Abstract
Objectives
To examine sex differences in prevalent comorbidity and frailty across age and European regions.
Methods
This is a cross-sectional study based on 113,299 Europeans aged 50+ participating in the Survey of Health, Ageing and Retirement in Europe from 2004–2005 to 2015. Sex differences in the Comorbidity Index and the Frailty Phenotype were investigated using ordinal logistic regressions.
Results
European women had generally higher odds of prevalent comorbidity (OR 1.11, 95% CI 1.07–1.15) and frailty (OR 1.56, 95% CI 1.51–1.62). Sex differences increased with advancing age. No overall sex difference in comorbidity was found in Western Europe, but women had more comorbidity than men in Eastern (OR 1.30, 95% CI 1.18–1.44), Southern (OR 1.23, 95% CI 1.15–1.30), and Northern (OR 1.08, 95% CI 1.01–1.16) Europe. Women were frailer than men in all regions, with the largest sex difference in Southern Europe (OR 1.84, 95% CI 1.72–1.96).
Conclusions
European women are frailer and have slightly more comorbidity than European men lending support for the male–female health survival paradox.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Women outlive men in most countries in the world (Barford et al. 2006). Despite the longer life expectancy, women generally report worse health than men (Dix 2014; Hubbard and Rockwood 2011; Van Oyen et al. 2013)—the so-called male–female health survival paradox (Oksuzyan et al. 2008). Women’s poorer health has been defined in many ways, for example, in terms of lower self-rated health, grip strength measures and worse physical functioning (Andersen-Ranberg et al. 2009; Crimmins et al. 2011; Oksuzyan et al. 2010); however, the direction and magnitude of sex differences vary across health measures and geographical regions (Oksuzyan et al. 2010).
Possible explanations for the paradox include sex differences in disease occurrence (Kingston et al. 2014). In general, men are reported to have more life-threatening conditions, such as cardiovascular diseases, whereas women tend to have more non-fatal chronic diseases, such as physical and psychological limitations, migraine, musculoskeletal and autoimmune diseases (Case and Paxson 2005; Gold et al. 2002; Whitacre 2001). In 11 European countries, and in England and the USA, arthritis and depressive symptoms were more common among women, whereas men were more likely to suffer from heart diseases (Crimmins et al. 2011). A study reporting the prevalence of chronic conditions in the National Social Life, Health and Aging Project found that men more frequently reported heart conditions, cancer, diabetes and stroke compared with women. In contrast, women had higher prevalence of arthritis, with the largest sex difference at older ages (Vasilopoulos et al. 2014).
The poorer physical health among women has also been defined in terms of frailty (Fried et al. 2001; Jones et al. 2004). Frailty is a well-validated measure of physical health and is associated with a considerably increased risk of adverse health outcomes such as falls, hospitalization, long-term care, institutionalization and mortality (Chen et al. 2014; Clegg et al. 2013). Studies of community-dwelling populations have found higher frailty scores among women than among men (Collard et al. 2012; Saum et al. 2014), but sex differences in frailty vary among studies (Gordon et al. 2017), perhaps reflecting differences in the measurements of frailty, or differences in the study base characteristics, such as age, country of origin or ethnicity (Gordon et al. 2017; Saum et al. 2014). Numerous definitions of frailty have been proposed, but the one that has gained most acceptance is the phenotypic definition of frailty proposed by Fried et al. (2001). This is a widely used tool to identify frailty reported in 69% of published papers (Bouillon et al. 2013). The Frailty Phenotype identifies frailty by the presence of three or more of the following criteria: weakness, slowness, low physical activity, low energy or self-reported exhaustion and unintentional weight loss. Santos-Eggimann et al. (2009) used the Frailty Phenotype in the first wave of the Survey of Health, Ageing and Retirement in Europe (SHARE) and found that the overall prevalence of frailty was 17% in Europe, with women being frail more frequently than men. The proportion of frailty was higher in Southern Europe than in Northern Europe (Santos-Eggimann et al. 2009) in accordance with findings of a north–south gradient for other health dimensions (Andersen-Ranberg et al. 2009; Cimas et al. 2018; Dahlin and Harkonen 2013; Van de Velde et al. 2010; Weber et al. 2014).
