Abstract
Although variation in body size has been recently reported in stingless bees (Meliponini), empirical evidence evaluating possible factors related to such variation is lacking, and thus it is not clear if it may have an adaptive significance. We evaluated if variation in the body size and weight of workers of stingless bees fluctuates across a seasonal pattern and if this could be related to characteristics of the food consumed during the larval stage. The weight of larval provisions, their protein, and sugar content were evaluated in four colonies of Nannotrigona perilampoides every 2 months across 1 year. Worker-destined larvae from the same combs were allowed to develop and were sampled as callow workers to determine their weight and size using morphometric data. The weight and size of workers were highly correlated and varied across the seasons in established colonies, suggesting that size variation cycles across the year in stingless bees. An increase in the protein content and, to a lesser degree, the quantity of larval food were positively linked to variation in body weight and size; food with richer protein content resulted in larger and heavier workers. This study provides the first evidence of an effect of the quantity and composition of larval food on the size of workers in stingless bees. Although body weight and size of workers differed across seasons, they were not readily noticeable as changes seem to occur as a continuum across the year. Since size polymorphism was of a larger magnitude across time but not within age cohorts and as it was highly determined by food resources, it may not be an adaptive feature in stingless bees. However, more studies are needed to determine the role of the cyclical change in worker body size on colony performance and thus its adaptive significance in stingless bees.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Body size shows significant variation in non-social bees, and it is frequently correlated with the reproductive success and fitness of both males and females (Smith and Weller, 1989; Richards and Packer, 1996; Bosch and Vicens, 2006; Zobel and Paxton, 2007). In non-apid highly social insects, such as leafcutter ants, variation in worker body size or alloethism is a common feature of colonies (Hölldobler and Wilson, 1990; Medan and Josens, 2005). A similar phenomenon is found in the primitively eusocial bumble bees (Goulson et al., 2002; Spaethe and Weidenmüller, 2002), a sister taxon to the highly social Apini and Meliponini (Cameron, 1993; Cameron and Mardulyn, 2001). Body size variation in bumble bees seems to be adaptive: foraging efficiency increases as a result of larger workers collecting resources whilst small ones concentrate on tasks within the nest (Goulson et al., 2002; Spaethe and Weidenmüller, 2002). Moreover, it does not depend on colony size or amount of food stores (Couvillon et al., 2010).
The Apini and Meliponini have not been thoroughly studied with respect to worker size variation. Nevertheless, body size variation has been acknowledged in honey bees and stingless bees, although to a lesser extent compared with non-social species (Waddington et al., 1986; Kolmes and Sam, 1991; Nazzi, 1992; Ramalho et al., 1998; Goulson et al., 2005; Quezada-Euán et al., 2008). However, size differences in eusocial bee workers may only be a result of environmental factors and may not result in environment-specific fitness trade-offs. For instance, in stingless bees, newly relocated colonies experience a reduction in worker population and produce smaller workers (Ramalho et al., 1998). Additionally, workers experience changes in weight during their adult life as they age due to glandular and ovarian development (León-Contrera et al., 2006).
In general few studies have addressed the effect of the composition of larval provisions on the resulting offspring in bees. In the halictid bee Lassioglossum zephyrum, Roulston and Cane (2002) experimentally manipulated provisioning gyne access to diets with different protein content; offspring showed up to 25% variation in body size depending on the composition of the food they received. These results suggest that seasonal variation in food composition should ultimately affect development and adult size. Although the quantity and composition of larval food could also influence body size in eusocial bees and thus explain worker polymorphism, their effect has not been evaluated.
In honey bees, in contrast with most other bee taxa, the worker larvae food is mainly of glandular origin, and larval provisioning is controlled by the adult workers, who progressively feed them small quantities of secretion (Huang and Otis, 1991). This may explain why adult worker honey bees are more uniform in size compared with other bee taxa (Roulston and Cane, 2000). First, the nutritional composition of glandular secretions may only vary across a very narrow range compared with the crude pollen and nectar provided to offspring of most other bees (Roulston and Cane, 2002; but see Sommeijer et al., 2009). Second, honey bee larvae are not mass provisioned but are rather progressively provisioned, giving adult workers the possibility to regulate food consumption by larvae (Huang and Otis, 1991).
