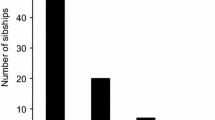Abstract
Neotropical orchid bees (Euglossini) are conspicuously different from other corbiculate bees (Apinae) in their lack of advanced sociality and in male use of acquired odors (fragrances) as pheromone-analogues. In both contexts, orchid bee mating systems, in particular the number of males a female mates with, are of great interest but are currently unknown. To assess female mating frequency in the genus Euglossa, we obtained nests from three species in Mexico and Panama and genotyped mothers and their brood at microsatellite DNA loci. In 26 out of 29 nests, genotypes of female brood were fully consistent with being descended from a singly mated mother. In nests with more than one adult female present, those adult females were frequently related, with genotypes being consistent with full sister–sister (r = 0.75) or mother–daughter (r = 0.5) relationships. Thus, our genetic data support the notions of female philopatry and nest-reuse in the genus Euglossa. Theoretically, single mating should promote the evolution of eusociality by maximizing the relatedness among individuals in a nest. However, in Euglossini this genetic incentive has not led to the formation of eusocial colonies as in other corbiculate bees, presumably due to differing ecological or physiological selective regimes. Finally, monandry in orchid bees is in agreement with the theory that females select a single best mate based on the male fragrance phenotype, which may contain information on male age, cognitive ability, and competitive strength.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hymenoptera are a favored group for studies on mating systems because of the presumed effect of female mating frequency on the evolution of sociality (Hamilton, 1964; Boomsma, 2007; Hughes et al., 2008). The majority of female Hymenoptera are thought to mate only once or have an effective mate number very close to one (Boomsma and Ratnieks, 1996; Strassmann, 2001). Well known exceptions are the highly eusocial honey bees (Apini), attine ants and vespine wasps, some of which are highly polyandrous (Foster and Ratnieks, 2001; Villesen et al. 2002; Tarpy et al., 2004). Queens of the European honey bee (Apis mellifera) have an effective mate number of 12 on average (Strassmann, 2001). Honeybees belong to the monophyletic clade of corbiculate bees (Apinae) that also includes the eusocial stingless bees (Meliponini) and bumblebees (Bombini) (Michener, 2000), both of which generally exhibit monandry (Strassmann, 2001), and the strictly neotropical orchid bees (Euglossini). Information on the mating frequency of female orchid bees is lacking. Orchid bees are generally described as solitary (Zucchi et al., 1969), but in some cases two or several females share a nest or nest cavity. For some species there are reports of reproductive dominance of one female (Garofalo, 1985), providing a potential opportunity for the evolution of more complex sociality (Augusto and Garofalo, 2004; Cameron, 2004; Cocom Pech et al., 2008; Otero et al., 2008). However, why eusociality as an obligate condition has not evolved in orchid bees is an open question (Roubik et al., 1996).
Kin selection theory predicts that female mating frequency affects the likelihood that eusociality evolves because it elevates relatedness to 0.75 among female hymenopteran offspring, increasing their inclusive fitness when helping their mother or sisters to reproduce (Queller and Strassmann, 1998; Boomsma, 2007). Female multiple mating, on the other hand, decreases relatedness among a mothers’ daughters (to nearly 0.25), and their theoretical incentive to cooperate. Thus, polyandry may reduce the likelihood of advanced sociality to evolve. Its existence in honey bees, attine ants and vespine wasps is therefore considered to be derived and to postdate the evolution of sociality and permanent worker castes (Boomsma, 2007).
Other evolutionary factors may favor polyandry over monandry. For example, in social species polyandry provides an increase in intracolonial genetic variation (Crozier and Fjerdingstad, 2001) that has been shown to result in a better colony phenotype that, for example, minimizes the adverse effects of parasitism (Baer and Schmid-Hempel, 1999; Tarpy and Seeley, 2006; Seeley and Tarpy, 2007). Furthermore, polyandry in social and non-social insects is thought to provide compensation for difficulties in identifying the single best mate. The latter hypothesis predicts that multiple mating occurs in species where male quality is difficult to assess, thus constraining the evolution of choosiness in females (Strassmann, 2001).
