Abstract
Ants of the genus Pheidole are abundant and hyperdiverse, particularly in Neotropical rainforests. Very little is known, however, about the degree of ecological and behavioral differentiation of coexisting species comprising Pheidole communities. Additionally, the ecological role of the major worker subcaste, thought to be significant to the diversification of Pheidole, is poorly understood. We investigated the ecology and behavior of a ground-foraging Pheidole community of at least 56 species in Amazonian Ecuador. Pheidole species differed strongly in tolerance to flooding, nest site usage, foraging range, major worker foraging, and control of baits, but not in daily activity or ability to discover baits. A molecular phylogeny based on mitochondrial DNA was characterized by poorly resolved basal relationships and long terminal branches, suggesting an ancient diversification of many Pheidole lineages. Comparison of well-supported sister species suggests that both phylogenetic history and ecologically induced differentiation contribute to interspecific variation in Amazonian Pheidole. Ground-nesting species had larger major workers than twig-nesting species, whereas dominant species with stronger recruitment had a higher proportional abundance of major workers at baits. Variation among species suggests the presence of behavioral groups within the Amazonian Pheidole community that appear to segregate according to nest site usage and/or tolerance to flooding disturbance. Our results suggest an important role for major worker differentiation in the diversification of Pheidole.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The ant genus Pheidole has great significance in social insect biology due to its diversity, abundance, and use as a model for behavioral and ecological research. The dominance of Pheidole, particularly in Neotropical, ground-dwelling ant communities, has long been recognized (Fowler, 1993; Byrne, 1994; Tobin, 1995; Longino et al., 2002). However, we know very little about the behavioral ecology of Pheidole or the organization of tropical communities of this genus. Community studies on Barro Colorado Island, Panama showed that seven omnivorous, diurnal Pheidole species were similar in their use of ground and wood nest sites and recruitment behavior (Levings and Franks, 1982). Five Pheidole species found in subtropical Chaco in Argentina divided into large-bodied species with low recruitment and small-bodied, mass-recruiting species (Bestelmeyer, 2000). Diet and activity did not significantly differ among the two most common twig-nesting Pheidole at La Selva, Costa Rica, although foraging distance varied among four Pheidole species (Byrne, 1994). These studies suggest that interacting Pheidole may show niche differentiation, but the ecological and behavioral differentiation in hyperdiverse tropical Pheidole communities and the phylogeny of niche divergence have not been examined.
The most striking characteristic of Pheidole is its completely dimorphic worker caste: the majority of species possess a major worker subcaste morphologically distinguished from the minor worker subcaste by its disproportionately large head. Major workers (“soldiers”) are thought to be behaviorally specialized for colony defense or food-processing (Wilson, 2003). By concentrating defensive morphology into one subcaste, the major workers have been hypothesized as a key innovation that led to the extraordinary diversification of Pheidole (Wilson, 2003). However, Pheidole majors exhibit strong interspecific variation in behavior, including their ability to contribute to brood care and engage in foraging (Hölldobler and Möglich, 1980; Fowler, 1984; Wilson, 1984; Detrain, 1990; Patel, 1990; Brown and Traniello, 1998; Sempo and Detrain, 2004; Mertl and Traniello, 2009). Therefore, although majors may have evolved as defensive specialists, we expect to find strong interspecific variation in their morphology and behavior in a diverse Pheidole community. Variation in the functional ecology of major workers could be associated with nest site defense, tolerance to flooding, and foraging strategy.
The most common nesting sites of Neotropical Pheidole include ground (underground chambers) and litter (inside hollow twigs or between leaves; Tobin, 1995; Wilson, 2003). Nesting ecology could correlate with traits of Pheidole majors. For example, litter nests are spatially limited (Kaspari and Vargo, 1995), which may restrict the size of major workers in litter-nesting Pheidole, as size variation in majors is greater than that of minors (Wilson, 2003; Pie and Traniello, 2007). Litter nests are also more ephemeral than ground nests, and litter-nesting colonies frequently relocate (Byrne, 1994), reducing the need for nest defense when the costs of absconding are low (Droual, 1983). Overall, reduced defensive needs could lead to reduced size in majors of litter-nesting species.
Disturbance, in particular periodic flooding, could also play a role in structuring Pheidole communities (Kaspari et al., 2003; Mertl et al., 2009). We previously identified at least two tropical Pheidole species showing strong preference for habitats that periodically flood (Mertl et al., 2009). Adaptations to flooding may be similar to those shown by ants in response to disturbance in general, such as frequent nest re-localization and opportunistic foraging (Adis, 1997; King et al., 1998). These traits may correspond with major worker morphology and behavior: majors of Pheidole species common in flood-disturbed habitats may be smaller due to a reduced need for nest defense, and could play a more prominent role in foraging to allow opportunistic feeding on a larger size range of items.
Finally, the foraging strategy employed by Pheidole species may directly involve traits of the major worker subcaste. Foraging strategy in ants often involves trade-offs between behaviorally dominant, mass-recruiting species, and subordinate species that can quickly discover and/or insinuate themselves into food resources (Traniello, 1989; Davidson, 1998; Delsinne et al., 2007). Dominant Pheidole species that use mass-recruitment to defend food resources would be expected to involve major workers in foraging, whereas subordinate species would not require foraging by majors, which would pose an unnecessary risk and energetic cost.
How do ecological and behavioral traits vary within a clade of coexisting Pheidole species? Here we present a study of the ground-dwelling Pheidole in a lowland primary rainforest in Amazonian Ecuador, as well as a phylogeny of species in this community. The primary goals of our study were to: (1) catalog species richness and abundance; (2) investigate the degree of interspecific variation along multiple ecological and behavioral axes (tolerance to flooding disturbance, nest site use, foraging range, activity cycles, food discovery, dominance at food sources, and major worker foraging) as well as phylogenetic patterns of variation; and (3) analyze the functional ecology of major workers in structuring the Pheidole community by testing three hypotheses: (a) litter-nesting Pheidole will have smaller major workers than ground-nesting species, (b) flood-tolerant species will have smaller majors and higher major worker foraging than terra firme specialists, and (c) dominant species utilizing mass-recruitment will have higher foraging participation by major workers.