In contrast, in the stingless bees, the sister group to the honey bees, pollen, and nectar/honey are the main components of the larval food and glandular secretions are not considered to make a significant contribution (Roulston and Cane, 2000; Velthuis et al., 2003). Moreover, stingless bees mass provision the brood cells, so adult worker size may be more directly affected by the size and composition of food resources compared with the situation in honey bees. Set against this, in proportion, stingless bees store larger quantities of pollen (Roubik, 1982), and worker body size may then be less affected by the amount of pollen that workers bring back to the colony because there is often a surfeit of pollen within the nest.
Size differences have been found between foragers and nest workers in three species of stingless bees but the causes of such pattern have not been determined (Goulson et al., 2005). Body size variation of workers has been recently reported in the stingless bee Nannotrigona perilampoides (López-Velasco, 2009, unpubl. data). We used this species as a model to evaluate if in stingless bees body size differs in workers produced at different times and if this can be attributed to variation in the characteristics of the larval food. To test this hypothesis we measured the provisions to larval cells and its composition at different periods of time and related these parameters with the size of the resulting individuals. The results from this study should provide information on the origin of worker body size variation and thus, on its possible adaptive significance in stingless bees.
Materials and methods
Nannotrigona perilampoides is a common stingless bee species that is found across the Mexican tropics, with the most northern distribution of any stingless bee species in the Americas (Ayala, 1999). A colony can contain up to 2,000 individuals and can be easily transferred to, and maintained in, wooden hives. Our studies were conducted at the Department of Apiculture of the Campus of Biological Sciences at the Universidad Autónoma de Yucatán, México, where N. perilampoides is native.
We evaluated four colonies across a whole year. All experimental colonies were strong, with a worker population >1,500 bees, and were housed in wooden hives (30 × 14 × 9 cm) for at least 6 months before the investigation started. The number of pollen and honey pots in all colonies was set to ca. 50 at the start of the experiments but, after that, no manipulation of colony food reserves was conducted nor was food provided to the colonies so they survived only on the resources that foragers collected in the area.
The study was divided into three steps. First, new combs were used to estimate the volume of larval provisions as well as the amount of protein and the percentage sugar of those larval provisions. Second, worker-destined larvae from the same combs were allowed to develop and the resulting adults were weighed and measured (morphometric data). Third, both sets of data were correlated to determine the relationship between the amount or composition of larval food and body size or weight of resultant adult workers.
Quantity and composition of the larval food
New combs were collected from each of the four experimental colonies every 2 months, starting in November 2007 and finishing in September 2008. Between 15–20 provisioned and sealed cells into which a queen had recently oviposited were randomly selected from each comb and opened, the eggs were carefully removed and all the larval food removed with a micropipette tip and weighed. Then the contents were analyzed to determine the percentage of total sugars and the amount of protein. The percentage sugars were calculated by means of a refractometer (PDX-95 Digital Refractometer Brix 0–95%). Protein estimation was conducted by fluorescent detection (Bio-Protein assay®) using a microplate reader (BECKMAN COULTER DTX 880 Multimode Detector) at 590 nm at the Laboratory of Molecular Genetics in the Universidad Autónoma de México. The amount of protein in μg/ml for each sampled cell was obtained using the general calculations of Bradford (1976). The total weight, the percentage sugars and the amount of protein in the larval food were compared between colonies and among the six sampling periods by a repeated measures ANOVA and Tukey tests.