To shed light on cross-country differences in health and aging, we investigated the direction and magnitude of the sex differences in prevalent comorbidity and frailty over age across European regions in a large sample of men and women aged 50+ who participated in SHARE between 2004–2005 and 2015. Because men die at higher rates than women throughout life, surviving men are likely to be more physically robust than women, because only the healthiest men live to older ages (Austad and Fischer 2016). We hypothesize that women are generally frailer and have more comorbidity than men at any given age, with sex differences increasing with advancing age.
Methods
Setting and study population
SHARE is a multinational database with information from sequential survey “waves” on health, socioeconomic status and social factors in European men and women aged 50 and older. Data were collected as personal interviews at the participants’ homes by trained interviewers, in accordance with the strict quality standards (Börsch-Supan et al. 2013). The data collection was drawn at the household level with response rates differing by waves and countries ranging between 40.3 and 97.6% in wave 1 (Bergmann et al. 2017). Proxy respondents were allowed for the interview if the respondent had physical or health limitations (Börsch-Supan et al. 2013).
We performed a large cross-sectional study of European men and women aged 50+ who participated in SHARE waves 1 (2004–2005), 2 (2006–2007), 4 (2011), 5 (2013) and 6 (2015) where health measures were available. This includes repeated measures of participants and thus the ability to include a larger proportion of the oldest people. We excluded individuals with unknown birth date (n = 19) and missing cross-sectional weights (n = 385, 0.3%). The numbers of observations by wave and country are provided in Supplementary Table 1.
Socio-demographic variables
Demographic variables included age at interview, wave, sex, European region, education and body mass index (BMI). Age was grouped into 5-year categories from age 50 with an open-ended category from age 90. European countries were categorized into four regions: Northern Europe (Denmark and Sweden), Western Europe (Austria, Germany, France, the Netherlands, Switzerland, Belgium, Ireland and Luxembourg), Southern Europe (Spain, Italy, Greece and Portugal) and Eastern Europe (Czech Republic, Poland, Hungary, Slovenia, Estonia and Croatia). Education was measured according to the International Standardized Classification of Education (ISCED) (UNESCO 1997), classified into low (ISCED groups 0–2), medium (ISCED groups 3–4) and high (ISCED groups 5–6). In SHARE, self-reported height and weight are converted into body mass index (BMI) and grouped into underweight, normal, overweight and obese.
Health variables
A Comorbidity Index was constructed based on ten major diseases available in all waves of SHARE. Information about chronic diseases is reported in response to the question: “Has a doctor ever told you that you had/do currently have one of the following conditions?” (heart attack, stroke, lung disease, arthritis, stomach/duodenal ulcer, diabetes, cancer, hypertension, high cholesterol and Parkinson) (Supplementary Table 2). The Comorbidity Index resulted in a score from 0 to 8 (i.e., number of comorbidities). Categories 7 and 8 were combined due to few observations.
We used a modified version of the Frailty Phenotype based on five items in the SHARE questionnaire (Romero-Ortuno et al. 2010; Santos-Eggimann et al. 2009), resulting in a score from 0 to 5. The model differed from Fried’s framework in that “weight loss” was replaced by appetite and “slowness” was measured by questions on functional limitation (Supplementary Table 2).
Statistical analyses
We estimated the difference in comorbidity and frailty between women and men over the five waves of SHARE by use of ordinal logistic regressions. This model considers the ordinal nature of the Comorbidity Index and the Frailty Phenotype, resulting in ORs for an increase of one in comorbidity and frailty, respectively. Using logistic regressions, sex differences for the specific items in the Comorbidity Index and the Frailty Phenotype were investigated for all countries combined. The analyses were adjusted for age, region and wave and separately investigated for age and regions since significant interactions for sex-by-age and sex-by-region were found. The models were extended by controlling for education and BMI.
By use of logistic regression, we investigated the association between the Frailty Phenotype and comorbidity and tested whether the association differed between men and women. In this case, comorbidity was defined as a binary variable (< 2 vs 2+ diseases). Additionally, the main model was extended by controlling for frailty in the analysis of comorbidity and vice versa. To investigate whether repeated measurements of individuals have influenced our results, we performed a sensitivity analysis examining sex differences in the Comorbidity Index and the Frailty Phenotype including participants at first intake only (i.e., participants were only included once, namely the first time they took part in the survey). In all models, we used robust standard errors from clustered analyses due to repeated measurements from individuals participating in more than one wave of SHARE, and we applied the calibrated cross-sectional weights supplied by SHARE (Börsch-Supan and Jürges 2005). We evaluated the assumptions of proportional odds using a generalized ordinal logistic regression model and found that the proportional odds assumption for gender was fulfilled except for two models of the Frailty Phenotype, where the gender difference seemed to increase when frailty increases (after Bonferroni correction of 18 tests). Model summary statistics for the regression models are given in Supplementary Table 3. Stata version 14.2 was used for all analyses.
