Abstract
Many social insects exhibit morphologically distinct worker and queen castes that perform different functions. These functional differences may generate unique selection regimes operating on body size. For example, queens may be under directional selection for large body size, whereas directional selection on worker body size may be limited. Such contrasting selection pressures may differentially affect levels of genetic variation associated with size variation in the two castes. This study sought to determine if genetic effects underlying phenotypic differences varied between the worker and queen castes of the social wasp Vespula maculifrons. We predicted that directional selection would remove genetic variation associated with size differences in the queen caste, whereas a lack of directional selection would tend to maintain genetic variation associated with size differences in the worker caste. We thus (1) calculated broad and narrow sense heritabilities for several morphological traits, (2) examined whether some paternal genotypes produced more morphologically diverse offspring than others, and (3) determined whether trait size variation was associated with genetic variation within colonies. We found that few morphological traits were significantly heritable, indicating that little genetic variance for those traits existed within our study population. We also found that some patrilines produced more morphologically variable offspring than others, suggesting a role of genotype in phenotypic plasticity. And finally, no significant correlations between genetic diversity arising from multiple mating by queens within colonies and trait variation in either caste were found. Overall, our findings indicate a weak effect of genotype on both worker and queen body size variation and are suggestive of a large environmental influence on morphological trait size. Moreover, our results do not indicate that levels of genetic variation underlying size variation differ substantially between castes in this species.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Social insects often display body size variation between castes. For example, social hymenopteran queens are generally larger than workers. These size differences likely reflect the different selective pressures encountered by the two castes, which arise because of their distinct societal functions and life histories. For instance, worker size often determines function within a colony (Oster and Wilson, 1978; Wheeler, 1986; 1991; Hölldobler and Wilson, 1990; Fjerdingstad and Crozier, 2006). Larger workers frequently undertake tasks such as colony defense or food processing, whereas smaller workers may be more efficient at completing tasks like brood tending. Thus, producing a diversity of worker body sizes may be selectively advantageous for a queen and her colony. In contrast, queen body size may be under directional selection. Large body size may increase a queen’s ability to survive colony founding as well as maintain a colony through to the production of new sexuals, particularly in species where queens found colonies independently (Abell et al., 1999; Peeters and Ito, 2001; DeHeer, 2002; Wiernasz and Cole, 2003; Bargum et al., 2004; Fjerdingstad, 2005).
If the opposing selective pressures operating in the two castes are strong enough, then differences in the genetic architecture controlling body size in the two castes may result (Lande, 1980; Phelan et al., 2003; Archer et al., 2003; Bonduriansky and Rowe, 2005; Bonduriansky and Chenoweth, 2009). Specifically, the different selective pressures operating on body size in the two castes may lead to differences in the magnitude of genetic variation underlying body size variation. For example, an absence of directional selection on worker body size would result in the maintenance of more genetic variation for worker body size. In contrast, little genetic variation is expected to underlie phenotypic differences in queen size, because such variation is expected to have been erased by the operation of directional selection over time. Therefore, since workers will be less likely to be under the same type of directional selection as queens, we predict that there would be greater genetic control of worker body size than queen body size.
To test this prediction, we used classical quantitative genetic methods to estimate broad and narrow sense heritabilities for several worker and queen traits in a social wasp. Heritability estimates provide information on the amount of genetic variation underlying phenotypic variation and can be used as indicators of the selective pressures that have acted on traits in the past (Roff, 1997). Generally, strong, multi-generational directional selection will erode additive genetic variance from a population, which will result in low narrow sense heritability (Falconer and Mackay, 1996). Therefore, we predicted that queens would display lower heritability for size than workers.
In addition to investigating the relative heritability of traits between castes, we were also interested in determining if genotype affected variation in trait size. We thus tested whether some genotypes produced more variable offspring than others, which would suggest that some genotypes were more phenotypically plastic than others. Again, due to the presumed directional selection faced by queens, we predicted that these phenotypically plastic genotypes would be expressed more often among workers than queens.
Finally, we examined whether increased genetic diversity within a colony resulted in increased phenotypic variation among the worker and queen castes. Genetic diversity may lead to phenotypic diversity if genotype influences morphology (Oster and Wilson, 1978). We predicted a positive correlation between genetic diversity and morphological variation within colonies for workers because we expected genetic variation to influence phenotypic variation in the worker caste. However, we did not predict such a correlation for queens because we did not expect genetic variation to strongly influence phenotypic variation in the queen caste (Crozier and Fjerdingstad, 2001).
We chose to investigate the influence of genetic variation on size variation in the social wasp Vespula maculifrons. V. maculifrons is a convenient taxon for investigating genetic effects on size for several reasons. First, workers and queens show strongly divergent morphology and behavior and therefore may be expected to be under distinct types of selection (V. maculifrons natural history reviewed by Spradbery, 1973; Macdonald and Matthews, 1981; Greene, 1991). Second, V. maculifrons colonies produce abundant workers and new queens each year, thus providing the material necessary for genetic studies. Finally, the social structure displayed by V. maculifrons is well understood; colonies are headed by a single multiply mated queen (range in queen mate number of 3–9; Ross, 1985; Goodisman et al., 2007a), and members of all patrilines are found in both castes (Goodisman et al., 2007b). The variation in mate number found across colonies provides the genetic variation necessary to undertake our study examining how genetic differences influence size differences.