Body weight and morphometric measurements
After the larval food was extracted, the comb was cleaned with water and the remaining cells containing sealed cells were returned to their mother colonies. Thirty days later, the same combs containing mature pupae were retrieved from their natal colonies, taken to the lab, and kept at 28 ± 1°C and 80–90% humidity in an incubator (Lab-Line Biotronette Chamber). The developmental period of workers in this species is 36–38 days at these conditions (pers. obs.). Between 12 and 15 workers were selected from among the emerging individuals in each comb; they were killed by keeping them in an ultra low freezer for 20 s, and then dried at 60°C for 24 h to obtain their dry weight. After weighing each individual, bees were rehydrated in Dietrich solution to soften the body. The head and the thorax were dissected from each worker and the size of the following variables, which are recognized as good indicators of size in bees (Cane, 1987; Hartfelder and Engels, 1992; Bullock, 1999), were recorded: head length (HL), head width (HW), lower interocular distance (LID), upper interocular distance (UID), intertegular distance (ITD), length (LM) and width of the mesonotum (MW). The measures were made with an inverted microscope and converted to micrometers with the software AFUSDA (W. Rubink, pers. comm.).
A Principal Components Analysis (PCA) was conducted on the morphometric variables to obtain a single measure of size (Wiley, 1981; Pignata and Diniz-Filho, 1996). The PCA was carried out based on a correlation matrix (verified by means of the Keiser–Meyer–Olkin measure of sampling adequacy and Bartlett’s test of sphericity) and analyzed using VARIMAX axis rotation. The correlation matrix procedure was selected as the derived Principal Component 1 (PC1) is better related with size (Wiley, 1981). A final measure of body size was given by the PC scores that were calculated for each individual as the product of the resulting coefficients in each PC. A repeated measure ANOVA was applied on body weight and size using the individual scores of PCs one to three across the six time periods. The repeated measure ANOVA would also provide evidence of the relative magnitudes of size variation within a period (the effect of colony) and across the whole study (the effect of period). These results were complemented with the distribution of individual PC scores that were plotted against PC1 and PC2.
Relationship between quantity and composition of larval food with body weight and size
The magnitude of the relationship between body sizes, as indicated by the scores of individuals at PC 1, with their dry weight was analyzed using Pearson’s correlation. We also used Pearson’s correlations to determine if a relationship existed between worker body size and the quantity or composition (protein and sugar content) of larval food.
All statistical analyses were conducted with the SAS statistical package (SAS, 2002).
Results
Quantity and composition of larval food
Significant variability across the year was found in the total amount of larval food deposited in cells (F 5,287 = 4.028, p < 0.001; Table 1 and Fig. 1). However, Tukey tests revealed similar quantities of food per cell in July, September and November (p > 0.01; Supplementary Table A). The mean weight of larval food per cell across the year was 15.4 ± 0.4 mg. The lowest quantities of larval food were collected in January (13.7 ± 0.2 mg) and the highest in May (17.13 ± 0.4 mg). Colonies also varied in how much larval food they provisioned cells at a given sampling period (colony effect, Table 1), and in their seasonal variation in larval food provisions (interaction effect, Table 1).
Significant variation in the composition of the food was also detected between sampling periods, both in the percentage of sugars (F 5,287 = 183.6, p < 0.001) and protein content (F 5,287 = 841.5, p < 0.001). Tukey tests for the percentage of sugars revealed similar values for the months of March and July (p > 0.01); however, the values for protein content differed across all six periods. The lowest percentage of sugar (41.2% ± 1.8) was seen in the larval food collected in May and the highest in November (44.7% ± 1.7). Protein content ranged from 7.19 (±1.6) ng/μl in November to 15.78 (±1.5) ng/μl in May (Fig. 1). There were again significant differences between colonies in the content of larval food provisions and in how that composition varied across the year (Table 1).
Body weight and morphometric measurements
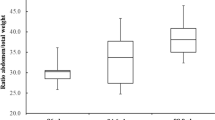
Worker dry weight showed significant differences between sampling periods, except for the measures taken in January and September (p > 0.01; Table 1 and Fig. 2). The lowest adult dry weight was seen in the individuals collected in November (1.54 mg ± 0.23) and the highest in May (3.06 mg ± 0.12) (Fig. 2 and Supplementary Table B), almost twice as heavy as in November. There were subtle between-colony differences in dry weight, but the interaction term colony × sampling period was not significant (Table 1).