Results
In total, 113,299 participants were included in the study corresponding to 244,258 observations. The total sample had an overall mean age of 66.2 years [standard deviation (SD) 10.1], and 54.7% were women. Higher educational attainment for men than women was found in all regions except Northern Europe, with Southern Europe having the highest proportion of participants with low education. More men than women were overweight (48.1 vs 36.3%), whereas the proportions of obesity were quite similar (Table 1). For the Comorbidity Index, men were more likely than women to have an index score of zero (32.8 vs 31.3%). The same pattern was found for the Frailty Phenotype, with a higher proportion of men than women with a score of zero (50.6 vs 41.2%). Eastern Europe had the highest percentage of individuals with a Comorbidity Index of two or more, whereas Southern Europe had the highest proportion of individuals with a frailty score of three or greater (Table 2).
Overall, women had slightly higher odds of comorbidity than men (OR 1.11, 95% CI 1.07–1.15). No sex-by-wave interaction was found (p = 0.141), i.e., the difference in comorbidity between men and women did not differ by wave; however, a sex-by-age interaction was found (p < 0.001). No sex difference was found at ages 50–59, but a female disadvantage was present from age 60, slightly increasing from ages 60–64 to ages 85–89 and becoming nonsignificant at age 90+ (Fig. 1a; Table 3). Sex differences varied by regions (p < 0.001). There were overall higher odds of comorbidity for women in Eastern Europe (OR 1.30, 95% CI 1.18–1.44), Southern Europe (OR 1.23, 95% CI 1.15–1.30) and Northern Europe (OR 1.08, 95% CI 1.01–1.16), but no sex difference in Western Europe (OR 1.01, 95% CI 0.96–1.01). Sex differences differed by age in Northern and Southern Europe. In Northern Europe, a female disadvantage was indicated from ages 50–59 and again from age 80 and above, whereas in Southern Europe, a sex difference was found from age 60 and above, but with fluctuating patterns over age (Fig. 1b; Table 3). When investigating sex differences for the specific diseases, we found overall higher odds of arthritis (OR 2.08, 95% CI 1.99–2.18), hypertension (OR 1.10, 95% CI 1.06–1.15) and high cholesterol (OR 1.06, 95% CI 1.01–1.11) for women than for men, whereas women had lower odds of heart attack (OR 0.60, 95% CI 0.56–0.63), stroke (OR 0.73, 95% CI 0.66–0.80), diabetes (OR 0.82, 95% CI 0.77–0.88), lung disease (OR 0.82, 95% CI 0.76–0.87), stomach/duodenal ulcer (OR 0.90, 95% CI 0.83–0.98) and Parkinson disease (OR 0.75, 95% CI 0.62–0.92). For all diseases, except for ulcer and Parkinson, sex differences varied by age. The male disadvantage in heart attack and diabetes was largest in the youngest age groups, whereas for lung disease, hypertension and high cholesterol, we found an increasing female advantage with rising age (Fig. 1c, Table 4).
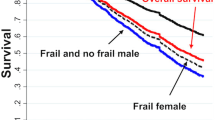
Sex differences in the Comorbidity Index and the Frailty Phenotype by age groups and European regions among participants in SHARE interviewed from 2004–2005 to 2015. a Sex differences in comorbidity by age groups for all countries combined. b Sex differences in comorbidity by age groups and regions. c Sex differences by age groups for the specific diseases included in the Comorbidity Index. d Sex differences in frailty by age groups for all countries combined. e Sex differences in frailty by age groups and regions. f Sex differences by age groups for the specific items included in the Frailty Phenotype
For frailty, higher odds for women than for men were found in all age groups with an overall OR of 1.56 (95% CI 1.51–1.62), but the difference was modified by age (p = 0.025). The OR was 1.49 at ages 50–54, slightly decreasing at age 60–64, but remaining stable from age 65 and above with ORs between 1.65 and 1.70 (Fig. 1d; Table 3). We found no sex-by-wave interaction (p = 0.440), but regional differences were present (p < 0.001). Higher odds of frailty were found for women at all ages in the four European regions, except for age 90+ in Northern Europe (Fig. 1e; Table 3). Overall, Southern Europe had the largest sex difference in frailty (OR 1.84, 95% CI 1.72–1.96), followed by Eastern Europe (OR 1.58, 95% CI 1.41–1.76) and Western Europe (OR 1.43, 95% CI 1.36–1.51) with stable patterns over age. In Northern Europe, sex differences varied by age, with the smallest sex difference at ages 65–69 and the largest difference at ages 80–84, but decreasing thereafter. When investigating the specific items in the Frailty Phenotype, we found higher odds for women than for men for all items, but with fluctuating patterns over age for all items except for “loss of appetite” (Fig. 1f, Table 4).