Methods and materials
Study site and sampling
Fieldwork was conducted at Tiputini Biodiversity Station (TBS), Orellana Province, Ecuador (0°37′55″S and 76°08′39″W, altitude 230 m, annual rainfall ≈ 3,000 mm) from January to April 2003, January–March 2004, July–August 2004, and December 2005–January 2006 (for site description, see Mertl et al. [2009]). Baiting and litter searching were used to locate colonies. Baiting involved establishing linear, 30 m transects at a minimum distance of 10 m from trails and other transects, sufficient to ensure Pheidole nests would not be sampled by multiple transects (Kaspari, 1996a). Bait (approx. 6 g peanut butter and 4 g cookie) was placed on thin plastic cards set out every meter. In preliminary tests, 6 g of canned tuna was also used, but Pheidole showed little attraction in comparison to other baits.
After bait placement, each station was observed at 15, 30, 60 and 90 min, and for each Pheidole species present, the number and subcaste of individuals was recorded, as was the distance between their nest and bait and the number of other ants present. If two nest entrances of the same species were found within 1 m (or within the same log or tree), ants from one entrance were transferred to the other to test for intraspecific aggression before recording entrances as separate nests. Each bait transect was repeated during 7:00–11:00 h. (morning), 13:00–18:00 h. (afternoon), and/or 20:00–24:00 h. (night), minimally 48 h apart to minimize colony satiation. Transects were established in terra firme and floodplain forest surrounding rivers and streams. In total, 26 bait transects were completed, 14 replicated in the morning and afternoon, 12 replicated in the morning, afternoon and night, 13 in terra firme, 13 in floodplain forest. Two 70 × 70 m baiting grids were also established (one in terra firme and one in floodplain) and baited every 10 m at intersections using similar methodology, including morning and night replicates.
We searched for litter nests to improve our Pheidole survey and abundance measurements for litter- and twig-nesting species (see Mertl et al., 2009 for search methodology). Samples of workers from all Pheidole nests found were collected. Only one twig-nesting species (P. amazonica) was polydomous. All nests of this species found within one m2 were therefore considered a single colony. The majority of plots (n = 84) were sampled January–March 2004, while 17 plots were sampled July–August 2004. These 101 plots were distributed among nine sites (three in terra firme, six in floodplain forest) and each site contained 10–14 plots, with the exception of one floodplain site which, due to small size, contained five plots. Each site was separated from other sites by a minimum of 300 m. In March 2003, three 5 × 5 m terra firme plots (minimally 300 m apart) were sampled using identical methods.
Specimens were identified to species or morphospecies using type specimens in the ant collection of the Harvard Museum of Comparative Zoology (MCZ). Some minor workers were too similar to be identified without a major; these samples were not included in assessing species characteristics. Vouchers of all species were deposited in the MCZ. A taxonomic key to the ground-foraging Pheidole of TBS based on major worker morphology is available from ALM. (http://www.amymertl.com/Keyindex.html).
Measuring body size
We quantified body size variation by measuring the head width (HW; across the top margin of the eyes) of a major and a minor from the same colony for each species (Pie and Traniello, 2007). Major worker and minor worker HW were tightly correlated across species (R 2 = 0.871, P < 0.0001, n = 55). For identified species, we compared our values to those of Wilson (2003) to validate HW measurements. We used IMAGE-J 1.38 (Rasband, 2007) and digital images taken with a JVC digital camera attached to a Leica MZ16 stereomicroscope. High-resolution images were created using Auto-Montage (Syncroscopy, Division of Synoptics, LTD, Frederick, MD). P. sp. nr. susannae was identified from a single major present at a bait; no minor was available to measure. Measurement data are available from ALM.
Phylogenetic analysis
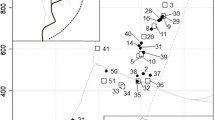
To control species-level ecological correlations for phylogenetic inertia (Felsenstein, 1985; Harvey and Pagel, 1991), we constructed a molecular phylogeny for 42 of the 56 species collected (Fig. 1). Three additional Pheidole species, P. ALM031, P. ALM033 and P. gilva collected previously by K. Ryder Wilkie and T. Erwin at TBS, were also included in the phylogeny. Fourteen of the 56 species were not included in the phylogeny due to rarity or the inability to obtain reliable sequence information despite repeated attempts with various primers. Total genomic DNA was isolated from single individuals stored in 95% EtOH using a DNeasy Tissue Kit (Qiagen Inc., Valencia, CA). We sequenced three mtDNA segments: (1) a portion of the large subunit (16S) ribosomal RNA gene (337–355 bp, primer pair 16SF1-16SR1); (2) a portion of the NADH dehydrogenase subunit 1 gene (481 bp, ND1F3–ND1R1, or ND1F4–ND1R1 and ND1F3–ND1R1a), and (3) a portion of the cytochrome b gene (433–436 bp, CB1–CB2; Chiotis et al., 2000). Primers for 16S, ND1, and revised primers for cytochrome b were designed based on available GenBank sequences for various ants and other hymenopterans and preliminary sequences of other Pheidole obtained with a number of additional primers (Pie, 2007; Sorenson et al., unpubl. data). Primer sequences are available from ALM. We amplified each gene region in 50 μl PCR reactions with 0.25 μl of AmpliTaq Gold DNA polymerase (Applied Biosystems, Foster City, CA) with a touchdown PCR cycle (annealing temperature ramped down from 58 to 52°C). PCR products were gel-purified in 1% agarose stained with ethidium bromide, excised from the gel, and then recovered using a Gel Extraction Kit (Qiagen Inc., Valencia, CA). Sequencing reactions were run on an Applied Biosystems 3100 automated DNA sequencers (Applied Biosystems, Foster City, CA). Electropherograms for both DNA strands were visualized and reconciled using Sequencher (v4.5; Gene Codes Corporation, Ann Arbor, MI). All sequences have been deposited in GenBank (accession numbers HM015935–HM016078).