The PCA of the seven morphometric meristic characters was justified, as indicated by the Kaiser–Meyer–Olkin measure of sampling adequacy of 0.79 (see comparisons for morphometric variables at the univariate level in Supplementary Table B). Bartlett’s test of sphericity was significant (<0.001), indicating that the matrix was not an identity matrix and confirming that the variables in the matrix were not correlated and, thus, suitable for a PCA.
The first three PCs explained 70.2, 15.1, and 12.7% of the variation in the morphometric data, respectively. The positive and high correlations of all seven variables with PC 1 confirmed that this factor was related with adult body size (see Supplementary Table C for PC correlation coefficients).
There were significant differences between the scores for PC1 across periods (Fig. 2, see Supplementary Table D) compared with the effect of colony and interaction terms that were non-significant (Table 1). The largest values of PC1 were seen in bees collected in May and the lowest in November, which is in accordance with the variation seen at the univariate level (Supplementary Table B). Similarly, plots of colony scores for PCs 1 and 2 made evident that body size differed significantly between sampling periods, with the November bees clustered around the lowest values of PC1 and the bees from May grouped at the other extreme (Fig. 3). It is thus evident that body size cycles across the year, with the peak in body size in the period March–July when resources were most abundant in this region (Flores, 1990). Moreover, body size variation within each period was much lower compared with the variation between contiguous periods as also seen in Fig. 3.
Relationship between quantity or composition of larval food and body weight or size
Body weight was closely correlated with PC1 scores (r 2 = 0.88), which indicates that there is a high association between the weight of a given individual and its overall body size (Fig. 4).
The relationships between characteristics of the larval food with body size are shown in Fig. 5. There was a highly significant correlation between the amount of protein and the size of individuals (r 2 = 0.93). The quantity of food also had a significant but lesser influence on body size (r 2 = 0. 429) than protein content. However, the percentage of sugar had no influence on the size of the resultant individuals (r 2 = −0.265).
Discussion
Our results reveal that body weight and size varied across the year in established colonies of the stingless bee N. perilampoides, suggesting that it is a cyclical phenomenon in this and possibly other species of stingless bees. Our findings also indicate that body weight and size were highly correlated, and that an increase in the protein content (and to a lesser degree the quantity) of larval food were positively linked to variation in both adult weight and size. In other words, larvae whose cell received a provision with richer protein became larger and heavier workers. It was also evident that body weight and size differed significantly amongst workers raised in different periods (2 months) compared with those raised within a short period. Moreover, although body weight and size changed across periods, they were relatively modest, up to twofold, compared with bumble bees, where a tenfold difference between sibling workers can be seen within a colony at any point in time and across its whole life cycle (Couvillon et al., 2010).
In stingless bees it had been proposed that the amount of larval food in a cell could affect the size of a worker (Ramalho et al., 1998; Goulson et al., 2005); this is clearly demonstrated by our results. The quantity and composition of larval food provisions in N. perilampoides varied markedly across the year. However, the effect of food quantity was moderate compared with the effect of protein content on adult body size. The effect of the amount of food during the larval stage on adult body size has been demonstrated in various bee taxa, such as Megachile (Klostermeyer et al., 1973), Ceratina (Johnson, 1990) and Osmia (Bosch and Vicens, 2006). Similarly, in bumble bees, in which progressive feeding of the larvae occurs, there is a strong correlation between the feeding rate (i.e. amount of food) and the biomass of the resultant workers (Pendrel and Plowright, 1981; Ribeiro, 1994; Ribeiro et al., 1999).
Protein was the larval food component that showed the largest variation, up to a threefold difference across the year and between N. perilampoides colonies. This is in contrast with earlier evaluations of larval food in stingless bees, in which protein content seemed constant within seven species of stingless bees (Hartfelder and Engels, 1989). However, Hartfelder and Engels’ (1989) food samples were taken within a short period of time, probably not allowing them to detect effects of seasonal variation in larval food quality. Moreover, we found that sugar and protein were inversely related, and larvae whose food contained proportionally more sugar than protein had lower weights and smaller bodies. This may occur when pollen input to the colony or pollen reserves decrease, and worker bees may use more nectar in the preparation of the larval food.