Orchid bees have a highly unusual mating biology, aspects of which are still poorly understood. Males forage for volatile chemicals (fragrances) in a time-consuming and risky manner (Eltz et al., 1999). Flowers of orchids and other plants, as well as decaying wood or fruits, serve as natural sources of such fragrances, which consist mostly of terpenoids and aromatics. For a great number of neotropical orchid species, male orchid bees act as specific pollinators, giving rise to the Euglossine pollination syndrome (Dodson et al., 1969; Williams, 1982; Cameron, 2004). Collected volatiles are stored by euglossine males in voluminous leg pockets (Vogel, 1966), where a species-specific blend of chemicals accumulates (Eltz et al., 2005a; Zimmermann et al., 2006). During courtship display these blends are actively released and ventilated at courtship territories (Bembé, 2004; Eltz et al., 2005b), where females have been observed to mate (Kimsey, 1980; Stern, 1991; Eltz et al., 2003). Although the attraction of females to these odors has yet to be demonstrated in behavioral experiments, the male perfume is likely to function as a species specific chemical signal analogous to endogenous sex pheromones (Vogel, 1966; Zimmermann et al., 2006). In addition, the individual perfume of a male could represent a fitness indicator, giving an approaching female the possibility to evaluate a male’s quality and to choose her best mate, making potentially costly multiple mating unnecessary.
Thus, information on orchid bee female mating frequency is desirable both in the context of social evolution in corbiculate bees as well as for a better understanding of the significance of euglossine fragrance collection. The genus Euglossa represents the largest and most widely distributed genus of orchid bees (more than 100 described species) (Roubik and Hanson, 2004), with females of some species accepting artificial trap nests for their brood rearing. To analyze female mating frequency in orchid bees we genetically analyzed brood from nests of three species of Euglossa—E. hemichlora, E. viridissima and E. sp. ‘2dentate’—using polymorphic microsatellites and calculated relatedness among brood, among cohabiting females and between brood and adults.
Materials and methods
Sample collection
We studied Euglossa hemichlora from Panama, Euglossa viridissima from Mexico and another so far undescribed species of Euglossa from Mexico, which is morphologically almost identical to E. viridissima, but males have two instead of three mandibular teeth. Population genetic analysis demonstrated that the lineage is reproductively isolated from E. viridissima (Eltz et al., 2008). For the purpose of this study, we refer to this species as Euglossa sp. ‘2dentate’.
We used wooden boxes (10 × 3 × 6 cm) as trap nests, which were placed around buildings in a private forest preserve in Santa Rita, Colon, Panama (8.39°N, 82.34°W) in October 2007 and at the campus of the Universidad Autónoma de Yucatán in Xmatkuil, Mexico (20.52°N, 87.37°W) in October 2006 and October 2007. The nest boxes were monitored over several months at each locality, and the number and condition of brood cells therein were recorded. Nests were collected when the boxes were populated with at least five (preferentially more) brood cells and at least one adult female was present (which was normally the case during afternoons). For microsatellite DNA analysis, adult females present in the nest boxes were immediately preserved in 99% ethanol and stored at +8°C. The brood cells were kept in a cabinet at 26°C and 70% humidity until the offspring emerged. We recorded the dates of eclosion of progeny, immediately preserved individuals in 99% ethanol and stored them at +8°C.
To obtain more precise estimates of allele frequencies, we also sampled males of all three species up to 3 km radius from the nest boxes. Males were attracted with fragrance baits (p-dimethoxy benzene for E. hemichlora, p-dimethoxy benzene, methyl cinnamate and eugenol for E. viridissima and E. sp. ‘2dentate’), caught and immediately preserved in 99% ethanol at +8°C.
DNA extraction and microsatellite DNA analysis
Microsatellite DNA analysis was conducted by using seven different microsatellite loci: ann02 (GenBank accession no. BV728898), ann04 (BV728900), ann08 (BV728902), ann24 (BV728906) (Paxton et al., 2009), Egc17 (EF451841), Egc18 (EF451842), and Egc37 (EF451846) (Souza et al., 2007). Not all of the samples were typed at all seven loci, but at the most polymorphic loci for the given species, with each individual being typed at an average of 3.36 loci (range 2–6). DNA was extracted from tissue from half of the thorax of each specimen using the method of Hunt and Page (Hunt and Page, 1995). After phenol/chloroform extraction, DNA pellets were dried at 37°C, resuspended in 40 μl of distilled water and stored at −20°C. All PCRs were performed as multiplex reactions with three loci 5′labeled with fluorescent dye (VIC, 6-FAM or NED; Applied Biosystems). Four μl of DNA template was used with 12.5 μl HotStar TaqTM Master Mix (Qiagen) in a final reaction volume of 25 μl, made up with RNase-free water (Qiagen). PCR reactions were performed in a Mastercycler gradient (Eppendorf), with the profile of an initial 95°C for 15 min (HotStar Taq Polymerase), followed by 22 cycles of 94°C for 30 s, 52°C for 90 s, 67°C for 90 s, and then a final extension step of 67°C for 10 min. Fragment analysis of PCR products was carried out with an ABI Prism 310TM Sequencer (PE Applied Biosystems) at the University of Düsseldorf (BMFZ). Allele lengths were assigned with the software GENEMARKER V1.71, using an internal standard run in every lane. Allele sizes were rounded to the nearest integer.