Bayesian consensus tree based on combined analysis of partial cytochrome b, ND1, and 16S rRNA gene sequences under a GTR + I + G model of sequence evolution. Values at nodes represent posterior probabilities. The tree was rooted using mid-point rooting, which places the root on the long branch leading to P. cephalica, which was highly divergent in sequence from all other taxa. Character mapping shows nest site use and flood tolerance of species (canopy nesting information from K. Ryder Wilkie). The 17 species in our subset are denoted with an asterisk; these species are also categorized into four behavioral groups based on their dominance (Dom. high dominance, foraging distance and recruitment) or subordinance (Sub. low/moderate dominance, foraging distance and recruitment) and degree of major worker foraging (MF). A blank space indicates character data was not available for that species
Sequences were manually aligned using Se-Al (Rambaut, 2007). The alignment of ND1 and cytochrome b coding sequences was straightforward, whereas two short, variable-length regions of the 16S gene (approximately 126 bp) were excluded from the analysis because their alignment could not be unambiguously determined. A tree was built using Bayesian inference as implemented in MrBayes (v3.1; Huelsenbeck and Ronquist, 2001). We used a GTR + I + G substitution model with default priors on all parameters. An analysis with model parameters partitioned by gene and codon position (results not shown) did not improve resolution or lead to any substantive change in phylogenetic inferences. Two replicate analyses, each with four MCMC chains were run for 4,400,000 generations, sampling trees and parameters every 2,000 generations after a burn-in of 400,000 generations. Examination of likelihood scores indicated that both runs had reached the stationary posterior distribution by 180,000 generations. A Bayesian consensus tree and posterior branch probabilities were based on the combined post-burn-in sample of trees from both runs, which yielded consistent results: only three nodes conflicted between the two trees, all of which had low posterior probabilities and are therefore collapsed in our phylogeny (Fig. 1).
Data analysis
Estimating actual species richness
The nonparametric Jack1 was used to estimate actual species richness of ground-dwelling Pheidole, as the most accurate estimator when the majority of estimators suggest 74–96% of species have been collected (Burnham and Overton, 1979; Brose et al., 2003). For baiting data, each transect or grid was considered one sample (n = 28). For litter-search data, we considered all litter plots within one site as one sample (n = 12). Three 5 × 5 m plots surveyed in 2003 were each considered one sample. Jack1 estimates were computed in EstimateS (Colwell, 2005) using the Mau Tao method (100 runs, no replacement).
Quantifying ecological and behavior characteristics
We characterized each species in terms of abundance, flood tolerance, nesting ecology, ability to discover/dominate baits, daily activity, foraging distance and major worker foraging. Abundance was estimated as: (1) proportional abundance of nests located by baiting; and (2) proportional abundance of nests found by litter searching. The higher of the two was used to categorize the abundance of a species. A histogram of species abundances was constructed and categories were chosen based on apparent groupings: species ≥9% were classified as “abundant,” 3–9% were “common”, 1–3% were “infrequent” and species ≤1% were “rare”. Not all Pheidole workers observed at baits could be tracked to nests, but the presence of a given Pheidole species was still considered indicative of a nest. Pheidole with undetermined nest locations were included in abundance estimates only after confirming that no workers from a known nest of the same species were observed at the same baiting station or the two adjacent bait stations during any census. We classified the relative abundance of each species in both floodplain and terra firme forest using this same method. We used the difference in abundance between habitats to categorize tolerance to flooding disturbance for species with at least three known nests. Species found in only terra firme habitat were categorized as flood intolerant, while those with higher abundance (>2×) in terra firme forest were categorized as having low tolerance. Species with similar relative abundances in both habitats were categorized as generalists, species with higher (>2×) abundance in floodplain forest were categorized as highly flood tolerant, and species found only in floodplain were categorized as floodplain specialists.
We classified primary nest type (occupied by >50% of colonies found) for species with at least three known nests. If there was no primary nest type, species were classified as “mixed” nesting. Nests found by litter searching were used to extend the range of nest types if a species had not already been identified as using twig or litter nests by baiting.
Foraging distance was defined as the mean distance between the nest and baits at which workers were observed. The ability to discover bait was measured as the average time after bait placement when the species was first seen. The ability of each species to dominate baits was measured as: (1) the proportional representation at baits: the number of workers of a certain species present at a bait at 90 min divided by the total number of ants at 90 min and averaged (this calculation included only baits where the species was present at 90 min or previously seen at 15, 30 or 60 min); and (2) recruitment ability: the average number of workers of a species present at a bait (since every bait was censused four times during each trial, we included only the census with the largest number of ants of that species for each bait at each trial for this calculation). Major worker foraging activity was quantified as the average proportional abundance of majors at bait (again including only the census with the largest number of ants of that species for each bait at each trial). Morning, afternoon and night baiting trials along the same transect were each treated as separate samples for these metrics and the average of all trials in which a species was present was taken to determine the metric value for each species. Species data are available from ALM.
Statistical analysis and sampling size
The species traits we measured were based on behavior at baits (discovery, proportional representation, recruitment, foraging range, and major worker foraging) or ecology (nesting site and flood tolerance). To examine the significance of interspecific differences in behavioral traits, ANOVA and Tukey’s HSD were used (with the exception of activity period, for which ANOSIM was more appropriate). Only the 17 species observed on a minimum of ten baits were included in statistical comparisons of interspecific variation, including P. aranoides, P. xanthogaster, P. huacana, P. sagax, P. laidlowi, P. deima, P. biconstricta, P. sp. nr. nesiota, P. pholeops, P. sp. nr. nitella, P. amazonica, P. fracticeps, P. gagates, P. astur, P. horribilis, P. peruviana, and P. fissiceps. To examine trait differences between species utilizing different nest sites or showing different flood tolerance, we used the nonparametric Kruskal–Wallis and Wilcoxon Sign Rank tests to compare groups in terms of major and minor worker HW, relative abundance, and species means for discovery, dominance, recruitment, proportion of majors at baits, and foraging distance. Only species for which a minimum of three nests could be classified were used in these comparisons: for flood tolerance this involved comparing 11 high tolerance and flood specialist species to 22 species showing little or no flood tolerance, while for nest sites we compared 13 ground-nesting to 11 twig-nesting and 12 mixed-nest species (Table 1). Pearson’s chi-square was used to compare primary nest site usage among the two flood tolerance species groups.