Considering that pollen is the main proteinaceous ingredient in the diet of stingless bee larvae (Velthuis et al., 2003), the high variability in protein content found in N. perilampoides larval food could be attributed to variation in the protein of the pollens that were used to feed the larvae. In a controlled experiment with the sweat bee L. zephyrum, the largest offspring were reared from cells that were provisioned with pollen of the highest protein content (Roulston and Cane, 2002). Nevertheless, since we did not evaluate the relative amount and protein content of pollen in N. perilampoides colony stores (pollen pots) or of pollen recently collected, it is difficult to determine the source of variation in larval protein provisions that we observed.
In Melipona stingless bees, Roubik (1982) found that the production of brood decreased during dearth, even in colonies with large food stores. These observations may reflect the fact that brood cells are provisioned only in proportion to the amount of incoming pollen. Alternatively, or in addition, they may reflect the lower protein quality (e.g. protein content or digestibility) of pollen sources, either those being actively collected or those stored in the colony at that time. Variation in body size may be subject to similar factors; when pollen of high quality (e.g. protein content or digestibility) is available, then colonies produce larger individuals. In honey bees there is no evidence for selection of pollens in accordance to their quality (Van der Moezel et al., 1987). It is not known whether stingless bees show a preference for the collection of pollen with respect to their quality. But since in general insects do not directly assay protein in their food (Chapman, 1998), pollen collection by stingless bees may be determined by its availability rather than its quality. Moreover, feeding of larvae in stingless bees is through a mass provision in a cell that is later sealed; thus, it is not possible to adjust the quantity or composition of the larval food as richer sources become available. High protein pollens that are available during a certain period of the year may directly influence the size of the bees produced at that time, as seen in our study, just because they are available then.
Although we found evidence of within-colony size variation across different periods in stingless bees its adaptive significance is not clear. In bumble bees several lines of evidence suggest that body size variation may be adaptive and that colonies benefit from rearing workers of a range of sizes (Goulson et al., 2002). First, body size differences in bumble bees are apparently not related to either food stores or the age of the colony, i.e., worker polymorphism is a permanent feature and not temporary (Couvillon et al., 2010). Second, it may increase colony fitness as larger workers perform foraging activities and collect more resources compared with small ones, which concentrate on tasks within the nest (Goulson et al., 2002; Spaethe and Weidenmüller, 2002), and when nectar is scarce, small bumble bee workers remain alive significantly longer than larger workers, possibly acting as insurance against times of nectar shortage (Couvillon and Dornhaus, 2010).
So far, most of those characteristics remain to be investigated in stingless bees. However, our evidence suggests that within-colony size polymorphism is a temporary feature in stingless bees mainly depending on food income which is not the case in bumble bees where size polymorphism is permanent and independent of colony food or age condition (Couvillon et al., 2010). Moreover, in stingless bees size differences in workers reared within a period were small compared with those between periods. In accordance with these evidences, the finding of large foragers and small within-nest bees in some species of stingless bees (Goulson et al., 2005) could merely reflect nutritional differences between the two groups at a point in time when older bees were better nourished during their larval development than their younger sisters. Thus, small workers in stingless bees may be the result of making the “best in a bad situation” as in other insects (Blanckenhorn, 2009) and not have an adaptive explanation.
Body size, however, can affect numerous traits associated with colony survival. For instance, during times of abundant resources, larger workers may be able to travel larger distances and collect more food and build materials which, coupled with a larger population of workers, could result in a better exploitation of resources in the surrounding areas. Larger food stores are associated with the production of sexuals and ultimately colony reproduction (Moo-Valle et al., 2001; Slaa, 2006; Quezada-Euán et al., 2008). As a corollary, in times of scarce floral resources, colonies reduce their worker population (Moo-Valle et al., 2004) and produce smaller workers, as evident in our study.