Data analysis
We tested for linkage disequilibrium between loci within each species with the program GENEPOP (Raymond and Rousset, 1995) (web version at http://genepop.curtin.edu.au/), using only the haploid male data because GENEPOP does not allow for a joint analysis of haploid and diploid data.
A standard suite of descriptive statistics was then calculated for each locus using Microsatellite Analyzer (MSA) version 4.05 (Dieringer and Schlotterer, 2003), which supports the option of jointly analyzing haploid and diploid data. To avoid pseudoreplication, only unrelated females were included in the calculation of population allele frequencies, which means one female per nest with one exception where a further unrelated female was present.
For each population of the three species we calculated the genetic non-detection error of the mating frequency, defined as the probability of an undetected second father among a female’s progeny, which occurs when two males have identical genotypes at all investigated loci (Boomsma and Ratnieks, 1996).
Estimation of mating frequency
Assignments of adult females as mothers of brood were first made by visual inspection of genotypes; all daughters of a mother had to carry one single maternal allele at all loci, and all sons had to carry one of the two maternal alleles at each locus. Under monandry, all female offspring of a mother should carry the same paternal allele at a locus. To support pedigree determination based on visual inspection of genotypes, we examined intra-nest relationships using the likelihood function of KINSHIP 1.3.1 (Goodnight and Queller, 1999), (http://www.gsoftnet.us/GSoft.html#Kinanchor), using the same data set as we used for the descriptive statistics. Female genotypes of each nest were analyzed separately in a group. In the first step we analyzed, whether one or more of the adult females present in a nest at the time of sampling were the mother of the brood. Specifically we tested whether her genotype allowed the rejection of the null hypothesis (no relationship to female brood; r = 0) in favor of the alternative hypothesis of a mother–daughter relationship (r = 0.5). In the second step, we tested whether the genotypes of female offspring in a nest allowed us to reject the null hypothesis that they were half sisters (r = 0.25 as expected with multiple mating of the mother) in favor of the alternative hypothesis that they were full sisters (r = 0.75 as expected by single mating). These tests were performed as pairwise comparisons calculated with 1,000 simulations between each female progeny pair within each nest.
With the relatedness function of KINSHIP 1.3.1 we performed pairwise relatedness (R) calculations between all female individuals to generate the mean value of relatedness between a mother and its female offspring and among all female offspring.
Results
In total, we collected 13 populated nest boxes from E. hemichlora (all in the year 2007), five from E. viridissima (two from 2006 and three from 2007) and 14 from Euglossa sp. ‘2dentate’ (eight from 2006 and six from 2007). Two types of nests were distinguished: newly founded nests (NFN) were in artificial boxes that had been colonized for the first time by a single female foundress, building one homogenous cell cluster. We found six NFNs for E. hemichlora, three for E. viridissima and 11 for E. sp. ‘2dentate’. The other nest type, older or re-used nests (RN), contained remains of previous brood (enclosed brood cells) and had obviously been re-used by other females, potentially by progeny of the original foundress. In RNs there was frequently more than one adult female and/or cell cluster, which made the assignment of mothers to progeny more difficult. For E. hemichlora we found four RNs, and for E. viridissima and E. sp. ‘2dentate’ we found two RNs each.
Three additional nests of E. hemichlora and one nest of E. sp. ‘2dentate’ were excluded from our analysis of mating frequency because they had both a low number of female progeny (n < 3) as well as more than one adult female present.
Microsatellite data analysis
No significant linkage disequilibrium between loci was detected in any of the three analysed species (n = 43 for E. hemichlora, n = 69 for E. sp. ‘2dentate’ and n = 73 for E. viridissima).
For the analysis of allelic variation at loci we genotyped males of each population (E. hemichlora n = 43, E. sp. ‘2dentate’ n = 69, E. viridissima n = 73) and pooled these data with the genotype data of unrelated females (see above; E. hemichlora n = 13, E. sp. ‘2dentate’ n = 15, E. viridissima n = 6). Genetic diversity estimates are given in Table 1. The expected heterozygosity (H e) of our markers ranged from 0.41 to 0.98 and we found between five and 49 alleles per locus. In every species H e was 0.8 at a minimum of 2 loci.