We used ANOSIM (Clarke, 1993) to examine differences between the species composition of Pheidole in flooded versus terra firme forest, and between activity periods. Each transect was considered a sample, and the presence or absence of each species was scored. Litter-search sites were also considered samples when comparing terra firme to floodplain (n = 20 for floodplain vs. terra firme, and n = 12 for morning vs. afternoon vs. night). ANOVA, Tukey’s HSD, Kruskal–Wallis, Wilcoxon Sign Rank test, and Chi-square were performed using JMP version 5.0.1 (SAS Institute Inc., Cary, NC, 1989-2002), ANOSIM was carried out on Bray–Curtis Similarity Index values of incidence data using PAST (version 1.57; Hammer et al., 2001).
Continuous variables showing significant interspecific variation were included in correlation analyses based on species means. Major and minor HW was also included, as significant interspecific variation in size is known among Pheidole at TBS (Mertl and Traniello, 2009). Proportion of majors, recruitment and foraging distance were log transformed to improve normality. A constant of one was added to all values prior to log transformation (Gotelli and Ellison, 2004). Correlation analysis using species as independent observations was completed in JMP version 5.0.1 (SAS Institute Inc., Cary, NC, 1989–2002). Correlations were run with the entire set of 56 species, as well as the 17 species subset listed above.
We applied the false discovery rate method of Benjamini and Hochberg (1995) to correct our 56 P values for multiple statistical comparisons. All P values greater than 0.0142 were considered non-significant based on the highest P value to satisfy the constraint P (16) = 0.014 ≤ (16/56) × 0.05 = 0.0142.
Results
Species richness and abundance
A total of 56 ground-dwelling Pheidole species were collected (Table 1). Jack1 estimated total richness at 70 species, suggesting our survey found approximately 80% of the Pheidole. Forty-six Pheidole species were found in terra firme forest and 30 species in floodplain forest, with an overlap of 21 species. Jack1 estimated actual Pheidole species richness at 56 for terra firme and 47 for floodplain forest. A total of 661 Pheidole nests were found or inferred from baiting transects: 524 nests were located by following foragers, 137 were of undetermined location. An additional 190 nests were found by litter searching. The majority of species (31 or 55%) were rare, whereas 13 species were infrequent (Table 1). Six species were common (P. fracticeps, P. sp. nr. nitella, P. lemnisca, P. biconstricta, P. ademonia and P. horribilis), and six were abundant (P. amazonica, P. allarmata, P. phoelops, P. astur, P. sp. nr. nesiota, P. laidlowi).
Pheidole was the dominant ground-foraging ant at TBS, present at 4,228 of 8,884 bait censuses (48%) and comprising over 50% of the ants present at 2,990 bait censuses (34%). All other ant genera combined were present at only 3,887 bait censuses (44%), while 2,744 (31%) censuses included no ants. The majority of baits attracting Pheidole included only one Pheidole species (72%); 23% included two, 5% included three and <1% included four. Intraspecific interactions were very rare; only three observations of workers from separate nests of the same species using the same bait were made.
Phylogenetics of Pheidole at TBS
A Bayesian consensus tree based on mtDNA sequence data is shown in Fig. 1. The phylogeny is characterized by long terminal branches, considerable genetic distances between most pairs of species, and poor resolution of basal relationships. Most basal nodes were left unresolved or had low posterior probabilities (<0.95). Nonetheless, several pairs of sister species and clades of three or four species were well supported (posterior probability > 0.95). Most of the species in these groups also had long terminal branches (e.g., P. peruviana and P. triplex; P. nr. sensitiva and P. ALM011; P. aranoides, P. cursor and P. 014; P. fissiceps and P. ALM013; P. fracticeps, P. scalaris and P. ALM022; Fig. 1). More recently derived species pairs included P. biconstricta and P. xanthogaster (minimum genetic distance 2.9%), and P. laidlowi and P. sagax (4.3%). P. cataractae and P. ALM031 (0.2%) had nearly identical sequences, and may in fact be sibling species.
Short basal internodes and long terminal branches suggest an ancient diversification for most of the Pheidole species and lineages in our analysis. Although not conducive to formal phylogenetic comparative analyses, Fig. 1 provides justification for treating species as independent observations in correlation analyses, given the long history of independent evolution represented by the long terminal branches for many species. Genetic distances among well-supported sister species (posterior probability > 0.95) ranged up to 31%, whereas the minimum genetic distance for 39 of 45 species exceeded 12.4%. In addition, well-supported sister species often differed in key traits (Fig. 1). Therefore, we use species as independent data points in the correlations reported below. However, the groupings of P. biconstricta/P. xanthogaster and P. laidlowi/P. sagax (genetic distance < 5%) could potentially bias these correlations. We therefore confirmed all significant correlations by sequentially removing one of the sister species from these pairs and re-analyzing the correlation. This had no effect on patterns of significance.
Foraging behavior
Mean foraging distance varied tenfold among Pheidole species, and showed significant interspecific variation among the 17 species subset (ANOVA, F 17,607 = 21.14, P < 0.0001). P. aranoides had a foraging distance of 5.49 ± 1.53 m, significantly greater than all other species (Table 2) and also had the maximum observed foraging distance (9.24 m). P. sagax and P. laidlowi also foraged at significantly greater distances than most other species (2.59 ± 3.06 and 0.99 ± 1.53 m, respectively; Table 2). Of the 17 species, P. horribilis, P. amazonica, P. pholeops, P. sp. nr. nitella, P. peruviana, and P. fracticeps foraged closest to their nests (mean distances approximately 0.5 m).
Pheidole were more active during day than night (ANOVA, F2,1845 = 8.21, P = 0.0003, Tukey’s HSD P < 0.001). One species (P. fimbriata) foraged nearly exclusively at night, but there were no significant differences in the composition of species active during the three time periods examined (ANOSIM, R = −0.04, P = 0.85).