When we further adjusted the analyses for education and BMI, the results were consistent with those of the main analyses for both comorbidity and frailty (Supplementary Tables 4 and 5). We found a strong association between frailty and comorbidity, with the probability of comorbidity increasing with more frailty, from an average of 26.1% for people with no frailty to 76.6% for the frailest individuals (Supplementary Figure 1). The association did not differ between men and women (p = 0.116). When adjusting the analysis of comorbidity for frailty, we found that the disadvantage for women was no longer present apart from the age group 70–74. Adjustment for comorbidity did not change sex differences in frailty (results not shown). When we repeated the analyses including respondents at first intake only (n = 113,299), results were overall consistent with the main analyses. There were still higher odds of comorbidity for women in Eastern Europe (OR 1.35, 95% CI 1.18–1.55) and Southern Europe (OR 1.18, 95% CI 1.10–1.27) and no sex difference in Western Europe (OR 0.97, 95% CI 0.92–1.03), but the estimate for Northern Europe changed slightly resulting in no significant sex difference in comorbidity (OR 1.04, 95% CI 0.97–1.12). The results for frailty remained unchanged (Supplementary Table 6).
Discussion
Our results from a large sample of European men and women confirm a sex gap in comorbidity and frailty, but sex differences varied across age and regions. The slightly higher odds of comorbidity were mainly caused by higher odds of arthritis, hypertension and high cholesterol among women aged 60+. No overall sex difference in comorbidity was found in Western Europe, but women had more comorbidity than men in Eastern, Southern and Northern Europe. Women were frailer than men in all regions, with the largest sex difference in Southern Europe.
As hypothesized, the overall sex gap in comorbidity and frailty increased with advancing age. A widening of the gap between the sexes may be consistent with a survival effect, and thus, the difference between men and women in prevalent comorbidity and frailty may have increased because the frailest men died, leaving the healthiest men in the sample (Austad and Fischer 2016). A survival effect would be expected to continue into the oldest age groups, which was also the case in this study. The findings agreed with the literature, identifying greater comorbidity and disability in the oldest women when compared with same-aged men (Hazra et al. 2015; Kingston et al. 2014; Nybo et al. 2001), and they were consistent with what would be expected based on the male–female health survival paradox.
In line with earlier research (Theou et al. 2012), we found a strong association between frailty and comorbidity, lending support to the idea that, despite different measures, the two concepts are interdependent. Similar to a recent review (Gordon et al. 2017), this study revealed consistent sex differences in frailty across age and European regions, whereas the pattern of sex differences in comorbidity was country specific and dependent on the specific items. The female disadvantage in comorbidity was to some degree explained by a sex gap in self-reported arthritis, hypertension and high cholesterol in agreement with earlier research (Crimmins et al. 2011; Kingston et al. 2014; Vasilopoulos et al. 2014). Contrarily, the women in this study reported less heart attack, stroke, diabetes, lung disease, stomach/duodenal ulcer and Parkinson disease compared with the men, lending support to previous international studies showing that women experience a greater number of comorbidities that are disabling, but non-fatal, whereas men experience more life-threatening conditions (Case and Paxson 2005; Crimmins et al. 2011). The incidence of coronary heart disease is reported to be about twice as high for men than for women in middle age, but the male excess declines after age 60 (Rich-Edwards et al. 1995). We found that self-reported heart attack had the largest disadvantage for men in the youngest age groups, but was on average 40% less likely to be reported by women, with stable sex differences also after controlling for education and BMI. In this study, women had a disadvantage in cancer from age 50–64, whereas a female advantage was found from age 75 and above. This agreed with a large study based on the Surveillance, Epidemiology and End Results program, which found that rates for all cancers combined and for cancers excluding the sex-specific sites during the period 1975–2004 were higher among adult women than among men below age 50 and higher among men than among women from age 60 and onward (Cook et al. 2009).