Methods
Sample collection
Between the months of July and November in 2004, 2005, and 2008, a total of 61 V. maculifrons colonies were collected near metropolitan Atlanta, Georgia, United States (2004, N = 33; 2005, N = 4; 2008, N = 24). All colonies contained workers and sixteen colonies also contained newly enclosed queens that had not yet mated (hereafter referred to as gynes; 2004, N = 7; 2005, N = 4; 2008, N = 5). In 2004, twelve collected colonies also contained the true reproductive foundress of the colony (hereafter referred to as the queen, cf. with gyne above). In 2008, the queen was identified in five colonies containing adult gynes, and an additional 19 queens were identified in colonies containing only adult workers. Thus, in total, 1,889 workers (\( \bar{x} \pm {\text{SD}} \); 34.35 ± 24.36 workers per colony), 503 gynes (31.44 ± 12.63 gynes per colony), and 36 queens were collected in this study.
Genetic analysis
DNA was extracted from single legs of workers and gynes collected in 2004 and 2005. Tubes containing the leg samples were submerged into liquid nitrogen for no more than 30 s. The legs were then immediately crushed using plastic pestles. Five hundred microliters of 5% Chelex solution was added to the samples, which were then incubated at 95°C in a heat block for 20 min (Walsh et al., 1991).
The worker genotypes were determined at eight microsatellite loci: LIST2003, LIST2004, LIST2013, LIST2019, LIST2020 (Daly et al., 2002), RUFA 5 (Foster et al., 2001), VMA-3, and VMA-6 (Hasegawa and Takahashi, 2002). The heterozygosities at these loci were sufficiently high so that the probability of two males having the same genotype (nondetection error; Boomsma, 1996) was low (≪ 0.0001; Goodisman et al., 2007b). Gyne genotypes were determined at a subset of loci (LIST2004, LIST2013, LIST2019, LIST2020, and VMA-6) that possessed sufficient variation to distinguish all patrilines within colonies.
All PCRs were conducted in a volume of 15 μl containing a final concentration of 4 mM MgCl2, 0.2 mM dNTPs, 1 × PCR buffer, and 0.1 U Taq DNA polymerase. In addition, six of the eight loci were multiplexed in pairs. Pair 1 consisted of the loci LIST2020 and VMA-6 combined at final primer concentrations of 0.47 μM for each locus. Pair 2 included RUFA 5 and VMA-3 combined at final primer concentrations of 0.8 and 0.13 μM, respectively. Pair 3 consisted of LIST2019 and LIST2013 combined at final primer concentrations of 0.33 and 0.67 μM, respectively. Finally, both LIST2004 and LIST2003 were amplified singly at final primer concentrations of 0.67 μM. The PCR cycle was run as follows: 2 min denaturation at 94°C, 30 cycles of 94°C for 30 s, primer optimized annealing temperatures (pair 1 = 60°C; pair 2 = 54°C; pair 3 = 60°C; LIST2004 = 60°C, LIST2003 = 48°C) for 30 s, 72°C for 30 s, and a final 72°C extension for 10 min. PCR samples were run on 2.5% agarose gels to confirm amplification. PCR products were visualized using an Applied Biosystems PRISM® 3100 Genetic Analyzer. Gynes and workers were assigned to patrilines within colonies based on their multilocus genotype as described by Hoffman et al. (2008).
Morphological measurements
After DNA extractions were completed, workers from 37 colonies (2004, N = 33; 2005, N = 4) and gynes from 11 of those colonies (2004, N = 7; 2005, N = 4) were dried at 60°C. Individual dry mass was then recorded (workers from 10 of the colonies collected in 2004 were not photographed and were therefore analyzed for dry mass only). Additionally, digital photos were taken of workers from 46 of the collected colonies (2004, N = 23; 2005, N = 4; 2008, N = 19), gynes from a total of 16 colonies (2004, N = 7; 2005, N = 4; 2008, N = 5), and the queens from 36 colonies (2004, N = 12; 2008, N = 24).
Each individual was photographed in a dorsally facing position with a Zeiss© millimeter micrometer to allow for calibration. We measured the linear dimensions of each of the following six traits: thorax width measured from the inside of the left wing tegula to the inside of the right wing tegula, i.e., the length of the mesoscutum (TW), thorax length (TL), length from the head to the end of the thorax (HTL), length of the third tergum (3TL), gaster length (GL), and total body length (BL). The length of each of these traits was measured using tools within the Canvas 9.0.4 computer package.
For this study we were particularly interested in the effects of genotype on general body size (represented by Mass and BL). Significant correlations have been found between both of these traits and survival and fecundity in a variety of social insects (Rüppell et al., 1998; Abell et al., 1999; Peeters and Ito, 2001; DeHeer, 2002; Wiernasz and Cole, 2003; Bargum et al., 2004; Fjerdingstad, 2005). The other measured traits represent components of overall body size. Moreover, several of these traits are likely to be of functional importance in one or both castes. For example, recent studies suggest that length (i.e., GL and BL) of V. maculifrons gynes is correlated with mating success (Kovacs et al., 2008). In addition, thoracic measurements (TW and TL) may potentially be related to flight capabilities (Marden, 2000).
Statistical analyses
Broad sense heritabilities Broad sense heritability (H2) is defined as the proportion of the total variation that can be attributed to genetic variation within a population, or the ratio of genetic variance (VG) to total phenotypic variance (VP; Falconer and Mackay, 1996). H2 was estimated using a sire-within-dam setup modified for haplodiploid organisms for both worker and gyne traits (Table 2 in Fjerdingstad, 2005). Information resulting from two-factor nested ANOVAs (patriline within colony, and colony) was used to estimate H2 for measured traits in each year separately due to significant differences between years (see below).