The extremely high variability of ann02 (H e = 0.98) in E. viridissima as well as the large mean allele size of 238 bp (minimum 150 bp and maximum 387 bp) led us to suspect non-specific PCR products. However, repeated independent processing (including DNA extraction) of the same samples confirmed fragment sizes in all cases (n = 14). Also, the marker was clearly inherited in a strictly Mendelian manner within families of bees.
As a result of the high variability of our markers, the population-wide probability of genetic non-detection of a second fathering male’s offspring among progeny genotypes was very small within each of the three species; the non-detection error (d p) varied from 0.002 to 0.00007.
Estimation of mating frequency
In 17 of the 20 analyzed NFNs, the single adult female present was clearly identified as mother of the whole nests female progeny, both by visual inspection of genotypes as well as by KINSHIP (Table 2). One exception was NFN 6 (E. hemichlora), where the adult female was unrelated to the offspring (r = −0.03 ± 0.09), but the female progeny still consisted solely of full-sisters (see below). In the second exception in NFN 24 (E. sp. ‘2dentate’), the single adult female was likely a daughter of the deceased foundress as its genotype was fully congruent with a full-sister relationship with all other female progeny (brood) of the nest. In the third exceptional case (NFN 25 in E. sp. ‘2dentate’) the adult female was identified as the mother of four of the emerging females, whereas the remaining four females (that emerged >21 days earlier) were unrelated to this mother.
Genotypes of female progeny were consistent with a full-sister relationship in 19 out of 20 NFNs, and this finding was confirmed by KINSHIP tests (Table 2). The single exception was NFN 25 (E. sp. ‘2dentate’). Here, the first four emerged females formed a group of full-sisters that was likely descended from the adult female present in the nest at the time of sampling. The remaining four females that emerged later were not related to them (r = −0.09 ± 0.12), but were in a full-sister relationship amongst each other. For reasons of clarity the results of KINSHIP tests are given separately for these two groups of offspring (NFN 25a and NFN 25b in Table 2).
The haploid genotypes of male progeny of the NFNs were consistent with being derived from unfertilized eggs laid by the identified nest mothers. Numbers of emerged males per nest are given in Table 2. We did not find a single male offspring that was unrelated to the mother of the diploid brood.
Within the RNs we always found more than one adult female per nest, but only in RN 15 (E. viridissima) was an adult female present which was unrelated to the brood (r = 0.009 ± 0.17) in addition to the clearly identified nest mother. It had no offspring. In RN 20 (E. hemichlora) we found two unrelated pairs of full sisters present (r = 0.08 ± 0.22). In all other cases, relatedness estimates were consistent with adults being either sisters or in a mother-sister relationship (Table 3). The close relatedness between the adult females made an assignment of the offspring more difficult. Nonetheless, full-sister relationships among female offspring, as an indication for single mating, were found in five RNs (RN 1 in E. hemichlora, RN 6 and 26 in E. sp. ‘2dentate’ and RN 15 and 20 in E. viridissima). In these cases one adult female was clearly identified as the mother of the entire female progeny. The other related female(s) had definitely produced no female offspring, but definite exclusion of their contribution to male progeny was impossible based on the available data. One exception was RN 1 (E. hemichlora), where a likely sister of the major reproductive female appeared to be responsible for at least three of the nine male offspring. RN 18 (E. hemichlora) consisted only of female progeny in a full-sister relationship (including four adult females), but the mother was apparently no longer present. The female offspring of RN 4, RN 20 (E. hemichlora), and RN 21 (E. sp. ‘2dentate’) were not explainable with one single mother (even taking into account multiple mating), and a full-sister relationship between the female progeny was not detected. The genetically mixed brood must have been the offspring of more than one reproductive female. In RN 4 and RN 21 we could identify one main reproductive female; these two nests are explainable as derived from multiple, closely related mothers. In RN 20 we were not able to explain the origin of the progeny with the adult females present, which were two pairs of full sisters. This “nest” was located in an old stingless bee nest box (larger than the artificial trap-nests we employed for Euglossa) and contained three cell clusters. Two of them belonged to E. viridissima (RN 20) and one outlying cell cluster to E. sp. ‘2dentate’ (RN 26).