Although we found a remarkable consistence in the direction of sex differences across European regions, the extent of the sex differences varied by regions. A recent SHARE study of 35,453 European men and women from Northern, Central and Southern Europe found that, although Southern Europe had the highest improvement in cognitive function and grip strength between 2004–2005 and 2013, they still had the lowest mean in 2013 and the lowest average proportion of people with no limitations of activities of daily living (Ahrenfeldt et al. 2018a). Other studies have also documented a north–south gradient in health for instance regarding grip strength and cognitive function (Ahrenfeldt et al. 2018b), musculoskeletal pain (Cimas et al. 2018), depression (Van de Velde et al. 2010) and self-rated health (Dahlin and Harkonen 2013) with sex gaps being largest in Eastern and Southern European countries. Besides the widespread inequalities in health across European countries, there are also large differences in life expectancy (World Health Organization 2013). When considering average remaining life expectancy at age 50 in the Eurostat database (Eurostat 2019) between 2004 and 2015 for the four European regions (Supplementary Table 7), Eastern Europe had the lowest remaining life expectancy at age 50 (29.0 years), but the largest sex difference in life expectancy (6.2 years). Southern and Western Europe had longer remaining life expectancy (32.8 and 32.6 years) and smaller sex differences (4.3 and 4.4 years), whereas Northern Europe had the lowest remaining life expectancy (32.0 years) and the smallest sex difference (3.5 years). Taking account of all waves in SHARE, this study confirms that the extent of sex differences in comorbidity and frailty varies across regions with the largest differences in Southern and Eastern Europe. When also considering life expectancy, we found that the male–female health survival paradox is most pronounced in Eastern Europe, which is in line with the findings in an earlier study investigating components of the paradox between Denmark and Moscow (Oksuzyan et al. 2014). This study suggested that potential reasons for the stronger male–female health survival paradox in Moscow than in Denmark could be the greater sex differences in lifestyle behavior in Russia, differences in economic situations and social support systems as well as sex differences in the prevalence of various diseases (Oksuzyan et al. 2014). Also, in agreement with that study, we found the largest sex differences in comorbidity in Eastern Europe. According to the World Health Organization (WHO), gender equity is important and differences in mortality and morbidity between men and women should be handled by adopting a gender equity approach in tackling social and economic inequities, which, for instance, includes equal access to education and the labor market and to health resources (World Health Organization 2013).
Notably, this study had very large sample size and multiple waves of data, and thus good power to detect sex-specific patterns in comorbidity and frailty over age across European regions. Further, we included elderly and very old individuals, for whom data are sparser (Christensen et al. 2013). A potential concern in this study could be the proportion of missing data for the Frailty Phenotype in which 11.6% were missing; however, the proportion of missing data was almost similar between men and women (10.1 vs 12.8%). Hence, we do not expect that the comparison of sex differences will be biased due to the missing data. Another limitation in this study was its considerable reliance on self-report. In general, validation studies have supported the accuracy of self-reports as a measure of prevalent chronic diseases (Beckett et al. 2000; Okura et al. 2004). However, regarding medication use, a study of 15,330 Danes aged 46–102 found that both men and women underreport the number of used medications when compared with register data, but the degree of underreporting was similar between sexes (Oksuzyan et al. 2009). We cannot exclude that underreporting of comorbidities in SHARE might have influenced the results, but potential misclassification is expected to be non-differential. Another concern in this study was the low response rate in SHARE, which may lead to sample selection bias (Börsch-Supan et al. 2013), and the large number of participants with unknown vital status, making it impossible to adjust for mortality and thus to perform longitudinal analyses. Hence, we were only able to estimate prevalence, and the observed patterns might be influenced by sex differences in disease survival (for instance, regarding cancer (Radkiewicz et al. 2017)), as surviving women have a higher probability for participating in SHARE, and thus to report their experienced disease. However, in all analyses we included the calibrated weights, which are constructed to reduce the impact of non-response and sample attrition on estimates (Börsch-Supan and Jürges 2005). In this study, we used the Frailty Phenotype as the measure of frailty, because we aimed to investigate sex differences in frailty and comorbidity separately, and comorbidity is a part of the Frailty Index (Jones et al. 2004). Even though the findings in this study are consistent with recent results based on the Frailty Index (Gordon et al. 2017), the results might have been slightly different if another frailty model had been applied.