There was no evidence of significant interspecific variation in discovery time among the 17 species subset (ANOVA, F 16,1058 = 1.67, P = 0.042). However, ability to dominate baits varied significantly as measured by proportional abundance at 90 min (F 16,1024 = 9.78, P < 0.0001) and recruitment ability (F 16,1183 = 11.41, P < 0.0001; Table 2). Species with the highest proportional abundance and recruitment at 90 min included P. aranoides, P. xanthogaster, P. huacana, P. sagax, and P. laidlowi; the lowest included P. fissiceps. P. biconstricta, P. peruviana, P. astur and P. horribilis.
The proportion of major workers at baits varied significantly among the 17 species subset (ANOVA, F 16,1183 = 11.47, P < 0.0001; Table 2). P. gagates, P. sp. nr. nesiota, P. amazonica and P. laidlowi had significantly more major workers at baits (>11%), whereas ≤1% of foragers were majors in P. horribilis, P. astur, and P. peruviana. P. deima majors were never seen at baits, although majors were occasionally observed along foraging trails.
Nesting ecology
A wide range of nest types were found, including spaces between leaves, cavities in living plants, bromeliads, epiphyte roots, and in clumps of mud. The most common nesting sites were underground, in twigs, or in decaying logs and tree stumps (wood nests). Thirty-eight species could be classified by nest site: 13 (34%) were primarily ground nesting, 11 (29%) were twig-nesting and 2 (5%) were wood nesting. The remaining 12 species (32%) were mixed-nesters (Table 1). Comparing ground-, twig- and mixed-nesting species, no significant differences in minor HW (χ 22 = 3.84, P = 0.147), relative abundance (χ 22 = 3.39, P = 0.183), dominance (χ 22 = 3.56, P = 0.168), recruitment (χ 22 = 1.96, P = 0.375), major foraging (χ 22 = 1.83, P = 0.400), or foraging distance (χ 22 = 2.48, P = 0.289) were found. Differences in time to discover baits approached significance (χ 22 = 6.97, P = 0.031), with twig-nesting species arriving faster (32.6 min ± 10.5) than ground-nesting species (43.0 min ± 9.7). Ground-nesting Pheidole also had significantly larger majors (1.40 mm ± 0.55) than twig-nesting species (0.82 mm ± 0.25; χ 22 = 8.49, P = 0.014).
Flood tolerance
Pheidole species showed varying degrees of flood tolerance (Table 1). Of the 39 species for which flood tolerance could be categorized, six species (P. phoelops, P. gagates, P. cramptoni, P. haskinsorum, P cataractae, and P. ALM013) had similar abundances in both habitats. In contrast, 15 species were found only in terra firme forest, including the abundant species P. astur and P. sp. nr. nitella, which are presumably completely intolerant to flooding. Other species were found only in floodplain forest: the abundant P. sp. nr. nesiota, as well as P. ALM022, P. ALM023 and P. sospes. Seven species showed low flooding tolerance, being twice as abundant in terra firme, and the remaining seven species showed high flood tolerance, being twice as abundant in floodplain (Table 1).
The species composition of the Pheidole community in terra firme forest was significantly different from the floodplain community (ANOSIM, R = 0.59, P < 0.0001). We found no significant differences in body size (minor HW: Wilcoxon Sign Rank test, Z = −0.36, P = 0.716; major HW: Z = −1.51, P = 0.131), abundance (Z = 0.15, P = 0.878), dominance (Z = −0.42, P = 0.674), discovery time (Z = −0.48, P = 0.633), proportion of majors at baits (Z = 1.27, P = 0.205), recruitment (Z = −0.15, P = 0.879), or foraging distance (Z = 0.36, P = 0.720) between flood-tolerant species (n = 11) and species showing little or no flood tolerance (n = 22). Floodplain species were 36% twig, 45% mixed, 9% wood and 9% ground nesting, whereas terra firme species were 55% ground, 23% mixed, 18% twig and 5% wood nesting. These differences were not significant (χ 23 = 6.35, P = 0.096).
Correlations between behavioral traits across Pheidole species
Species with a higher proportional dominance at baits showed stronger recruitment to baits (R 2 = 0.626, P < 0.0001; Table 3); this relationship was also significant within the subset of 17 species (Table 3). Species with higher proportional dominance also traveled further to forage among all species (R 2 = 0.444, P = 0.002) and among the 17 species subset (R 2 = 0.640, P = 0.006). Overall, species with higher recruitment also traveled further to forage (R 2 = 0.400, P = 0.005), but this pattern was not seen among the subset of 17 species (Table 3). Conversely, among the 17 species subset those with higher recruitment also had a higher proportion of majors at baits (R 2 = 0.616, P = 0.009), a result not seen in analyses of the complete species set (Table 3).
Discussion
We found a total of 56 species (38 identified, 18 morphospecies) of ground-foraging Pheidole; this is 80% of the estimated richness of the ground-foraging Pheidole fauna. At least six additional ground-foraging Pheidole species have been identified at TBS, including P. ALM025, P. sabella, P. exigua, P. ALM026, P. ALM032, and P. ALM031 (Ryder Wilkie et al., 2009 and unpublished data). Our methods did not target subterranean or arboreal species, which are relatively rare. Only one species at TBS (P. fimbriata) appears to forage deeper than 25 cm in soil (Ryder Wilkie et al., 2007) and extensive canopy-fogging yielded only five Pheidole species (P. floricola, P. gilva, P. pubiventris, P. ALM033 and P. ALM034) not found in our survey (Ryder Wilkie et al., unpublished data). Pheidole species varied tenfold in abundance, with the majority (59%) being rare (each comprising 1% or less of nests).
Interspecific variation among ground-foraging Pheidole
Variation in nest type, flood tolerance, foraging distance, ability to dominate baits, and major worker foraging appear important to the structuring of the ground-foraging Pheidole community at Tiputini. Species varied in their preferred nest site (Table 1). Although data suggest twig nesters may find food before ground nesters, we found no significant behavioral differences among species utilizing difference nest types. It therefore seems likely that species with similar dominance and recruitment segregate by microhabitat by utilizing different nest sites. For example, P. aranoides nests in soil, P. xanthogaster in decayed logs, whereas P. laidlowi and P. sagax use a range of nest sites.