We effectively assumed that epistatic variance makes a negligible contribution to our estimates of broad sense heritability due to difficulties in calculating epistatic variance in sib analysis (Falconer and Mackay, 1996). Therefore, only the combined additive and dominance components were calculated. Estimates of broad sense heritability for all gyne and worker traits were calculated as twice the patriline variance (\( \sigma_{\text{patriline}}^{2} = {\frac{1}{2}}V_{\text{A}} + {\frac{1}{2}}V_{\text{D}} \)) divided by the total variance (\( \sigma_{\text{total}}^{2} = V_{\text{A}} + V_{\text{D}} + V_{\text{EC}} + V_{\text{EW}} \); Fjerdingstad, 2005). H 2 estimates were deemed to be significant when the lower 95% confidence interval did not include zero (Falconer, 1989).
In addition to determining the broad sense heritability for gyne and worker morphological traits, we estimated the proportion of the observed variance explained by each level (i.e., colony, patriline within colony, and offspring within patriline) by dividing the observational pure component of variance (\( \sigma^{2} \)) by the total variance (\( \sigma_{\text{total}}^{2} \)) and multiplying by 100 (Sokal and Rohlf, 1995). We also used a three-factor nested ANOVA to determine the influence of patriline within colony, colony within sampling year, and sampling year on trait size.
Narrow sense heritabilities Narrow sense heritability (h2) is an expression of the extent to which offspring phenotypes are determined by additive genetic effects. The value of h2 is expressed by the ration VA/VP and can be estimated as twice the slope (b) of the regression of mean offspring size on the mothers’ sizes (parent–offspring regression). Narrow sense heritabilities could be calculated for morphological traits for both workers and gynes belonging to colonies that were collected with their mother queen. However, when offspring and parent differ in trait size, as is the case for traits in workers and queens, then trait sizes must be standardized (Lynch and Walsh, 1998). Therefore, prior to analyses, both worker and gyne offspring as well as queen trait sizes were standardized to a mean of zero and unit variance within each year. We then took the mean of the standardized trait values for all workers (or gynes) within a colony and used this mean, in conjunction with the trait value of the queen of the colony, as a single datum point in our regression. These paired worker (gyne) and queen values were then used to estimate h2.
Influence of genotype on variation in trait size In addition to determining if workers and gynes produced by different males differed in mean trait size, we also investigated whether the variance for each trait differed among workers and gynes sired by different males. To explore this issue, we tested if the variances in trait size for each patriline were equal within each colony separately using Levene’s test for equality of variances. We then combined the information by performing a Z-transform test on the colony P values to determine overall significance across all colonies (Whitlock, 2005).
Influence of genetic variability on morphological variability within colonies To test for an effect of queen mate number on phenotypic variation among both worker and gyne offspring, we used Spearman’s correlation coefficients to determine if the standard deviation for each trait was directly related to the effective number of times the queen of each colony mated (ke3) in 2004. Effective paternity (ke3) for each colony was calculated using the sample size correction proposed by Nielsen et al. (2003). ke3 combines information on the number of times a queen mates and the unequal contributions of a queen’s male mates to offspring, thereby providing a measure of the amount of genetic variability of diploid individuals within colonies (Boomsma and Ratnieks, 1996).
Results
A total of 1,427 worker and 397 gynes were assigned to 203 and 70 patrilines using genetic techniques, respectively. The means of all seven worker traits differed significantly between colonies (P < 0.0001 for all worker traits; Table 1). In contrast, only two worker traits (GL and Mass) differed significantly between years (Table 1). Most gyne traits also differed significantly between colonies (P < 0.05 for all gyne traits except 3TL; Table 1), as well as years (P < 0.05 for gyne traits except TW, HTL, and 3TL; Table 1). These results suggest that environmental variation associated with the development of different colony environments, genetic variation associated with colony makeup, or interactions of these factors generated size variability between colonies.
Broad sense heritabilities
To gain a better understanding of whether genetic variation specifically affected phenotypic variation, we quantified broad sense heritabilities (H 2) for all traits. Our analysis of worker Mass incorporated information from 1,427 workers from 36 colonies, whereas our analysis of other worker traits included 774 individuals from 17 colonies. All traits were analyzed from 397 gynes from 11 colonies. Mass in 2004 was the only trait with an H 2 estimate significantly greater than zero in workers (Table 2). In contrast, H 2 estimates for TW, GL, and Mass in 2004 and TW and BL in 2005 were significantly different from zero in gynes (Table 2). The lower 95% confidence intervals for all other worker and gyne H 2 estimates contained zero and therefore were not considered significant (Table 2). Thus, genetic variation contributed through male sires had only weak effects on size variation in the two castes.
The proportion of variance explained by colony was greater than that explained by patriline within colony for all worker traits, and most gyne traits (Table 2). Most notably the percentages of variance explained by offspring within patrilines were much larger than both the percentages of variance explained by either colony or patriline within colony for both the worker and gyne castes (Table 2). This suggests a high proportion of the observed variance in trait sizes in both castes is due to non-genetic factors.
Narrow sense heritabilities
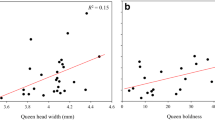
Offspring phenotypes can be influenced by both dam and sire genotypes. We quantified the effect of dam genotype on offspring phenotype by calculating narrow sense heritabilities for worker and gyne traits for colonies collected with the queen. Our analyses included 587 workers from 31 colonies and 80 gynes from 5 colonies. We found that maternal queen phenotype had little correlation with mean worker phenotype, resulting in mostly non-significant h 2 estimates in both 2004 and 2008 (Fig. 1; in 2008 only worker GL and BL displayed significant heritabilities). Interestingly, worker h 2 estimates showed large differences between years, in both direction (negative or positive) and significance. In fact, the parent–offspring regressions for GL were significantly different between years (ANCOVA, F 3,27 = 4.47, P = 0.01). The association between queen size and gyne size was also investigated for five colonies collected in 2008. No gyne traits displayed significant h 2 estimates (Fig. 2), although our sample size was very limited for these calculations. Thus, our results must be viewed with caution and only as suggestive of possible trends.