In conclusion, our results confirm that European women are frailer and have slightly more comorbidity than European men with the most pronounced sex differences in Southern and Eastern Europe. The sex gaps increased with advancing age, which corresponds to what we expected based on the male–female health survival paradox.
References
Ahrenfeldt LJ, Lindahl-Jacobsen R, Rizzi S, Thinggaard M, Christensen K, Vaupel JW (2018a) Comparison of cognitive and physical functioning of Europeans in 2004–05 and 2013. Int J Epidemiol. https://doi.org/10.1093/ije/dyy094
Ahrenfeldt LJ, Scheel-Hincke LL, Kjaergaard S, Moller S, Christensen K, Lindahl-Jacobsen R (2018b) Gender differences in cognitive function and grip strength: a cross-national comparison of four European regions. Eur J Public Health. https://doi.org/10.1093/eurpub/cky266
Andersen-Ranberg K, Petersen I, Frederiksen H, Mackenbach JP, Christensen K (2009) Cross-national differences in grip strength among 50+ year-old Europeans: results from the SHARE study. Eur J Ageing 6:227–236. https://doi.org/10.1007/s10433-009-0128-6
Austad SN, Fischer KE (2016) Sex differences in lifespan. Cell Metab 23:1022–1033. https://doi.org/10.1016/j.cmet.2016.05.019
Barford A, Dorling D, Davey SG, Shaw M (2006) Life expectancy: women now on top everywhere. BMJ 332:808
Beckett M, Weinstein M, Goldman N, Yu-Hsuan L (2000) Do health interview surveys yield reliable data on chronic illness among older respondents? Am J Epidemiol 151:315–323
Bergmann M, Kneip T, Luca D, Giuseppe, Scherpenzeel, Annette (2017) Survey participation in the Survey of Health, Ageing and Retirement (SHARE), wave 1–6. Based on Release 6.0.0 (March 2017)
Börsch-Supan A, Jürges H (2005) The survey of health, aging, and retirement in Europe—methodology. MEA, Mannheim
Börsch-Supan A, Brandt M, Hunkler C et al (2013) Data resource profile: the Survey of Health, Ageing and Retirement in Europe (SHARE). Int J Epidemiol 42:992–1001. https://doi.org/10.1093/ije/dyt088
Bouillon K, Kivimaki M, Hamer M et al (2013) Measures of frailty in population-based studies: an overview. BMC Geriatr 13:64. https://doi.org/10.1186/1471-2318-13-64
Case A, Paxson C (2005) Sex differences in morbidity and mortality. Demography 42:189–214
Chen X, Mao G, Leng SX (2014) Frailty syndrome: an overview. Clin Interv Aging 9:433–441. https://doi.org/10.2147/cia.s45300
Christensen K, Thinggaard M, Oksuzyan A et al (2013) Physical and cognitive functioning of people older than 90 years: a comparison of two Danish cohorts born 10 years apart. Lancet 382:1507–1513. https://doi.org/10.1016/s0140-6736(13)60777-1
Cimas M, Ayala A, Sanz B, Agullo-Tomas MS, Escobar A, Forjaz MJ (2018) Chronic musculoskeletal pain in European older adults: cross-national and gender differences. Eur J Pain 22:333–345. https://doi.org/10.1002/ejp.1123
Clegg A, Young J, Iliffe S, Rikkert MO, Rockwood K (2013) Frailty in elderly people. Lancet 381:752–762. https://doi.org/10.1016/s0140-6736(12)62167-9
Collard RM, Boter H, Schoevers RA, Oude Voshaar RC (2012) Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc 60:1487–1492. https://doi.org/10.1111/j.1532-5415.2012.04054.x
Cook MB, Dawsey SM, Freedman ND et al (2009) Sex disparities in cancer incidence by period and age. Cancer Epidemiol Biomark Prev 18:1174–1182. https://doi.org/10.1158/1055-9965.epi-08-1118
Crimmins EM, Kim JK, Sole-Auro A (2011) Gender differences in health: results from SHARE, ELSA and HRS. Eur J Public Health 21:81–91. https://doi.org/10.1093/eurpub/ckq022
Dahlin J, Harkonen J (2013) Cross-national differences in the gender gap in subjective health in Europe: does country-level gender equality matter? Soc Sci Med 98:24–28. https://doi.org/10.1016/j.socscimed.2013.08.028
Dix D (2014) The female health-survival advantage: paradox unwarranted. Int J Public Health 59:213. https://doi.org/10.1007/s00038-013-0505-y