The Pheidole communities of terra firme and floodplain forest were significantly different, reflecting interspecific variation in tolerance to flood-disturbed habitat. There was little evidence of structural differences between these two communities, and it again seems likely that differences in species composition between terra firme and floodplain forest are due to a segregation of behaviorally similar species. For example, P. sp. nr. nesiota, and P. laidlowi are behaviorally dominant in floodplain forest, but P. aranoides, P. xanthogaster, and P. sagax replace them as the most dominant species in terra firme. Physical disturbance created by flooding appears to be the most important determinant of the distribution of flood-tolerant species (Mertl et al., 2009). We cannot eliminate the possibility that additional ecological factors could be influencing the distribution of species among flooding and non-flooding habitat; however, the differences in community composition are clearly present.
Pheidole species varied significantly in their ability to dominate baits, but not in their ability to discover baits. Distance from the nest and/or colony size may be important determinants of food discovery, rather than differences in search strategy enabling quick localization. Indeed, we found a significant correlation between discovery time and distance between nest and bait (Spearman’s Rho = 0.159, P = 0.002). Our results suggest that a trade-off between the ability of ants to find food and the ability to secure it from competitors may not be significant in the foraging ecology of Amazonian Pheidole. Significant interspecific differences in mean foraging distance and a significant positive correlation with dominance at baits suggests that dominant Pheidole species control relatively large territories, while subordinate species may avoid competition by foraging closer to their nests. This relationship is also supported by a positive correlation between recruitment and foraging distance: dominant species traveled further and recruited more ants. Although Pheidole were more active during the day, daily activity patterns did not appear to differentiate Pheidole species. Only P. fimbriata was more active at night.
We found no significant correlation between body size and dominance, although colony size and subcaste demography were not measured and could impact dominance, recruitment and foraging distance (Wilson, 1984; Gordon, 1992; Palmer, 2004). The difficulty of collecting whole colonies precluded the collection of such data for all species. Significant variation in colony size and proportion of major workers has been found among litter-nesting Pheidole at TBS (Mertl and Traniello, 2009). We did collect colony size and caste ratio data for the 11 twig-nesting species included this study, though an exploratory analysis of the relationship between these two variables and the foraging strategy metrics presented in this paper showed no significant correlations among these species. However, the role of sociometrics at the community level remains largely unstudied.
Functional ecology of Pheidole major workers
Major workers and nest ecology
Ground-nesting species had significantly larger majors than litter-nesting species, while minors showed no size difference. This subcaste-specific selection suggests size differences may reflect more than space constraints in a twig nest. Ground nests may indeed require larger majors to defend comparatively diffuse and more permanent nests.
Major workers and flood tolerance
We found no significant differences in major worker size or participation in foraging between flood-tolerant and flood-intolerant species. The behavioral response of these Pheidole species to flooding is not known, and it may be that nest evacuation is not always primary. Ground-nesting species could seal nest entrances and wait out floods; indeed, ground-nesting Pheidole show a weaker evacuation response to flooding than twig- or wood-nesting species (Wilson, 1986). In this case, an overall decrease in major worker size would not be expected. Species may deal with opportunistic feeding by increasing group transport among minors, rather than increased foraging by majors, or, as noted, factors other than the flooding itself may be influencing the distribution of Pheidole species that appear to specialize on flooded habitat.
Major workers and foraging strategy
Pheidole species varied significantly in the proportion of major workers recruited to baits. Majors comprised 10% or more of workers present in some species, while majors of other species were rarely or never seen at baits. Among the 17 species subset, degree of recruitment correlated with the proportion of majors at baits, suggesting that majors may play a greater role in foraging in mass-recruiting species. However, there were no correlations between major worker recruitment and dominance; therefore, major workers are not necessarily required to aggressively defend food sources. Some species may recruit majors to process or transport food rather than to defend it.
These results show that majors of Amazonian Pheidole differ in their functional ecology. Wilson (2003) proposed that the major worker subcaste was one of the principle causes of Pheidole hyperdiversity. Our results do not support or refute this hypothesis, although the clear interspecific variation in major worker foraging behavior suggests their behavioral specialization may increase ecological differentiation in Pheidole.
Evolution of behavioral species groups in Pheidole
Our phylogeny suggests an ancient and rapid diversification of many Pheidole lineages, consistent with Moreau’s (2008) analysis indicating that Pheidole is much older than previously recognized. A striking pattern of long terminal branches and the relative paucity of closely related species at TBS suggests that most sister species are allopatric. The generally poor resolution of basal relationships renders the mtDNA phylogeny less than ideal for comparative analyses, but provides justification for using species as units of observation. Independent evolution along the generally long terminal branches in the phylogeny should reduce the effects of shared ancestry. More importantly, comparisons of distantly related taxa and well-supported sister species suggest that the trait correlations identified among our 56 Pheidole species are largely due to ecological diversification rather than phylogenetic inertia. Phylogenetically disparate species such as P. aranoides, P. xanthogaster and P. sagax shared the traits of large foraging range, mass-recruitment, dominance at baits, and recruitment of a high proportion of major workers to food. P. huacana is also similar to these species, although characterized by lower major worker foraging. Similarly, species such as P. deima, P. astur, P. peruviana, and P. horribilis, having small foraging ranges, low dominance and low recruitment of majors, occur throughout the phylogeny. Further support stems from the clear behavioral and ecological variation among well-supported sister groups: for example P. amazonica (twig nesting, low flood tolerance) and P. cramptoni (mixed nesting, generalist); P. xanthogaster (wood nesting, dominant, high major worker foraging) and P. biconstricta (mixed nesting, subordinate; low major worker foraging), and finally P. sp. nr. nesiota (ground nesting, flood specialist) and P. haskinsorum (mixed nesting, generalist; Fig. 1). Overall, our studies suggest the presence of behavioral groups within the Pheidole community, based partly on the behavior of the major worker caste (Fig. 1).