Paternal effects on variation in trait size
Genotype, rather than just influencing mean trait size, may also affect the amount of variability in trait size. That is, individuals sired by different males may differ in the variability of their traits, with individuals from some patrilines displaying a wider range of trait sizes than those from others (i.e., greater phenotypic plasticity). To test this hypothesis, we analyzed 578 workers and 397 gynes sampled from 11 colonies. Our analyses revealed significant differences in the amount of variation displayed by both workers and gynes sired by different males for several traits (Table 3). Worker Mass in 2004 as well as worker 3TL in both years showed significantly different amounts of variation between patrilines (Table 3). Among gyne traits, Mass in both 2004 and 2005 and TW and HTL in 2005 displayed significantly different levels of variation between patrilines. Thus, certain genotypes do lead to more variable phenotypes than others.
Effect of genetic variability on trait variation
A queen increases the genetic variation among her offspring by mating multiply. A possible consequence of multiple mating is increased size variation among offspring if size has a genetic basis. To test if there was a relationship between levels of polyandry and size variation, we analyzed worker mass in 1,425 workers from 32 colonies. All other worker morphological traits were analyzed in 713 workers from 13 colonies. All gyne analyses include information from 274 gynes sampled from 7 colonies. However, no worker or gyne traits standard deviations were significantly correlated with queen effective mate number (Fig. 3, Spearman’s correlations, P > 0.05 for all traits). Thus, total genetic variation found among colony mates does not appear to affect total phenotypic variation in V. maculifrons colonies.
Discussion
Social insect colonies are often composed of individuals belonging to distinct worker and queen castes. To determine the effects of genetic variation on variation in worker and queen body size and test predictions regarding the nature of genetic variation underlying morphological traits, we (1) calculated broad and narrow sense heritabilities for several morphological traits in both castes of a social wasp, (2) examined whether some paternal genotypes produced more morphologically diverse offspring than others, and (3) determined whether there was a positive correlation between trait size variation within castes and the amount of genetic variation within colonies.
Genetic effects on mean trait size
We found evidence that variation in genotype influenced variation in phenotype for a few worker and gyne traits (Tables 1, 2; Fig. 1; workers: gaster length, body length, and mass; gynes: thorax width, head to thorax length, gaster length, body length, and mass). Interestingly, some of these traits may be associated with individual caste function and may be under selection. For example, thorax width is set early in pupal development and may be linked to flight, which may have important implications for mating and colony founding in V. maculifrons. Moreover, a previous study in this species revealed that gyne gaster length and body length were correlated with female mating success (Kovacs et al., 2008).
The existence of genetic variation for traits potentially linked to fitness components (i.e., thorax width, gaster length, and body length, especially in gynes) is somewhat surprising, because directional selection may be expected to remove additive genetic variation for that trait from the population (Mousseau and Roff, 1987; Falconer, 1989). However, recent research suggests that the magnitude of heritability for fitness-related traits may not always be so clear due to the influence of non-additive genetic effects (Wolf et al., 1998; Merila and Sheldon, 1999). Thus, the significant heritabilities for these morphological traits, which may be linked to variation in fitness, may result from dominance and epistatic variance. This hypothesis is bolstered by the fact that all significant heritability estimates for gynes were broad sense estimates, which include dominance variance. If directional selection quickly erodes additive genetic variation for a fitness trait then most of the remaining genetic variation would be due to dominance, and therefore the contribution of dominance is expected to be greater in fitness traits than non-fitness traits (Roff et al., 1997; Merila and Sheldon, 1999). Additionally, some studies have revealed that fitness-related traits are often controlled by more loci than non-fitness related traits (Falconer, 1989; Armbruster et al., 1997) and therefore epistatic interactions may substantially contribute to variation in fitness-related traits (Lynch and Walsh, 1998).
We uncovered significant and strong differences in mean trait size between colonies for both workers and gynes (Tables 1, 2). Additionally, more trait variance was explained by differences between colonies, which represent a combination of both maternal and environmental effects, than by the amount of variance within patrilines (Table 2). Thus, variation in individual size in both gynes and workers is largely determined by environmental conditions in V. maculifrons.
The heritability estimates calculated for morphological traits in V. maculifrons in this study were generally smaller than those calculated for morphological traits in previous studies of social insects (Oldroyd and Moran, 1983; Milne, 1985; Oldroyd et al., 1991; Diniz-Filho et al., 1994; Diniz-Filho and Pignata, 1994; Poklukar and Kezic, 1994; Fraser et al., 2000; Rüppell et al., 2001; Hughes et al., 2003; Bargum et al., 2004; Fjerdingstad, 2005; Rheindt et al., 2005; Schwander et al., 2005; Jaffe et al., 2007; Fournier et al., 2008). For example, the magnitudes of head width heritabilities for both ant workers and queens range from 0.10 (workers, Fournier et al., 2008) to 0.88 (queens; Rüppell et al., 2001). These differ markedly from estimates in V. maculifrons, which were generally quite low (Table 2; broad sense heritability estimates for gyne thorax width in both years and body length in 2005 being the notable exceptions). One explanation for this result is that the traits measured in this study are more closely related to fitness in wasps than head width, for example, is in ants. Thus, selection may have removed genetic variation from the system, as discussed above. Indeed, estimates of broad sense heritability for body weight, a trait also measured in our current study, in Lasius niger queens were non-significant and close to the broad sense heritability estimates obtained in this study (Fjerdingstad, 2005). This may indicate that similar traits experience similar selective pressures across species.