Altogether, we found strong evidence for single mating in 26 out of 29 nests. Even if multiple mating occurred among females that produced the three problematic nests, the average number of effective mates of the three Euglossa species would be close to one. Within the nine nests that were populated by multiple adult females, we found four cases with progeny that were definitely derived from more than one female. These contributing females were close relatives in three cases, most likely in a full sister relationship (N = 1) or in a mother-daughter relationship (N = 2).
Discussion
Our results represent strong evidence for a predominance of single mating in three Euglossa species and suggest that single mating is the rule in this genus and perhaps in the Euglossini as a whole. Single mating (monandry) within the Euglossini is consistent with the idea that females select a single best mate based on male fragrance phenotypes. However, as single mating is common among bees (Boomsma and Ratnieks, 1996; Roubik, 2006; Soro et al., 2009), and probably ancestral for corbiculate bees (Hughes et al., 2008), monandry in orchid bees is unlikely to be an adaptation resulting from fragrance-based mate selection by females. In any case, single mating is fully consistent with the rarity with which matings are observed at euglossine display sites. Males of most if not all species of orchid bees establish non-resource based display sites for fragrance signaling (Eltz et al., 2005b). These display sites are usually centered around perches (trunks of trees or tree lets, often in the forest understory), where males perform series of hovering flights during which they release their fragrances. Extensive studies of displaying males in the field have resulted in only a handful of observed matings (Kimsey, 1980; Stern, 1991). In most cases the female suddenly appeared and quickly landed on the perch, where it was mounted by the resident male. Such rare events may seem to be lucky strikes in the long lives of males, which otherwise appear to display unsuccessfully for weeks or more. Given single mating in females, the rarity of observed matings is understandable. Orchid bee males devote much of their life to fragrance collection, a behavior that requires specialized morphological features (Bembé, 2004), an intricate metabolic recycling mechanism (Eltz et al., 2007), and certainly a lot of energy and risk-taking in order to create their perfume. Although the composition of the male’s fragrance mixture is broadly species specific, there is substantial individual variation in quantity and complexity of the blends (Eltz et al., 1999; Zimmermann et al., 2006). It is, therefore, conceivable that females evaluate male fragrance phenotypes to obtain information on male suitability as a mate. If so, females may not need to mate more than once to obtain good genes for their offspring.
Our results fuel the discussion on how bee mating systems evolved (Paxton, 2005) and how they might affect the evolution of advanced sociality. The Euglossini were the last group of corbiculate bees for which information on female mating frequency was lacking. Our study confirms the prevalence of single mating among corbiculate bees: with the exception of honeybees, all other corbiculate clades (bumblebees, stingless bees, and orchid bees) have been demonstrated to be predominantly monandrous. Although the exact phylogenetic relationship among corbiculate bees is still controversial (Ascher et al., 2001; Kawakita et al., 2008), monandry in orchid bees confirms the view that single mating was the ancestral state in this clade. Correspondingly, polyandry in honeybees is likely derived (Hughes et al., 2008).
Kin selection theory predicts that single mating promotes the evolution of eusociality, because it increases the genetic relatedness among offspring and, accordingly, their incentive to cooperate in the care of brood (Trivers and Hare, 1975; Cole, 1983; Boomsma, 2007). This is suggested because non-reproductive individuals can gain greater inclusive fitness by functioning as helpers of close relatives (Hamilton, 1964; Queller and Strassmann, 1998). As orchid bees seem to be mostly singly mated, the mating system cannot account for the conspicuous lack of advanced sociality in this group. Female offspring were full sisters in most of the analyzed nests of this study, and adult females present in a given nest were almost always close relatives. This would seem to represent the ideal genetic background for reproductive division of labor and eusocial behavior to evolve. Indeed, some early stages of sociality and reproductive division of labor exist in orchid bees. Previous studies (Dressler, 1982; Santos and Garofalo, 1994) as well as our own observations here have provided evidence for the frequent occurrence of multi-female nests, which seem to result predominantly from nest re-use by bees of the next generation (Garofalo, 1985; Soucy et al., 2003; Augusto and Garofalo, 2004). In some species of Euglossa there is evidence for individual females gaining reproductive dominance over their nest mates that allocate more time to foraging (Michener, 1974; Garofalo, 1985; Cocom Pech et al., 2008; Otero et al., 2008). There is also a suggestion of reproductive conflict, e.g. evidenced by the frequent occurrence of oophagy, usually by the reproductively dominant female (Garofalo, 1985; Roubik and Hanson, 2004; Cocom Pech et al., 2008; Otero et al., 2008). In natural nest cavities, nest sharing may even be more common than in artificial trap-nests, because natural cavities are potentially larger and alternative nest sites perhaps more difficult to find. The benefits of nest sharing may include the avoidance of mortality risks associated with searching for new nest cavities and nest construction material. Furthermore, multi-female nests may be better protected against nest parasites, which regularly enter the nest while the female is out foraging (Soucy et al., 2003; Cocom Pech et al., 2008). However, while the presence of host females in the nest has been observed to deter attacking cleptoparasitic bees, such effects may not necessarily translate into better protection of multiple-female nests (Garofalo and Rozen, 2000).