Interspecific interactions in the Pheidole community
Interference competition between Pheidole species was not often observed, as more than 70% of baits were occupied by a single Pheidole colony. Intraspecific interactions at baits were extremely rare. This does not necessarily imply that inter- and intra-specific competition are not important in structuring the ground-foraging Pheidole community, as competition during colony establishment may lead to nest overdispersion (Ryti and Case, 1992; Andersen, 2008) and reduce interactions between mature colonies. The most aggressive behaviors occurred at baits occupied by three to four Pheidole colonies: aggression was seen at 12% of these baits, compared to 3% of baits with two Pheidole species present and 2% of baits with Pheidole species and other ant genera.
Although Pheidole was by far the most numerically dominant genus at baits, interactions with other ant genera could also impact the structure of Pheidole communities. Camponotus femoratus is behaviorally dominant among ground-foraging ants at TBS, and was observed in more aggressive interspecific interactions with Pheidole than any other species. C. femoratus workers have relatively large body size (≥1 mm HW), but the extent of its dietary overlap with Pheidole is unknown. Workers of smaller ground-foraging ant genera such as Crematogaster (HW range 0.6–0.9 mm at TBS), Paratrechina (HW 0.5–0.9 mm) and Solenopsis (HW 0.3–0.8 mm) may be the more important resource competitors. Pheidole species are often generalist scavengers (Wilson 2003), or granivores (Levey and Byrne, 1993; Kaspari, 1996b; Pizo, 2007); but we were unable to quantify diet due to the difficulty of collecting and identifying the minute food particles carried by minor workers. More investigations into resource use among Pheidole species are needed to determine the degree of interspecific and intergeneric competition.
Conclusions
The identification of significant interspecific variation in ecology and behavior indicates that Pheidole species do not correspond to a single genus-typical phenotype. Major worker characteristics also directly relate to species differentiation; ground-nesting species have larger majors and dominant species utilizing mass-recruitment to defend food sources recruit proportionally more majors. The structure of the exceptionally diverse Pheidole community includes groups of species with shared behavioral traits that are segregated by nest type and/or flood tolerance. Wilson (2003) hypothesized that the use of litter nests, small colony size, and the development of the major worker subcaste contributed to the ecological dominance of Pheidole. Our identification of significant interspecific variation in micro- and macrohabitat, foraging strategy and major worker behavior suggest that these types of ecological and behavioral niche differentiation in Pheidole may have been associated with subsequent speciation in Neotropical habitats. Comparisons between Pheidole and other ant genera in a robust phylogenetic context will be necessary to determine if these or other characteristics may have served as key innovations in the diversification of Pheidole, and improve our understanding of the causes of hyperdiversity.
References
Adis J. 1997. Survival strategies of terrestrial invertebrates in central Amazonian inundation forests: A response to long-term flooding. Acta amazon. 27: 43-54
Andersen A.N. 2008. Not enough niches: Non-equilibrial processes promoting species coexistence in diverse ant communities. Austral Ecol. 33: 211-220
Benjamini Y. and Hochberg Y. 1995. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B. 57: 289-300
Bestelmeyer B.T. 2000. The trade-off between thermal tolerance and behavioural dominance in a subtropical South American ant community. J. Anim. Ecol. 69: 998-1009
Brose U., Martinez N.D. and Williams R.J. 2003. Estimating species richness: Sensitivity to sample coverage and insensitivity to spatial patterns. Ecology 84: 2364-2377
Brown J.J. and Traniello J.F.A. 1998. Regulation of brood-care behavior in the dimorphic castes of the ant Pheidole morrisi (Hymenoptera: Formicidae): Effects of caste ratio, colony size, and colony needs. J. Insect Behav. 11: 209-219
Burnham K.P. and Overton W.S. 1979. Robust estimation of population size when capture probabilities vary among animals. Ecology 60: 927-936
Byrne M.M. 1994. Ecology of twig-dwelling ants in a wet lowland tropical forest. Biotropica 26: 61-72
Chiotis M., Jermiin L.S. and Crozier R.H. 2000. A molecular framework for the phylogeny of the ant subfamily Dolichoderinae. Mol. Phylogenet. Evol. 17: 108-116
Clarke K.R. 1993. Nonparametric multivariate analysis of changes in community structure. Austral Ecol. 18: 117-143
Colwell R.K. 2005. EstimateS: Statistical Estimation of Species Richness and Shared Species from Samples. 7.5 edn
Davidson D.W. 1998. Resource discovery versus resource domination in ants: A functional mechanism for breaking the trade-off. Ecol. Entomol. 23: 484-490
Delsinne T. Roisin Y. and Leponce M. 2007. Spatial and temporal foraging overlaps in a Chacoan ground-foraging ant assemblage. J. Arid Environ. 71: 29-44
Detrain C. 1990. Field study on foraging by the polymorphic ant species, Pheidole pallidula. Insect. Soc. 37: 315-332
Droual R. 1983. The organization of nest evacuation in Pheidole desertorum Wheeler and P. hyatti Emery (Hymenoptera: Formicidae). Behav. Ecol. Sociobiol. 12: 203-208
Felsenstein J. 1985. Phylogenies and the comparative method. Am. Nat. 125: 1-15
Fowler H.G. 1984. Recruitment, group retrieval and major worker behavior in Pheidole oxyops. Rev. Bras. Biol. 44: 21-24
Fowler H.G. 1993. Relative representation of Pheidole (Hymenoptera: Formicidae) in local ground ant assemblages of the Americas. Anales Biol. 19: 29-37
Gordon D.M. 1992. How colony growth affects forager intrusion between neighboring harvester ant colonies Behav. Ecol. Sociobiol. 31: 417-427