In contrast, after predation or when colonies are transported between sites, the population of forager bees may dwindle. In turn, food income may decrease and thus brood cells may be provisioned with lesser food and smaller individuals may be produced. It seems that such small workers could be more efficient foragers per unit body weight that could help in the recovery of the population of the colony (Ramalho et al., 1998). These arguments assume a relatively short lifespan of adult stingless bee workers.
Stingless bees have a low translocation capacity both at the individual (physogastric queens cannot fly, workers have limited flight ranges) and colony levels (colonies are dependent on their food and resin stores), making it difficult for them to migrate (unlike the case of the honey bee) in search for better environmental conditions in the event of food stress (Engels and Imperatriz-Fonseca, 1990; Biesmeijer et al., 1999). Decrease in the production of sexuals, reduction in the queen’s egg-laying rate, increased longevity of workers, cannibalism of larvae and eggs, reproductive diapause observed in some species, and reduction in body size are some of the responses of stingless bee colonies to food deprivation (Roubik, 1982; Moo-Valle et al., 2001, 2004; Alves et al., 2009).
Further studies are needed to determine the role of cyclical changes in worker body size on colony performance and its value as an adaptive strategy in stingless bees.
References
Alves D.A., Imperatriz-Fonseca V.L. and Santos-Filho P.S. 2009. Production of workers, queens and males in Plebeia remota colonies (Hymenoptera, Apidae, Meliponini), a stingless bee with reproductive diapauses. Gen. Mol. Res. 8: 672-683
Ayala R. 1999. Revisión de las abejas sin aguijón de México (Hymenoptera: Apidae: Meliponini). Folia Entomol. Mex. 106: 1-123
Biesmeijer J.C., Born M., Lukacs S. and Sommeijer M.J. 1999. The response of the stingless bee Melipona beecheii to experimental pollen stress, worker loss and different levels of information input. J. Apic. Res. 38: 33-41
Blanckenhorn W.U. 2009. Causes and consequences of phenotypic plasticity in body size: the case of the yellow dung fly Scathophaga stercoraria (Diptera: Scathophagidae) In: Phenotypic Plasticity of Insects: Mechanism and Consequences (Whitman D.W. and Ananthakrishnan T.N., Eds) Enfield NH, USA. pp 369-422
Bosch J. and Vicens N. 2006. Relationship between body size, provisioning rate, longevity and reproductive success in females of the solitary bee Osmia cornuta. Behav. Ecol. Sociobiol. 60: 26-33
Bradford M.M. 1976. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72: 248-254
Bullock S.H. 1999. Relationships among body size, wing size and mass in bees from a tropical dry forest in Mexico. J. Kansas Entomol. Soc. 72: 426-439
Cameron S. 1993. Multiple origins of advanced eusociality in bees inferred from mitochondrial DNA sequences. Proc. Natl Acad. Sci. USA 90: 8687-8691
Cameron S.A. and Mardulyn P. 2001. Multiple data sets suggest independent origins of highly eusocial behavior in bees (Hymenoptera: Apinae). Syst. Biol. 50: 194-214
Cane J.H. 1987. Estimation of bee size using intertegular span (Apoidea). J. Kansas Entomol. Soc. 60:145-147
Chapman R.F. 1998. The Insects: Structure and Function. Cambridge, Univ. Press, UK. 770 pp
Couvillon M.J. and Dornhaus A. 2010. Small worker bumble bees (Bombus impatiens) are hardier against starvation than their larger sisters. Insect. Soc. 57: 193-197
Couvillon M.J., Jandt J.J., Duong N. and Dornhaus A. 2010. Ontogeny of worker body size distribution in bumble bee (Bombus impatiens) colonies. Ecol. Entomol. 35: 424-435