Eurostat (2019) Life expectancy by age and sex. Eurostat. https://appsso.eurostat.ec.europa.eu/nui/show.do?dataset=demo_mlexpec&lang=en. Accessed 27 Feb 2019
Fried LP, Tangen CM, Walston J et al (2001) Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 56:M146–M156
Gold CH, Malmberg B, McClearn GE, Pedersen NL, Berg S (2002) Gender and health: a study of older unlike-sex twins. J Gerontol B Psychol Sci Soc Sci 57:S168–S176
Gordon EH, Peel NM, Samanta M, Theou O, Howlett SE, Hubbard RE (2017) Sex differences in frailty: a systematic review and meta-analysis. Exp Gerontol 89:30–40. https://doi.org/10.1016/j.exger.2016.12.021
Hazra NC, Dregan A, Jackson S, Gulliford MC (2015) Differences in health at age 100 according to sex: population-based cohort study of centenarians using electronic health records. J Am Geriatr Soc 63:1331–1337. https://doi.org/10.1111/jgs.13484
Hubbard RE, Rockwood K (2011) Frailty in older women. Maturitas 69:203–207. https://doi.org/10.1016/j.maturitas.2011.04.006
Jones DM, Song X, Rockwood K (2004) Operationalizing a frailty index from a standardized comprehensive geriatric assessment. J Am Geriatr Soc 52:1929–1933. https://doi.org/10.1111/j.1532-5415.2004.52521.x
Kingston A, Davies K, Collerton J et al (2014) The contribution of diseases to the male-female disability-survival paradox in the very old: results from the Newcastle 85+ study. PLoS ONE 9:e88016. https://doi.org/10.1371/journal.pone.0088016
Nybo H, Gaist D, Jeune B, McGue M, Vaupel JW, Christensen K (2001) Functional status and self-rated health in 2,262 nonagenarians: the Danish 1905 Cohort Survey. J Am Geriatr Soc 49:601–609
Oksuzyan A, Juel K, Vaupel JW, Christensen K (2008) Men: good health and high mortality. Sex differences in health and aging. Aging Clin Exp Res 20:91–102
Oksuzyan A, Petersen I, Stovring H, Bingley P, Vaupel JW, Christensen K (2009) The male-female health-survival paradox: a survey and register study of the impact of sex-specific selection and information bias. Ann Epidemiol 19:504–511. https://doi.org/10.1016/j.annepidem.2009.03.014
Oksuzyan A, Crimmins E, Saito Y, O’Rand A, Vaupel JW, Christensen K (2010) Cross-national comparison of sex differences in health and mortality in Denmark, Japan and the US. Eur J Epidemiol 25:471–480. https://doi.org/10.1007/s10654-010-9460-6
Oksuzyan A, Shkolnikova M, Vaupel JW, Christensen K, Shkolnikov VM (2014) Sex differences in health and mortality in Moscow and Denmark. Eur J Epidemiol 29:243–252. https://doi.org/10.1007/s10654-014-9893-4
Okura Y, Urban LH, Mahoney DW, Jacobsen SJ, Rodeheffer RJ (2004) Agreement between self-report questionnaires and medical record data was substantial for diabetes, hypertension, myocardial infarction and stroke but not for heart failure. J Clin Epidemiol 57:1096–1103. https://doi.org/10.1016/j.jclinepi.2004.04.005
Radkiewicz C, Johansson ALV, Dickman PW, Lambe M, Edgren G (2017) Sex differences in cancer risk and survival: a Swedish cohort study. Eur J Cancer 84:130–140. https://doi.org/10.1016/j.ejca.2017.07.013
Rich-Edwards JW, Manson JE, Hennekens CH, Buring JE (1995) The primary prevention of coronary heart disease in women. N Engl J Med 332:1758–1766. https://doi.org/10.1056/nejm199506293322607
Romero-Ortuno R, Walsh CD, Lawlor BA, Kenny RA (2010) A frailty instrument for primary care: findings from the Survey of Health, Ageing and Retirement in Europe (SHARE). BMC Geriatr 10:57. https://doi.org/10.1186/1471-2318-10-57
Santos-Eggimann B, Cuenoud P, Spagnoli J, Junod J (2009) Prevalence of frailty in middle-aged and older community-dwelling Europeans living in 10 countries. J Gerontol A Biol Sci Med Sci 64:675–681. https://doi.org/10.1093/gerona/glp012