Ecological factors such as limited nest sites and economy of nest material have already been hypothesized to favor nest sharing in studies about communal bees (Paxton et al., 1996), where females tolerate unrelated conspecifics as well. Nest reuse by succeeding generations would avoid the search for a suitable nesting place and allow the recycling of nest material from hatched brood cells (Dressler, 1982). Thus, given the right ecological conditions, females might benefit from cooperation with conspecifics or even heterospecifics. However, there is no evidence for highly eusocial behavior in orchid bees, or for the formation of long-lived colonies with highly skewed reproduction among individual females. This evolutionary avenue may be barred by several constraining factors: First, euglossine bees have relatively long generation times, with development from egg to adult taking at least 6 weeks and often longer (Roubik and Hanson, 2004). This probably reduces the amount of time that mother and offspring overlap in their adult lives and reduces the opportunities for daughters to help their mothers (but see (Cocom Pech et al., 2008). Second, female reproduction may be limited by physiological factors, even when help from related individuals is available. Possible physiological constraints include the number of sperm that can be stored in the female spermatheca (euglossine spermatozoa are exceptionally large, see (Zama et al., 2005)) or, more likely, the availability of protein for egg production.
In our study, we found little evidence for communal nesting of unrelated females. There were only three cases where an unrelated conspecific female was present in a nest box, and in two cases these had no own progeny. It is conceivable that the unrelated females were in search of nest cavities or even for nest building material, since for example E. viridissima females are known to glean resin from populated nests of stingless bees (J. Quezada-Euan, unpubl. data). Detailed behavioral observations of individually marked females coupled to genetic analysis of offspring will help resolve the uncertainty over the degree to which Euglossines exhibit social behavior.
References
Ascher J.S., Danforth B.N. and Ji S.Q. 2001. Phylogenetic utility of the major opsin in bees (Hymenoptera: Apoidea): A reassessment. Mol. Phylogenet. Evol. 19: 76–93
Augusto S.C. and Garofalo C.A. 2004. Nesting biology and social structure of Euglossa (Euglossa) townsendi Cockerell (Hymenoptera, Apidae, Euglossini). Insect. Soc. 51: 400–409
Baer B. and Schmid-Hempel P. 1999. Experimental variation in polyandry affects parasite loads and fitness in a bumble-bee. Nature 397: 151-154
Bembé B. 2004. Functional morphology in male euglossine bees and their ability to spray fragrances (Hymenoptera, Apidae, Euglossini). Apidologie 35: 283-291
Boomsma J.J. 2007. Kin selection versus sexual selection: Why the ends do not meet. Curr. Biol. 17: 673-683
Boomsma J.J. and Ratnieks F.L.W. 1996. Paternity in eusocial Hymenoptera. Phil. Trans. R. Soc. Lond. 351: 947–975
Cameron S.A. 2004. Phylogeny and biology of neotropical orchid bees (Euglossini). Annu. Rev. Entomol. 49: 377–404
Cocom Pech M.E., May-Itza W.D., Medina Medina L.A. and Quezada-Euan J.J.G. 2008. Sociality in Euglossa (Euglossa) viridissima Friese (Hymenoptera, Apidae, Euglossini). Insect. Soc. 55: 428-433
Cole B.J. 1983. Multiple mating and the evolution of social-behavior in the Hymenoptera. Behav. Ecol. Sociobiol. 12: 191–201
Crozier R.H. and Fjerdingstad E.J. 2001. Polyandry in social Hymenoptera - disunity in diversity? Ann. Zool. Fenn. 38: 267–285
Dieringer D. and Schlotterer C. 2003. MICROSATELLITE ANALYSER (MSA): a platform independent analysis tool for large microsatellite data sets. Mol. Ecol. Notes 3: 167–169