Gotelli N.J. and Ellison A.M. 2004. A Primer of Ecological Statistics. Sinauer Associates, Inc. Sunderland, MA. 510 pp
Hammer Ø., Harper D.A.T. and Ryan P.D. 2001. PAST: Paleological Statistics Software Package for Education and Data Analysis. Palaeontologia Electronica 4: 9 pp
Harvey P.H. and Pagel M.D. 1991. The Comparative Method in Evolutionary Biology. Oxford University Press, Oxford, UK. 239 pp
Hölldobler B. and Möglich M. 1980. The foraging system of Pheidole militicida (Hymenoptera: Formicidae). Insect. Soc. 27: 237-264
Huelsenbeck J.P. and Ronquist F. 2001. MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17: 754-755
Kaspari M. 1996a. Testing resource-based models of patchiness in four Neotropical litter ant assemblages. Oikos 76: 443
Kaspari M. 1996b. Worker size and seed size selection by harvester ants in a Neotropical forest. Oecologia 105: 397-404
Kaspari M. and Vargo E. 1995. Does colony size buffer environmental variation? Bergmann’s rule and social insects. Am. Nat. 145: 610-632
Kaspari M., Yuan M. and Alonso L. 2003. Spatial grain and the causes of regional diversity gradients in ants. Am. Nat. 161: 459-477
King J.R., Andersen A.N. and Cutter A.D. 1998. Ants as bioindicators of habitat disturbance: Validation of the functional group model for Australia’s humid tropics. Biodivers. Conserv. 7: 1627-1638
Levey D.J. and Byrne M.M. 1993. Complex ant–plant interactions: Rain-forest ants as secondary dispersers and post-dispersal seed predators. Ecology 74: 1802-1812
Levings S.C. and Franks N.R. 1982. Patterns of nested dispersion in a tropical ground ant community. Ecology 63: 338-344
Longino J.T., Coddington J. and Colwell R.K. 2002. The ant fauna of a tropical rain forest: Estimating species richness three different ways. Ecology 83: 689-702
Mertl A.L., Ryder Wilkie K.T. and Traniello J.F.A. 2009. Impact of flooding on the species richness, density and composition of Amazonian litter-nesting ants. Biotropica 41: 633-641
Mertl A.L. and Traniello J.F.A. 2009. Behavioral evolution in the major worker subcaste of twig-nesting Pheidole (Hymenoptera: Formicidae): Does morphological specialization influence task plasticity? Behav. Ecol. Sociobiol. 63: 1411-1426
Moreau C.S. 2008. Unraveling the evolutionary history of the “hyperdiverse” ant genus Pheidole (Hymenoptera: Formicidae). Mol. Phylogenet. Evol. 48: 224-239
Palmer T.M. 2004. Wars of attrition: Colony size determines competitive outcomes in a guild of African acacia ants. Anim. Behav. 68: 993-1004
Patel A.D. 1990. An unusually broad behavioral repertory for a major worker in a dimorphic ant species: Pheidole morrisi (Hymenoptera, Formicidae). Psyche 97: 181-192
Pie M.R. 2007. Morphological Evolution in a Hyperdiverse Clade: The Ant Genus Pheidole. Ph.D. Dissertation. Boston University, Boston, MA. 129 pp
Pie M.R. and Traniello J.F.A. 2007. Morphological evolution in a hyperdiverse clade: The ant genus Pheidole. J. Zool. 271: 99-109
Pizo M.A. 2007. The use of seeds by a twig-dwelling ant on the floor of a tropical rain forest. Biotropica 40: 119-121
Rambaut A. 2007. Se-al: Application for creating multiple sequence alignments from nucleotide and amino acid sequences. 2.0 edn
Rasband W.S. 2007. ImageJ. U.S. National Institutes of Health, Bethesda, Maryland. 1.38 edn
Ryder Wilkie K.T., Mertl A.L. and Traniello J.F.A. 2007. Biodiversity below ground: Probing the subterranean ant fauna of Amazonia. Naturwissenschaften 94: 725-731
Ryder Wilkie K.T., Mertl A.L. and Traniello J.F.A. 2009. Diversity of ground-dwelling ants in primary and secondary forests in Amazonian Ecuador. Myrmecol. News 12: 139-147
Ryti R.T. and Case T.J. 1992. The role of neighborhood competition in the spacing and diversity of ant communities. Am. Nat. 139: 355-374
Sempo G. and Detrain C. 2004. Between-species differences of behavioural repertoire of castes in the ant genus Pheidole: A methodological artefact? Insect. Soc. 51: 48-54
Tobin J.E. 1995. Ecology and Diversity of Neotropical Rainforest Canopy Ants. Ph.D. Dissertation. Harvard University, Cambridge, MA. 149 pp
Traniello J.F.A. 1989. Foraging strategies of ants. Annu. Rev. Entomol. 34: 191-210
Wilson E.O. 1984. The relation between caste ratios and division of labor in the ant genus Pheidole (Hymenoptera: Formicidae). Behav. Ecol. Sociobiol. 16: 89-98
Wilson E.O. 1986. The organization of flood evacuation in the ant genus Pheidole (Hymenoptera: Formicidae). Insect. Soc. 33: 458-469
Wilson E.O. 2003. Pheidole in the New World: A Dominant, Hyperdiverse Ant Genus. Harvard University Press, Cambridge, MA. 794 pp
Acknowledgments
We thank the directors and staff of TBS for assistance and support in the field. We thank Clifton Meek, Megan Johnson, Brian Henry, Noah Reid, Scott Appleby, Elise Koncsek, Frank Azorsa Salazar and Wendy Mertl for assistance in the field, and Winston McDonald for laboratory assistance. We thank Dr. Kari Ryder Wilkie for specimen donations. We are very grateful to Stefan Cover for confirming Pheidole identifications and to Dr. Gary Alpert for instruction on digital imaging. We thank Dr. Marcio Pie and Jeff Tetrault for advice and assistance with sequencing methods, and Dr. Corrie Moreau for help with PCR primers. This work was funded by a National Science Foundation Graduate Research Fellowship awarded to ALM, and NSF Grant IOB 0725013 to J.T. Voucher samples were collected and transported under permit 017-IC-FA-PNY-MA issued to A.L.M. by the Ecuadorian Ministry of the Environment and this work complied with all current laws of Ecuador and the United States of America.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mertl, A.L., Sorenson, M.D. & Traniello, J.F.A. Community-level interactions and functional ecology of major workers in the hyperdiverse ground-foraging Pheidole (Hymenoptera, Formicidae) of Amazonian Ecuador. Insect. Soc. 57, 441–452 (2010). https://doi.org/10.1007/s00040-010-0102-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00040-010-0102-5