Another reason for our overall low heritabilities may be indirect genetic effects (Wolf et al., 1998). Social insects are defined by the extensive interactions among colony members (Hölldobler and Wilson, 1990). These interactions may result in indirect genetic effects on phenotypic trait expression (Wolf et al., 1998), which result when the genes expressed in a social partner affect an individual’s phenotype (Wolf et al., 1998; Wolf, 2000). The interaction of social conspecifics can create a genotype-by-genotype interaction that in addition to contributing to environmental variance, may also affect non-additive genetic variance (Via and Lande, 1985, 1987). Under certain conditions these interactions can distort heritability estimates (Meffert, 1995). Due to the design of our study, we were unable to estimate indirect genetic effects, but Linksvayer (2006) detected significant maternal and sib-social effects in ants, indicating that genetic variation possessed by mothers and sibs of individuals within colonies affected phenotypic variation of those focal individuals (see also Bienefeld and Pirchner, 1991; Rüppell et al., 2001; Linksvayer and Wade, 2005). Thus, social environment likely plays a large role in determining phenotype in social insects and may have a genetic basis as well. Finally, we note that accurate estimates of heritability may be difficult to obtain because they require large sample sizes. Although our sample sizes for broad sense heritability estimates were relatively large, our sample sizes for narrow sense heritability estimates were more modest (particularly for gyne–queen regression analyses) and likely insufficient to accurately estimating quantitative genetic parameters. Thus, our results may best be taken as indicative of the magnitude of genetic effects rather than as strong evidence against their existence. Nevertheless, we suggest future studies incorporating parent–offspring regressions of individuals belonging to different castes may provide valuable information on the genetic architecture underlying caste phenotypes in social insects.
We found a considerable amount of variation in heritability estimates between years, as was the case in previous studies documenting variation in heritability across years or environments (Larsson et al., 1997; Merila, 1997; Sgro and Hoffmann, 1998; Bargum et al., 2004; but see Fjerdingstad, 2005). This variation between years may be due to changes in either the amount of genetic variance or the total phenotypic variance present in different years (Price and Schluter, 1991). Regardless, the observed variation between years suggests that caution be used when interpreting heritability estimates obtained from only a single experiment or breeding season.
Our original motivation for this study was to determine if the influence of genetic variation on phenotypic variation differed between castes. In particular, we expected that variation in worker morphological traits would show evidence of genetic control, and would therefore display considerable amounts of genetic variation and significant heritabilities. However, our results ran somewhat contrary to this prediction. We failed to document substantial amounts of additive genetic variation or strong heritabilities for most worker traits. We also predicted that variation in gyne traits would not be strongly influenced by genotype due to putative positive directional selection on reproductive female size. We did indeed find a general lack of additive genetic control for many gyne traits, although variation in some traits was significantly influenced by non-additive genetic variation. Moreover, more gyne than worker traits were influenced by genetic variation. Thus, our results did not strongly follow our predictions, thereby casting some doubt on the presumed types of selection acting on morphological traits in social insect castes.
We also note that the evolution of the genetic architecture underlying size differences in the two castes may be affected by intralocus caste conflict, similar to intralocus sexual conflict (Bonduriansky and Chenoweth, 2009). Intralocus sexual conflict results in the displacement of sexes from their optimal phenotype as a result of opposing selection pressure in the sexes. It is possible that similar conflicts occurred during the evolution of social insect castes and that such conflicts could affect the evolution of both the caste phenotypes, as well as the genetic architecture underlying phenotypic differences in the castes.
Genetic affect on variation in trait size
Vespula maculifrons wasps of all genotypes show considerable levels of phenotypic plasticity because they can develop into either gynes or workers (Goodisman et al., 2007b). Nevertheless, our analyses revealed significant differences in the variance of trait sizes exhibited by workers and gynes sired by different males (Table 3). We believe that these are among the first analyses to demonstrate a genetic component to morphological trait variability within social insect castes. However, previous studies in other nonsocial animal taxa have also found significant heritabilities for plasticity in traits such as egg laying date and seasonal body mass (Newman, 1994; Nussey et al., 2005a; 2005b; Stinchcombe et al., 2004; Pelletier et al., 2007; Jensen et al., 2008).
Our results suggest that some genotypes may be more plastic than others. One might expect that genotypes showing more phenotypic plasticity would be selectively advantageous under some circumstances (Scheiner and Goodnight, 1984; Scheiner, 2002). Indeed due to the general life histories of social insects, heritable phenotypic plasticity for morphological traits may be advantageous since it would allow for colonies to respond to varying environmental conditions by producing individuals of different sizes.
Relationship between genetic and phenotypic variation
Both polyandry (female multiple mating) and polygyny (multiple reproductive females within a colony) generally result in increased genetic variation within colonies relative to colonies headed by one, singly mated queen. If genotype has a strong influence on morphology, increased genetic variation may lead to an increase in phenotypic variation. For example, if an increase in phenotypic variation results in increased worker task efficiency, polyandry and polygyny may be selectively advantageous (Crozier and Page, 1985; Crozier and Fjerdingstad, 2001; Fjerdingstad and Crozier, 2006). In fact, Fjerdingstad and Crozier (2006) found a significant negative correlation between relatedness, which is relatively low in colonies headed by multiple reproductives, and variation in worker body mass across 35 ant species supporting the hypothesis that genetic variability is associated with phenotypic variability in social insects.