Dodson C.H., Dressler R.L., Hills H.G., Adams R.M. and Williams N.H. 1969. Biologically active compounds in orchid fragrances. Science 164: 1243–1249
Dressler R.L. 1982. Biology of the orchid bees (Euglossini). Annu. Rev. Ecol. Syst. 13: 373–394
Eltz T., Roubik D.W. and Lunau K. 2005a. Experience-dependent choices ensure species-specific fragrance accumulation in male orchid bees. Behav. Ecol. Sociobiol. 59: 149–156
Eltz T., Roubik D.W. and Whitten W.M. 2003. Fragrances, male display and mating behaviour of Euglossa hemichlora - a flight cage experiment. Physiol. Entomol. 28: 251–260
Eltz T., Sager A. and Lunau K. 2005b. Juggling with volatiles: exposure of perfumes by displaying male orchid bees. J. Comp. Physiol. 191: 575–581
Eltz T., Whitten W.M., Roubik D.W. and Linsenmair K.E. 1999. Fragrance collection, storage, and accumulation by individual male orchid bees. J. Chem. Ecol. 25: 157–176
Eltz T., Zimmermann Y., Haftmann J., Twele R., Francke W., Quezada-Euan J.J.G. and Lunau K. 2007. Enfleurage, lipid recycling and the origin of perfume collection in orchid bees. Proc. R. Soc. B 274: 2843–2848
Eltz T., Zimmermann Y., Pfeiffer C., Ramirez Pech J., Twele R., Francke W., Quezada-Euan J.J.G. and Lunau K. 2008. An olfactory shift is associated with male perfume differentiation and sibling species divergence in orchid bees. Curr. Biol. 18: 1844–1848
Foster K.R. and Ratnieks F.L.W. 2001. Paternity, reproduction and conflict in vespine wasps: a model system for testing kin selection predictions. Behav. Ecol. Sociobiol. 50: 1–8
Garofalo C.A. 1985. Social structure of Euglossa cordata nests (Hymenoptera, Apidae, Euglossini). Entomol. Gen. 11: 77–83
Garofalo C.A. and Rozen J.G. 2000. Parasitic behavior of Exaerete smaragdina with descriptions of its mature oozyte and larval instars (Hymenoptera: Apidae: Euglossini)., Am. Mus. Novitates New York. 26 pp
Goodnight K.F. and Queller D.C. 1999. Computer software for performing likelihood tests of pedigree relationship using genetic markers. Mol. Ecol. 8: 1231–1234
Hamilton W.D. 1964. Genetical evolution of social behaviour I., II. J. Theor. Biol. 7: 1–52
Hughes W.O.H., Oldroyd B.P., Beekman M. and Ratnieks F.L.W. 2008. Ancestral monogamy shows kin selection is key to the evolution of eusociality. Science 320: 1213–1216
Hunt G.J. and Page R.E. 1995. Linkage map of the honey-bee, Apis mellifera, based on RAPD markers. Genetics 139: 1371–1382
Kawakita A., Ascher J.S., Sota T., Kato M. and Roubik D.W. 2008. Phylogenetic analysis of the corbiculate bee tribes based on 12 nuclear protein-coding genes (Hymenoptera: Apoidea: Apidae). Apidologie 39: 163–175
Kimsey L.S. 1980. The behaviour of male orchid bees (Apidae, Hymenoptera, Insecta) and the question of leks. Anim. Behav. 28: 996–1004
Michener C.D. 1974. The Social Behavior of the Bees: a Comparative Study. Belknap Press of Harvard University Press, Cambridge. 404 pp
Michener C.D. 2000. The Bees of the World. The Johns Hopkins University Press, Baltimore. 913 pp
Otero J.T., Ulloa-Chacon P., Silverstone-Sopkin P. and Giray T. 2008. Group nesting and individual variation in behavior and physiology in the orchid bee Euglossa nigropilosa Moure (Hymenoptera, Apidae). Insect. Soc. 55: 320–328
Paxton R.J. 2005. Male mating behaviour and mating systems of bees: an overview. Apidologie 36: 145–156
Paxton R.J., Thoren P.A., Tengö J., Estoup A. and Pamilo P. 1996. Mating structure and nestmate relatedness in a communal bee, Andrena jacobi (Hymenoptera, Andrenidae), using microsatellites. Mol. Ecol. 5: 511–519
Paxton R.J., Zobel M.U., Steiner J. and Zillikens A. 2009. Microsatellite loci for Euglossa annectans (Hymenoptera: Apidae) and their variability in other orchid bees. Mol. Ecol. Resour. doi:10.1111/j.1755-0998.2009.02612.x
Queller D.C. and Strassmann J.E. 1998. Kin selection and social insects. Bioscience 48: 165–175