Saum KU, Dieffenbach AK, Muller H, Holleczek B, Hauer K, Brenner H (2014) Frailty prevalence and 10-year survival in community-dwelling older adults: results from the ESTHER cohort study. Eur J Epidemiol 29:171–179. https://doi.org/10.1007/s10654-014-9891-6
Theou O, Rockwood MR, Mitnitski A, Rockwood K (2012) Disability and co-morbidity in relation to frailty: how much do they overlap? Arch Gerontol Geriatr 55:e1–e8. https://doi.org/10.1016/j.archger.2012.03.001
United Nations Educational, Scientific and Cultural Organisation (UNESCO) (1997) International standard classification of education (ISCED). UNESCO, Paris
Van de Velde S, Bracke P, Levecque K (2010) Gender differences in depression in 23 European countries. Cross-national variation in the gender gap in depression. Soc Sci Med 71:305–313. https://doi.org/10.1016/j.socscimed.2010.03.035
Van Oyen H, Nusselder W, Jagger C, Kolip P, Cambois E, Robine JM (2013) Gender differences in healthy life years within the EU: an exploration of the “health-survival” paradox. Int J Public Health 58:143–155. https://doi.org/10.1007/s00038-012-0361-1
Vasilopoulos T, Kotwal A, Huisingh-Scheetz MJ, Waite LJ, McClintock MK, Dale W (2014) Comorbidity and chronic conditions in the National Social Life, Health and Aging Project (NSHAP), Wave 2. J Gerontol B Psychol Sci Soc Sci 69(Suppl 2):S154–S165. https://doi.org/10.1093/geronb/gbu025
Weber D, Skirbekk V, Freund I, Herlitz A (2014) The changing face of cognitive gender differences in Europe. Proc Natl Acad Sci USA 111:11673–11678. https://doi.org/10.1073/pnas.1319538111
Whitacre CC (2001) Sex differences in autoimmune disease. Nat Immunol 2:777–780. https://doi.org/10.1038/ni0901-777
World Health Organization (2013) Review of social determinants and the health divide in the WHO European Region: final report. Denmark, Copenhagen
Acknowledgements
This paper uses data from SHARE Waves 1, 2, 4, 5 and 6, see Börsch-Supan et al. (2013) for methodological details (Börsch-Supan et al. 2013). The SHARE data collection has been primarily funded by the European Commission through FP5 (QLK6-CT-2001-00360), FP6 (SHARE-I3: RII-CT-2006-062193, COMPARE: CIT5-CT-2005-028857, SHARELIFE: CIT4-CT-2006-028812) and FP7 (SHARE-PREP: No. 211909, SHARE-LEAP: No. 227822, SHARE M4: No. 261982). Additional funding from the German Ministry of Education and Research, the Max Planck Society for the Advancement of Science, the U.S. National Institute on Aging (U01_AG09740-13S2, P01_AG005842, P01_AG08291, P30_AG12815, R21_AG025169, Y1-AG-4553-01, IAG_BSR06-11, OGHA_04-064, HHSN271201300071C) and from various national funding sources is gratefully acknowledged (see www.share-project.org).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Ethical approval
This study was part of the Survey of Health, Ageing and Retirement in Europe (SHARE), a cross-national panel database of microdata on health, socioeconomic status and social and family networks of more than 120,000 individuals aged 50 or older covering 27 European countries and Israel. The SHARE study is under continuous ethics review. Waves 1–4 were approved by the Ethics Committee of the University of Mannheim. Wave 4 and onwards were approved by the Ethics Council of the Max Planck Society for the Advancement of Science (MPG). Further information on SHARE is presented in Börsch-Supan et al. (2013) and Börsch-Supan and Jürges (2005) and on the SHARE webpage (www.share-project.org).
Informed consent
Written informed consent was obtained from all participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Major Revision: 22 February 2019.
Minor Revision: 29 May 2019.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Ahrenfeldt, L.J., Möller, S., Thinggaard, M. et al. Sex Differences in Comorbidity and Frailty in Europe. Int J Public Health 64, 1025–1036 (2019). https://doi.org/10.1007/s00038-019-01270-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00038-019-01270-9