However, we found no correlation between queen mate number and worker or gyne size variation in V. maculifrons. This lack of correlation is not surprising given the generally low heritability estimates of the analyzed traits and the low variation in queen mate number in this species. A lack of correlation between genetic diversity and trait variability has also been reported in a few polyandrous and polygyne ants (Brown and Schmid-Hempel, 2003; Schwander et al., 2005). We note that other mechanisms, such as polygyny syndrome resulting from competition among reproductives (Keller, 1993), may lead to the lack of correlation between genetic variation and phenotypic variation within polygyne taxa. However, such explanations cannot account for the lack of correlation between genetic and phenotypic variation in polyandrous taxa. Thus, these data suggest that polyandry would not necessarily evolve in response to selection for a morphologically variable worker force given the present-day association between genotype and worker size in V. maculifrons.
Conclusions
This study sought to understand the evolutionary and ecological factors affecting size in social insects by investigating genetic influences on size variation in a natural social wasp population. We determined the genetic effects underlying size variation in V. maculifrons workers and gynes, and found a weak effect of genotype, but a strong effect of environment, on variation in most traits. Additionally, we detected significant differences between offspring belonging to different patrilines in the levels of morphological variation expressed, suggesting that some genotypes may be more phenotypically plastic than others. Finally, we uncovered no evidence of correlations between the amount of variation in worker and gyne traits and queen mate number, indicating that high genetic variation within colonies did not necessarily translate into high phenotypic variation among colony mates. Overall, our study suggests a strong environmental influence on morphological trait size in this taxon. We suggest that future research aimed at understanding the effect of genotype on morphology in social insects should investigate a broad range of traits, including those associated with caste and sex function, in order to provide a better picture of which traits are under selection in different phenotypic forms. Overall, such data would help provide insight into the factors that have led to the domination of terrestrial communities by social insects.
References
Abell A.J., Cole, B.J., Reyes R. and Wiernasz D.C. 1999. Sexual selection on body size and shape in the western harvester ant, Pogonomyrmex occidentalis cresson. Evolution 53: 535–545
Archer M.A., Phelan J.P., Beckman K.A. and Rose M.R. 2003. Breakdown in correlations during laboratory evolution. II. Selection on stress resistance in Drosophila populations. Evolution 57: 536–543
Armbruster P., Bradshaw W.E. and Holzapfel C.M. 1997. Evolution of the genetic architecture underlying fitness in the pitcher-plant mosquito, Wyeomyia smithii. Evolution 51: 451–458
Bargum K., Boomsma J.J. and Sundström L. 2004. A genetic component to size in queens of the ant, Formica truncorum. Behav. Ecol. Sociobiol. 57: 9–16
Bienefeld K. and Pirchner F. 1991. Genetic correlations among several colony characters in the honey-bee (Hymenoptera, Apidae) taking queen and worker effects into account. Ann. Entomol. Soc. Am. 84: 324–331
Bonduriansky R. and Chenoweth S.F. 2009. Intralocus sexual conflict. Trends Ecol. Evol. 24: 280–288
Bonduriansky R. and Rowe L. 2005. Intralocus sexual conflict and the genetic architecture of sexually dimorphic traits in Prochyliza xanthostoma (Diptera: Piophilidae). Evolution 59: 1965–1975
Boomsma J.J. 1996. Split sex ratios and queen-male conflict over sperm allocation. Proc. R. Soc. Lond. B. 263: 697–704
Boomsma J.J. and Ratnieks F.L.W. 1996. Paternity in eusocial Hymenoptera. Phil. Trans. R. Soc. Lond. B. 351: 947–975
Brown M.J.F. and Schmid-Hempel P. 2003. The evolution of female multiple mating in social Hymenoptera. Evolution 57: 2067–2081
Crozier R.H. and Fjerdingstad E.J. 2001. Polyandry in social Hymenoptera: disunity in diversity? Annls Zool. Fenn. 38: 267–285
Crozier R.H. and Page R.E. 1985. On being the right size: male contributions and multiple mating in social Hymenoptera. Behav. Ecol. Sociobiol. 18: 105–115
Daly D., Archer M.E., Watts P.C., Speed M.P., Hughes M.R., Barker F.S., Jones J., Odgaard K. and Kemp S.J. 2002. Polymorphic microsatellite loci for eusocial wasps (Hymenoptera : Vespidae). Mol. Ecol. Notes 2: 273–275
DeHeer C.J. 2002. A comparison of the colony-founding potential of queens from single- and multiple-qeen colonies of the fire ant Solenopsis invicta. Anim. Behav. 64: 655–661
Diniz-Filho J.A., Malaspina O. and Cavalheri D.H. 1994. Genetic and within-colony environmental components of variation in the hamuli number of Africanized honey bees (Apis mellifera L.). Rev. Brasil. Genet. 17: 255–258
Diniz-Filho J.A. and Pignata M.I.B. 1994. Quantitive genetics of multivariate morphometric variation in the neotropical stingless bee, Scaptotrigona postica (Hymenoptera: Meliponinae) Rev. Brasil. Genet. 17: 259–265
Falconer D.S. 1989. Introduction to Quantitative Genetics. Longman Wiley, Essex, UK. pp 456
Falconer D.S. and Mackay T.F.C. 1996. Introduction to Quantitative Genetics. Longman, Harlow, UK. pp 480.