Engels W. and Imperatriz-Fonseca V.L. 1990. Caste development, reproductive strategies and control of fertility in honey bees and stingless bees. In: Social Insects an Evolutionary Approach to Castes and Reproduction (Engels W., Ed). Springer, Berlin. pp 166-230
Flores J.S. 1990. The flowering periods of Leguminosae in the Yucatan peninsula in relation to honey flows. J. Apic. Res. 29: 82-88
Goulson D., Peat J., Stout J., Tucker J., Darvill B., Derwent L.C. and Hughes W.O.H. 2002. Can alloethism in workers of the bumblebee, Bombus terrestris, be explained in terms of foraging efficiency? Anim. Behav. 64: 123-130
Goulson D., Derwent L.C. and Peat J. 2005. Evidence for alloethism in stingless bees (Meliponinae). Apidologie 36: 411-412
Hartfelder K. and Engels W. 1989. The composition of larval food in stingless bees: evaluating nutricional balance by chemosystematic methods. Insect. Soc. 36: 1-14
Hartfelder K. and Engels W. 1992. Allometric and multivariate analysis of sex and caste polymorphism in the neotropical stingless bee, Scaptotrigona postica. Insect. Soc. 39: 251-266
Hölldobler B. and Wilson E.O. 1990. The Ants. Harvard University Press, Cambridge, Mass. 732 pp
Huang Z. and Otis G.W. 1991. Inspection and feeding of larvae by worker honey bees (Hymenoptera: Apidae): effect of starvation and food quantity. J. Insect Behav. 4: 305-317
Johnson M.D. 1990. Female size and fecundity in the small carpenter bee, Ceratina calcarata (Robertson) (Hymenoptera: Antophoridae). J. Kansas Entomol. Soc. 63: 414-419
Kolmes S.A. and Sam Y. 1991 Relationship between sizes of morphological features in worker honey bees (Apis mellifera). J. N.Y. Entomol. Soc. 99: 684-690
Klostermeyer E.C., Mech J.S. Jr. and Rasmussen W.B. 1973. Sex and weight of Megachile rotundata (Hymenoptera: Megachilidae) progeny associated with provision weights. J. Kansas Entomol. Soc. 46: 536-548
León-Contrera F.A., Imperatriz-Fonseca V.L. and Koedam D. 2006. Age-dependent mass variation in the stingless bee Melipona quadrifasciata (Apidae, Meliponini). Braz. J. Morphol. Sci. 23: 321-324
Medan V. and Josens R.B. 2005. Nectar foraging behaviour is affected by ant body size in Camponotus mus. J. Insect Physiol. 51: 853-860
Moo-Valle H., Quezada-Euán J.J.G. and Wenseleers T. 2001. The effect food reserves on the production of sexual offspring in the stingless bee Melipona beecheii (Apidae: Meliponini). Insect. Soc. 48: 398-403
Moo-Valle H., Quezada-Euán J.J.G., Canto-Martín J. and González-Acereto J.A. 2004. Caste ontogeny and distribution of reproductive cells on the combs of Melipona beecheii (Apidae: Meliponini). Apidologie 35: 587-594
Nazzi F. 1992. Morphometric analysis of honey bees from an area of racial hybridization in northeastern Italy. Apidologie 23: 89-96
Pendrel B.A. and Plowright R.C. 1981. Larval feeding by adult bumble bee workers (Hymenoptera: Apidae). Behav. Ecol. Sociobiol. 8: 71-76
Pignata M.I.B. and Diniz-Filho J.A.F. 1996. Spatial autocorrelation and evolutionary constraints in worker body size of some Neotropical stingless bees (Hymenoptera: Apidae). Heredity 76: 222-228
Quezada-Euán J.J.G., May-Itzá W.J., Valladares P. and De la Rúa P. 2008. Variación fenotípica en obreras y su relación con la producción de miel en colonias de Melipona beecheii B. (Hymenoptera:Meliponini). In: Memorias V Congr. Mesoamericano Abejas sin Aguijón. Univ. Aut. Yucatán. pp 106-112
