamid A.A. and Inoue T. 1998. Pollination biology in a lowland dipterocarp forest in Sarawak, Malaysia. I. Characteristics of the plant-pollinator community in a lowland dipterocarp forest. Am. J. Bot. 85: 1477-1501
Nadelhoffer K. and Fry B. 1994. Nitrogen isotope studies in forest ecosystems. In: Stable Isotopes in Ecology and Environmental Science (Lajtha K. and Michener R.H., Eds). Blackwell Scientific Publications, Oxford. pp 22-44
O’Neil K.M. 2001. Solitary Wasps: Behavior and Natural History. Cornell University Press, Ithaca. 406 pp
Pfeiffer M. and Linsenmair K.E. 2000. Contributions to the life history of the Malaysian giant ant Camponotus gigas (Hymenoptera, Formicidae). Insect. Soc. 47: 123-132
Pollierer M.M., Langel R., Scheu S. and Maraun M. 2009. Compartmentalization of the soil animal food web as indicated by dual analysis of stable isotope ratios (15N/14N and 13C/12C). Soil Biol. Biochem. 41: 1221-1226
Post D.M. 2002. Using stable isotopes to estimate trophic position: models, methods, and assumptions. Ecology 83: 703-718
Richter M.R. 2000. Social wasp (Hymenoptera: Vespidae) foraging behavior. Annu. Rev. Entomol. 45: 121-150
Roubik D.W. 1989. Ecology and Natural History of Tropical Bees. Cambridge University Press, New York. 514 pp
Tanaka H.O., Inui Y. and Itioka T. 2009. Anti-herbivore effects of an ant species, Crematogaster difformis, inhabiting myrmecophytic epiphytes in the canopy of a tropical lowland rainforest in Borneo. Ecol. Res. 24: 1393-1397
Tayasu I. 1998. Use of carbon and nitrogen isotope ratios in termite research. Ecol. Res. 13: 377-387
Tayasu I., Abe T., Eggleton P. and Bignell D.E. 1997. Nitrogen and carbon isotope ratios in termites: an indicator of trophic habit along the gradient from wood-feeding to soil-feeding. Ecol. Entomol. 22: 343-351
Tayasu I., Hyodo F., Takematsu Y., Sugimoto A., Inoue T., Kirtibutr N. and Abe T. 2000. Stable isotope ratios and uric acid preservation in termites belonging to three feeding habits in Thailand. Isotopes Environ. Health Stud. 36: 259-272
Tillberg C.V. and Breed M.D. 2004. Placing an omnivore in a complex food web: Dietary contributions to adult biomass of an ant. Biotropica 36: 266-272
Tillberg C.V., McCarthy D.P., Dolezal A.G. and Suarez A.V. 2006. Measuring the trophic ecology of ants using stable isotopes. Insect. Soc. 53: 65-69
Tilman D. 1982. Resource Competition and Community Structure. Princeton University Press, Princeton, N.J. 296 pp
Van Mele P. and Cuc N.T.T. 2000. Evolution and status of Oecophylla smaragdina (Fabricius) as a pest control agent in citrus in the Mekong Delta, Vietnam. Int. J. Pest Manage. 46: 295-301
Vanderklift M.A. and Ponsard S. 2003. Sources of variation in consumer-diet δ15N enrichment: a meta-analysis. Oecologia 136: 169-182
Wäckers F.L. 2004. Assessing the suitability of flowering herbs as parasitoid food sources: flower attractiveness and nectar accessibility. Biol. Cont. 29: 307-314
Watanabe H. and Tokuda G. 2010. Cellulolytic systems in insects. Annu. Rev. Entomol. 55: 609-632
Wilson E.O. 1958. The beginnings of nomadic and group-predatory behavior in the Ponerine ants. Evolution 12: 24-31
Yumoto T. and Nakashizuka T. 2005. The canopy biology program in Sarawak: Scope, methods, and merit. In: Pollination Ecology and the Rain Forest (Roubik D.W., Sakai S. and Hamid A.A., Eds). Springer, New York. pp 13-21
Acknowledgments
We thank Josef Kendawang (Forest Department, Sarawak) and Lucy Chong (Sarawak Forestry Corporation) for permission for research in Sarawak. We also thank Tohru Nakashizuka (Tohoku University) and Het Kaliang (Sarawak Forestry Corporation) for kind arrangement to conduct the field sampling, Seiki Yamane (Kagoshima University) for identification of ants and sphecid wasps and helpful discussion, Shuichi Ikudome (Kagoshima Women’s Junior College) for identification of bees and two anonymous reviewers and the Associate Editor for constructive comments on an earlier draft of this manuscript. This study was supported by Research Institute for Humanity and Nature, Japan (P3-1 and P3-5), by Grant-in-Aids (no. 17405006 to T.I., and no. 16405009 to Y.T.) and partly by Special Coordination funds for Promoting Sciences and Technology from the Japanese Ministry of Education, Science, and Culture. F.H. and T.M. were supported by the Research Fellowships of the Japan Society for the Promotion of Science (JSPS) for Young Scientists.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
δ15N and δ13C values of Hymenoptera and Isoptera examined in this study.