Fjerdingstad E.J. 2005. Control of body size of Lasius niger ant sexuals: worker interests, genes and environment. Mol. Ecol. 14: 3123–3132
Fjerdingstad E.J. and Crozier R.H. 2006. The evolution of worker caste diversity in social insects. Am. Nat. 167: 390–400
Foster K.R., Ratnieks F.L.W., Gyllenstrand N. and Thoren P.A. 2001. Colony kin structure and male production in Dolichovespula wasps. Mol. Ecol. 10: 1003–1010
Fournier D., Battaille G., Timmermans I. and Aron S. 2008. Genetic diversity, worker size polymorphism and division of labour in the polyandrous ant Cataglyphis cursor. Anim. Behav. 75: 151–158
Fraser V.S., Kaufmann B., Oldroyd B.P. and Crozier R.H. 2000. Genetic influence on caste in the ant Camponotus consobrinus. Behav. Ecol. Sociobiol. 47: 188–194
Goodisman M.A.D., Kovacs J.L. and Hoffman E.A. 2007a The significance of multiple mating in the social wasp Vespula maculifrons. Evolution 61: 2260–2267
Goodisman M.A.D., Kovacs J.L. and Hoffman E.A. 2007b. Lack of conflict during queen production in the social wasp Vespula maculifrons. Mol. Ecol. 16: 3063–3065
Greene A 1991. Dolichovespula and Vespula. In: The Social Biology of Wasps (Ross K.G. and Matthews R.W., Eds), Cornell University Press, Ithaca, NY. pp 263–309
Hasegawa E. and Takahashi J. 2002. Microsatellite loci for genetic research in the hornet Vespa mandarinia and related species. Mol. Ecol. Notes 2: 306–308
Hoffman E.A., Kovacs J.L. and Goodisman M.A.D. 2008. Genetic structure and breeding system in a social wasp and its social parasite. BMC Evol. Biol. 8: Art. 239
Hölldobler B. and Wilson E.O. 1990. The Ants. Belknap Press of Harvard University Press, Cambridge, MA. pp 746.
Hughes W.O.H., Sumner S., Van Borm S. and Boomsma J.J. 2003. Worker caste polymorphism has a genetic basis in Acromyrmex leaf-cutting ants. Proc. Natl. Acad. Sci. USA 100: 9394–9397
Jaffe R., Kronauer D.J.C., Kraus F.B., Boomsma J.J. and Moritz R.F.A. 2007. Worker caste determination in the army ant Eciton burchellii. Biol. Lett. 3: 513–516
Jensen L.F., Hansen M.M., Pertoldi C., Holdensgaard G., Mensberg K.L.D. and Loeschcke V. 2008. Local adaptation in brown trout early life-history traits: implications for climate change adaptability. Proc. R. Soc. Lond. B. 275: 2859–2868
Keller L. 1993. The assessment of reproductive success of queens in ants and other social insects. Oikos 67: 177–180
Kovacs J.L., Hoffman E.A. and Goodisman M.A.D. 2008. Mating success in the polyandrous social wasp Vespula maculifrons. Ethology 114: 340–350
Lande R. 1980. Sexual dimorphism, sexual selection, and adaptation in polygenic characters. Evolution 34: 292–305
Larsson K., Rattiste K. and Lilleleht V. 1997. Heritability of head size in the common gull Larus canus in relation to environmental conditions during offspring growth. Heredity 79: 201–207
Linksvayer T.A. 2006. Direct, maternal, and sibsocial genetic effects on individual and colony traits in an ant. Evolution 60: 2552–2561
Linksvayer T.A. and Wade M.J. 2005. The evolutionary origin and elaboration of sociality in the aculeate Hymenoptera: maternal effects, sib-social effects, and heterochrony. Q. Rev. Biol. 80: 317–336
Lynch M. and Walsh B. 1998. Genetics and Analysis of Quantitative Traits. Sinauer Associates, Inc., Sunderland, MA. pp 980
Marden J.H. 2000. Variability in the size, composition, and function of insect flight muscles. Annu. Rev. Phys. 62: 157–178
Macdonald J.F. and Matthews R.W. 1981. Nesting biology of the eastern yellowjacket, Vespula maculifrons (Hymenoptera, Vespidae). J. Kansas Entomol. Soc. 54: 433–457
Meffert L.M. 1995. Bottleneck effects on genetic variance for courtship repertoire. Genetics 139: 365–374
Merila J. 1997. Expression of genetic variation in body size of the collared flycatcher under different environmental conditions. Evolution 51: 526–536
Merila J. and Sheldon B.C. 1999. Genetic architecture of fitness and nonfitness traits: empirical patterns and development of ideas. Heredity 83: 103–109
Milne C.P. 1985. An estimate of the heritability of the corbicular area of the honeybee. J. Apic. Res. 24: 137–139
Mousseau T.A. and Roff D.A. 1987. Natural-selection and the heritability of fitness components. Heredity 59: 181–197
Newman R.A. 1994. Genetic-variation for phenotypic plasticity in the larval life-history of spadefoot toads (Scaphiopus couchii). Evolution 48: 1773–1785
Nielsen R., Tarpy D.R. and Reeve H.K. 2003. Estimating effective paternity number in social insects and the effective number of alleles in a population. Mol. Ecol. 12: 3157–3164
Nussey D.H., Clutton-Brock T.H., Elston D.A., Albon S.D. and Kruuk L.E.B. 2005a. Phenotypic plasticity in a maternal trait in red deer. J. Anim. Ecol. 74: 387–396
Nussey D.H., Postma E., Gienapp P. and Visser M.E. 2005b. Selection on heritable phenotypic plasticity in a wild bird population. Science 310: 304–306
Oldroyd B. and Moran C. 1983. Heritability of worker characters in the honeybee (Apis mellifera). Aust. J. Biol. Sci. 36: 323–332
Oldroyd B., Rinderer T. and Buco S. 1991. Heritability of morphological characters used to distinguish European and Africanized honeybees. Theor. Appl. Genet. 82: 499–504
Oster G.F. and Wilson E.O. 1978. Caste and ecology in the social insects. Monogr. Popul. Biol. 12: 1–352
Peeters C. and Ito F. 2001. Colony dispersal and the evolution of queen morphology in social Hymenoptera. Annu. Rev. Entomol. 46:601–630
Pelletier F., Reale D., Garant D., Coltman D.W. and Festa-Bianchet M. 2007. Selection on heritable seasonal phenotypic plasticity of body mass. Evolution 61: 1969–1979
Phelan J.P., Archer M.A., Beckman K.A., Chippindale A.K., Nusbaum T.J. and Rose M.R. 2003. Breakdown in correlations during laboratory evolution. I. Comparative analyses of Drosophila populations. Evolution 57: 527–535
Poklukar J. and Kezic N. 1994. Estimation of heritability of some characteristics of hind legs and wings of honeybee workers (Apis mellifera carnica Polm) using the half-sibs method. Apidologie 25: 3–11
Price T. and Schluter D. 1991. On the low heritability of life-history traits. Evolution 45: 853–861
Rheindt F.E., Strehl C.P. and Gadau J. 2005. A genetic component in the determination of worker polymorphism in the Florida harvester ant Pogonomyrmex badius. Insect. Soc. 52: 163–168
Roff D.A. 1997. Evolutionary Quantitative Genetics. Chapman and Hall, New York, NY. pp 516.