Ramalho M., Imperatriz-Fonseca V.L. and Giannini T.C. 1998. Within-colony size variation of foragers and pollen load capacity in the stingless bee Melipona quadrifasciata anthidioides Lepeletier (Apidae, Hymenoptera). Apidologie 29: 221-228
Ribeiro M.F. 1994. Growth in bumble bee larvae: relation between development time, mass, and amount of pollen ingested. Can. J. Zool. 72: 1978-1985
Ribeiro M.F., Velthuis H.H.W., Duchateau M.J. and Tweel I. van D. 1999. Feeding frequency and caste differentiation in Bombus terrestris larvae. Insect. Soc. 46: 306-314
Richards M.H. and Packer L. 1996. The sociology of body size variation in the primitively eusocial sweat bee Halictus ligatus (Hymenoptera: Halictidae). Oikos 77: 68-76
Roubik D.W. 1982. Seasonality in colony food storage, brood production and adult survivorship: studies of Melipona in tropical forest (Hymenoptera: Apidae). J. Kansas Entomol. Soc. 55: 189-800
Roulston T.H. and Cane J.H. 2000. The effect of diet breadth and nesting ecology on body size variation in bees (Apoidea). J. Kansas Entomol. Soc. 73: 129-142
Roulston T.H. and Cane J.H. 2002. The effect of pollen protein concentration on body size in the sweat bee Lasioglossum zephyrum (Hymenoptera: Apiformes). Evol. Ecol. 16: 49-65
SAS 2002. Ver. 9. SAS Institute, Cary, NC
Smith B.H. and Weller C. 1989. Social competition among gynes in halictine bees: the influence of bee size and pheromones on behavior. J. Insect Behav. 2: 397-411
Sommeijer M.J., Rooijakkers E.F., Jacobusse C. and Kerkvliet J.D. 2009. Larval food composition and food plants of the solitary bee Colletes halophilus (Hymenoptera: Colletidae). J. Apic. Res. and Bee World 48: 149-155
Slaa E.J. 2006. Population dynamics of a stingless bee community in the seasonal dry lowlands of Costa Rica. Insect. Soc. 53: 70-79
Spaethe J. and Weidenmüller A. 2002. Size variation and foraging rate in bumblebees (Bombus terrestris). Insect. Soc. 49: 142-146
Van der Moezel P.G., Delfs J.C., Pate J.S., Loneragan W.A. and Bell D.T. 1987. Pollen selection by honeybees in shrublands of the Northern Sandplains of Western Australia. J. Apic. Res. 26: 224-232
Velthuis H.H.W., Cortopassi-Laurino M., Pereboom Z. and Imperatriz-Fonseca V.L. 2003. Speciation, development, and the conservative egg of the stingless bee genus Melipona. Proc. Exper. Appl. Entomol. NEV Amsterdam 14: 53-57
Waddington K.D., Herbst L.H. and Roubik D.W. 1986. Relationship between recruitment systems of stingless bees and within-nest worker size variation. J. Kansas Entomol. Soc. 59: 95-102
Wiley E.O. 1981. Phylogenetics: the Theory and Practice of Phylogenetic Systematics. Wiley, New York. 456 pp
Zobel M. and Paxton R.J. 2007. Is big the best? Queen size, usurpation and nest closure in a primitively eusocial sweat bee (Lasioglossum malachurum). Behav. Ecol. Sociobiol. 61: 435-447
Acknowledgments
This work was supported by grant CONACYT-SAGARPA-2002-C01-1556/A-1. We thank Dr. Rogelio Alonso Morales and the Universidad Nacional Autónoma de México (UNAM) for the facilities to perform the analyses of food quality. ALV’s MSc studies were supported by grant CONACYT 206972. We are also grateful to Dr. Luis Rodriguez for statistical suggestions.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Quezada-Euán, J.J.G., López-Velasco, A., Pérez-Balam, J. et al. Body size differs in workers produced across time and is associated with variation in the quantity and composition of larval food in Nannotrigona perilampoides (Hymenoptera, Meliponini). Insect. Soc. 58, 31–38 (2011). https://doi.org/10.1007/s00040-010-0113-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00040-010-0113-2