No. | Taxa | n | δ15N (‰) | δ13C (‰) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
Mean (SE) | Range (min–max) | Mean (SE) | Range (min–max) | |||||||
Hymenoptera | ||||||||||
Ants | ||||||||||
Formicidae | ||||||||||
1 | Crematogaster borneensis | 5 | −1.2 (0.6) | D | hi | −2.3 to 0.8 | −33.9 (0.7) | C | g | −35.7 to −31.7 |
2 | Crematogaster difformis | 5 | 2.6 (0.5) | B | de | 1.0 to 3.7 | −26.4 (0.2) | A | ab | −27.0 to −25.9 |
3 | Camponotus gigas | 9 | 1.8 (0.4) | B | def | 0.5 to 4.3 | −26.9 (0.2) | A | ab | −27.7 to −26.2 |
4 | Leptogenys diminuta | 3 | 6.3 (0.6) | A | ab | 5.0 to 7.0 | −26.6 (0.2) | A | abc | −26.9 to −26.3 |
5 | Tetraponera attenuata | 16 | 0.3 (0.1) | C | gh | −1.1 to 1.3 | −28.4 (0.2) | B | cdef | −29.8 to −26.3 |
6 | Oecophylla smaragdina | 4 | 3.1 (0.2) | B | d | 2.6 to 3.5 | −26.5 (0.2) | A | ab | −27.1 to −26.0 |
Bees | ||||||||||
Apidae | ||||||||||
7 | Apis spp. | 3 | −2.0 (0.1) | B | ij | −2.2 to −1.7 | −25.4 (0.3) | A | a | −25.8 to −24.7 |
8 | Trigona spp. | 10 | −0.6 (0.1) | A | ghi | −1.5 to 0.0 | −27.2 (0.3) | B | abcd | −28.2 to −25.8 |
Anthophoridae | ||||||||||
9 | Amegilla sp. | 1 | −0.7 | −28.9 | ||||||
Halictidae | ||||||||||
10 | Nomia sp. | 1 | 1.0 | −31.0 | ||||||
11 | Megachilidae sp. | 1 | 1.8 | −23.2 | ||||||
Wasps | ||||||||||
12 | Braconidae sp. | 2 | −0.1 | −0.9 to 0.7 | −26.2 | −26.8 to −25.5 | ||||
13 | Ichneumonidae sp. | 4 | 5.9 (0.9) | AB | bc | 4.0 to 8.0 | −26.9 (0.7) | A | abcd | −28.6 to −24.9 |
Nyssonidae | ||||||||||
14 | Bembix sp. | 3 | 8.3 (0.9) | A | a | 6.7 to 9.9 | −25.4 (0.0) | A | a | −25.5 to −25.3 |
15 | Bembecinus sp. | 3 | 3.6 (0.7) | BC | cd | 2.2 to 4.6 | −26.5 (0.0) | A | ab | −26.6 to −26.5 |
16 | Pompilidae sp. | 2 | 7.4 | 5.4 to 9.4 | −25.3 | −26.1 to −24.5 | ||||
17 | Scoliidae sp. | 1 | 2.8 | −27.3 | ||||||
Sphecidae | ||||||||||
18 | Chalybion bengalense | 1 | 8.3 | −28.4 | ||||||
Vespidae | ||||||||||
19 | Polybioides pescas | 2 | 2.0 | 1.8 to 2.2 | −26.2 | −26.4 to −26.0 | ||||
20 | Provespa anomala | 6 | 2.3 (0.3) | C | def | 1.3 to 3.3 | −26.8 (0.1) | A | ab | −27.3 to −26.0 |
21 | Ropalidia spp. | 3 | 1.1 (0.3) | C | defg | 0.5 to 1.5 | −27.3 (0.4) | A | abcdef | −28.0 to −26.7 |
22 | Vespa affinis | 2 | 6.1 | 5.5 to 6.7 | −26.5 | −26.9 to −26.2 | ||||
Termites | ||||||||||
Isoptera | ||||||||||
Termitidae | ||||||||||
23 | Hospitalitermes hospitalis | 4 | −3.4 (0.3) | E | j | −4.0 to −2.6 | −28.9 (0.4) | B | def | −29.7 to −27.9 |
24 | Macrotermes malaccensis | 5 | −1.6 (0.2) | D | ij | −2.2 to −1.1 | −27.0 (0.4) | A | abcd | −28.4 to −26.1 |
25 | Odontotermes sarawakensis | 1 | 0.3 | −25.4 | ||||||
26 | Odontotermes denticulatus | 1 | 2.1 | −25.9 | ||||||
27 | Microcerotermes sabahensis | 4 | −0.4 (0.3) | CD | ghi | −0.9 to 0.6 | −29.2 (0.3) | B | e | −30.0 to −28.2 |
28 | Longipeditermes longipes | 3 | 0.5 (0.2) | BC | efghi | 0.1 to 0.9 | −29.3 (0.2) | B | ef | −29.6 to −29.0 |
29 | Homallotermes foraminifer | 9 | 0.7 (0.4) | B | fg | −1.0 to 2.6 | −27.6 (0.4) | A | bcdef | −29.6 to -25.9 |
30 | Prohamitermes mirabilis | 9 | 5.9 (0.2) | A | b | 5.1 to 6.6 | −27.5 (0.2) | A | bcdf | −28.1 to −26.7 |
31 | Termes rostratus | 2 | 5.6 | 4.9 to 6.4 | −25.3 | −25.4 to −25.2 | ||||
32 | Dicuspiditermes nemorosus | 4 | 6.7 (0.2) | A | ab | 6.3 to 7.2 | −26.7 (0.2) | A | ab | −27.1 to −26.2 |
Rights and permissions
About this article
Cite this article
Hyodo, F., Takematsu, Y., Matsumoto, T. et al. Feeding habits of Hymenoptera and Isoptera in a tropical rain forest as revealed by nitrogen and carbon isotope ratios. Insect. Soc. 58, 417–426 (2011). https://doi.org/10.1007/s00040-011-0159-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00040-011-0159-9