Roff D.A., Stirling G. and Fairbairn D.J. 1997. The evolution of threshold traits: a quantitative genetic analysis of the physiological and life-history correlates of wing dimorphism in the sand cricket. Evolution 51: 1910–1919
Ross K.G. 1985. Aspects of worker reproduction in 4 social wasp species (Insecta, Hymenoptera, Vespidae). J. Zool. 205: 411–424
Rüppell O., Heinze J. and Hölldobler B. 1998. Size-dimorphism in the queens of the North American ant Leptothorax rugatulus (Emery). Insect. Soc. 45: 67–77
Rüppell O., Heinze J. and Hölldobler B. 2001. Complex determination of queen body size in the queen size dimorphic ant Leptothorax rugatulus (Formicidae : Hymenoptera). Heredity 87: 33–40
Scheiner S.M. 2002. Selection experiments and the study of phenotypic plasticity. J. Evol. Biol. 15: 889–898
Scheiner S.M. and Goodnight C.J. 1984. The comparison of phenotypic plasticity and genetic-variation in populations of the grass Danthonia spicata. Evolution 38: 845–855
Schwander T., Rosset H. and Chapuisat M. 2005. Division of labour and worker size polymorphism in ant colonies: the impact of social and genetic factors. Behav. Ecol. Sociobiol. 59: 215–221
Sgro C.M. and Hoffmann A.A. 1998. Heritable variation for fecundity in field-collected Drosophila melanogaster and their offspring reared under different environmental temperatures. Evolution 52: 134–143
Sokal R.R. and Rohlf F.J. 1995. Biometry. W.H. Freeman, New York, NY. pp 880.
Spradbery J.P. 1973. Wasps: An Account of the Biology and Natural History of Solitary and Social Wasps. University of Washington Press, Seattle, WA. pp 408
Stinchcombe J.R., Dorn L.A. and Schmitt J. 2004. Flowering time plasticity in Arabidopsis thaliana: a reanalysis of Westerman & Lawrence (1970). J. Evol. Biol. 17: 197–207
Via S. and Lande R. 1985. Genotype-environment interaction and the evolution of phenotypic plasticity. Evolution 39: 505–522
Via S. and Lande R. 1987. Evolution of genetic-variability in a spatially heterogeneous environment: effects of genotype-environment interaction. Genet. Res. 49: 147–156
Walsh P.S., Metzger D.A. and Higuchi R. 1991. Chelex-100 as a medium for simple extraction of DNA for PCR-based typing from forensic material. Biotechniques 10: 506–513
Wheeler D.E. 1986. Polymorphism and division-of-labor in Azteca chartifex laticeps (Hymenoptera, Formicidae). J. Kansas Entomol. Soc. 59: 542–548
Wheeler D.E. 1991. The developmental basis of worker caste polymorphism in ants. Am. Nat. 138: 1218–1238
Whitlock M.C. 2005. Combining probability from independent tests: the weighted Z-method is superior to Fisher’s approach. J. Evol. Biol. 18: 1368–1373
Wiernasz D.C. and Cole B.J. 2003. Queen size mediates queen survival and colony fitness in harvester ants. Evolution 57: 2179–2183
Wolf J.B. 2000 Gene interactions from maternal effects. Evolution 54: 1882–1898
Wolf J.B., Brodie E.D., Cheverud J.M., Moore A.J. and Wade M.J. 1998. Evolutionary consequences of indirect genetic effects. Trends Ecol. Evol. 13: 64–69
Acknowledgments
We thank D. Bhatka for her diligent lab support, M. Chapuisat, E. Fjerdingstad, T. Linksvayer, and T. Schwander for their generous advice on statistical analyses, and B. G. Hunt for helpful comments on the manuscript. We also thank the Georgia Institute of Technology and NSF grant DEB #0640690 for providing the funds necessary to conduct this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kovacs, J.L., Hoffman, E.A., Marriner, S.M. et al. Environmental and genetic influences on queen and worker body size in the social wasp Vespula maculifrons . Insect. Soc. 57, 53–65 (2010). https://doi.org/10.1007/s00040-009-0050-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00040-009-0050-0