Raymond M. and Rousset F. 1995. Genepop (Version-1.2) - population-genetics software for exact tests and ecumenicism. J. Hered. 86: 248–249
Roubik D.W. 2006. Stingless bee nesting biology. Apidologie 37: 124-143
Roubik D.W. and Hanson P.E. 2004. Orchid Bees of Tropical America: Biology and Field Guide. Instituto Nacional de Biodiversidad Press (INBio), Heredia, Costa Rica. 370 pp
Roubik D.W., Weigt L.A. and Bonilla M.A. 1996. Population genetics, diploid males, and limits to social evolution of euglossine bees. Evolution 50: 931–935
Santos M.L. and Garofalo C.A. 1994. Nesting biology and nest re-use of Eulaema nigrita (Hymenoptera: Apidae, Euglossini). Insect. Soc. 41: 99–110
Seeley T.D. and Tarpy D.R. 2007. Queen promiscuity lowers disease within honeybee colonies. Proc. R. Soc. B 274: 67–72
Soro A., Ayasse M., Zobel M.U. and Paxton R.J. 2009. Complex sociogenetic organization and the origin of unrelated workers in a eusocial sweat bee, Lasioglossum malachurum. Insect. Soc. 56: 55–63
Soucy S.L., Giray T. and Roubik D.W. 2003. Solitary and group nesting in the orchid bee Euglossa hyacinthina (Hymenoptera: Apidae). Insect. Soc. 50: 248–255
Souza R.O., Cervini M., Del Lama M.A. and Paxton R.J. 2007. Microsatellite loci for euglossine bees (Hymenoptera: Apidae). Mol. Ecol. Notes 7: 1352–1356
Stern D.L. 1991. Male territoriality and alternative male behaviors in the euglossine bee, Eulaema meriana (Hymenoptera: Apidae). J. Kansas Entomol. Soc. 64: 421–437
Strassmann J. 2001. The rarity of multiple mating by females in the social Hymenoptera. Insect. Soc. 48: 1–13
Tarpy D.R., Nielsen R. and Nielsen D.I. 2004. A scientific note on the revised estimates of effective paternity frequency in Apis. Insect. Soc. 51: 203–204
Tarpy D.R. and Seeley T.D. 2006. Lower disease infections in honeybee (Apis mellifera) colonies headed by polyandrous vs monandrous queens. Naturwissenschaften 93: 195–199
Trivers R.L. and Hare H. 1975. Haplodiploidy and evolution of social insects. Science 191: 249–263
Villesen P., Murakami T., Schultz T.R. and Boomsma J.J. 2002. Identifying the transition between single and multiple mating of queens in fungus-growing ants. Proc. R. Soc. Lond. B-Biol. 269: 1541–1548
Vogel S. 1966. Parfümsammelnde Bienen als Bestäuber von Orchidaceen und Gloxinia. Österr. Bot. Z. 113: 302–361
Williams N.H. 1982. The biology of orchids and euglossine bees. In: Orchid Biology: Reviews and Perspectives. (Arditti J., Ed) Cornell University Press, Ithaca, NY, pp 119–171
Zama U., Lino-Neto J., Mello S.M., Campos L.A.O. and Dolder H. 2005. Ultrastructural characterization of spermatozoa in euglossine bees (Hymenoptera, Apidae, Apinae). Insect. Soc. 52: 122–131
Zimmermann Y., Roubik D.W. and Eltz T. 2006. Species-specific attraction to pheromonal analogues in orchid bees. Behav. Ecol. Sociobiol. 60: 833–843
Zucchi R., Sakagami S.F. and Camargo J.M.F.d. 1969. Biological observations on a neotropical parasocial bee, Eulaema nigrita, with a review on the biology of Euglossinae (Hymenoptera, Apidae). A comparative study. J. Fac. Sci. Hokkaido Univ. Ser. VI, Zool. 17: 271–380
Acknowledgments
We thank Tobias Wiesenfahrt for providing a Macintosh Computer and Manuel Breuer for supporting the literature search. Klaus Lunau and the members of the Sensory Ecology Group in Düsseldorf helped to improve the manuscript; Martin Hasselmann patiently answered methodological questions. Funding was provided to T.E. by the Deutsche Forschungsgemeinschaft (DFG, EL 249/3).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zimmermann, Y., Roubik, D.W., Quezada-Euan, J.J.G. et al. Single mating in orchid bees (Euglossa, Apinae): implications for mate choice and social evolution. Insect. Soc. 56, 241–249 (2009). https://doi.org/10.1007/s00040-009-0017-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00040-009-0017-1