Abstract
Objectives
To identify predictors of nicotine withdrawal symptoms among smokers who participated in a randomized cessation trial in a low-income country.
Methods
We analyzed data from 269 smokers who participated in a randomized, placebo-controlled smoking cessation trial conducted in primary healthcare in Aleppo, Syria. All participants received behavioral counseling and were randomized to receive either 6 weeks of nicotine or placebo patch and were followed for one year.
Results
Throughout the study, lower total withdrawal score was associated with greater education (p = 0.044), older age of smoking initiation (p = 0.017), lower nicotine dependence (p = 0.024), higher confidence in ability to quit (p = 0.020), lower reported depression (p < 0.001), higher adherence to patch (p = 0.026), belief of receiving nicotine patches rather than placebo (p = 0.011), and waterpipe use (p = 0.047).
Conclusions
Lower nicotine dependence, greater educational attainment, higher confidence in ability to quit and waterpipe use predict lower withdrawal severity. Waterpipe smoking may serve as a barrier to smoking cessation efforts in countries where its use is highly prevalent. Further, expectancies about the effects of pharmacotherapy appear to mediate the experience of nicotine withdrawal.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The need for tobacco cessation interventions in low-income developing countries is evident, particularly in countries where tobacco consumption is high and represents a major health problem (Ward et al. 2006; World Health Organization 2011). The spread of heavy smoking combined with lack of tested cessation programs makes quitting more challenging for smokers in those countries (Maziak et al. 2014). Quitting smoking is known to be difficult, even when aided with pharmacological and behavioral therapies (Ray et al. 2009). In fact, especially among chronic users, smoking cessation results in unpleasant physiological and psychological symptoms collectively known as nicotine withdrawal syndrome. This syndrome is a central constituent of tobacco dependence (Hughes et al. 1990). It includes a combination of subjective, cognitive, and physiological symptoms that manifest when a smoker tries to quit. These symptoms hinder efforts to stop and maintain long-term abstinence from smoking (Shiffman et al. 2004); therefore, the inability to cope with withdrawal symptoms when quitting seems to account for a large number of failed cessation attempts (West et al. 1989).
Although accomplishing complete abstinence from smoking is the ultimate goal of cessation efforts, nicotine withdrawal symptoms are also of a great interest for two reasons. First, withdrawal symptoms have a substantial role in inducing smoking relapse that compromises cessation outcomes (Shiffman et al. 2004). In fact, several studies show that the pattern, duration, and severity of withdrawal symptoms experienced during cessation attempts are important predictors of abstinence among smokers (Allen et al. 2008; Hughes 2007; Piasecki et al. 2003). Second, withdrawal symptoms are considered clinically significant as they cause discomfort and distress among smokers who are trying to quit. Therefore, closer examination of the individual characteristics that could influence the intensity of nicotine withdrawal symptoms is necessary to improve our understanding and management of these symptoms (Shiffman et al. 2004).
Prior research highlighted some of the important factors influencing nicotine withdrawal symptoms. These include smokers’ baseline serum cotinine levels (measure of nicotine levels), number of cigarettes smoked per day, motivation to smoke, and depression (Morrell et al. 2008;
West and Russell 1985). Despite the extensive literature on evaluating nicotine withdrawal symptoms in populations from high-income countries (Bidwell et al. 2013; Gritz et al. 1991; Hendricks and Leventhal 2013; Hendricks et al. 2014; Morrell et al. 2008; Neiro et al. 2014; Piasecki et al. 2003; West and Russell 1985; West et al. 2008), such information in low-income countries lags behind. Findings from the first cessation trial that was conducted in a low-income country setting (Aleppo, Syria) show that having fewer nicotine withdrawal symptoms during smoking cessation treatment was associated with a greater likelihood of smoking abstinence (Ward et al. 2013). Therefore, there is a great need for further examination of the nature of nicotine withdrawal symptoms, and individual factors (e.g., demographic, smoking related and psychosocial) that may influence the severity of these symptoms in low-income countries such as Syria, which as many other Middle Eastern countries has a different smoking profile in comparison to western developed high-income countries (Maziak et al. 2004).
This study aims to address this knowledge gap by investigating predictors of nicotine withdrawal symptoms among smokers who participated in a randomized cessation trial in a low-income country (Syria). Our findings will provide insights about factors that influence nicotine withdrawal symptomology among smokers in the Syrian context, and will likely be useful for other developing countries in the Middle East that share similar cultural and tobacco use patterns.
Methods
Study design
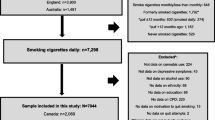
We analyzed data from a multi-site two-group, parallel-arm, double-blind, randomized, placebo-controlled trial that was conducted in four primary healthcare centers in Aleppo, Syria in 2008. All eligible and interested smokers received physician-delivered face-to-face behavioral counseling and brief telephone support, and then randomized to receive either active transdermal nicotine (TN) patches or placebo TN patches. A total of 269 smokers (age 18–65 years), who had smoked >5 cigarettes/day for at least 1 year were recruited. Exclusion criteria were (1) a diagnosis of generalized dermatology disease, liver failure, hyperthyroidism or pheochromocytoma; (2) current use of psychotropic drugs; (3) past year history of drug or alcohol abuse; (4) current unstable cardiovascular or psychiatric illness, or any other debilitating disease based on their physician’s assessment; (5) currently pregnant, lactating or intending to become pregnant during the next 3 months. Participants were patients who resided within the catchment area of one of the four centers included in the study. Each center had a primary care physician who delivered the smoking cessation intervention, served as the study coordinator, and liaised between the centers’ physicians and the research staff to ensure adherence to the study protocol. The study was approved by the Institutional Review Boards of The University of Memphis and Syrian Center for Tobacco Studies. Full details of the trial design and its methods are published elsewhere (Ward et al. 2013).
Procedures and intervention delivery
Patients who were interested in quitting were referred by their primary physician to the cessation coordinator. Upon referral, the cessation coordinator described the study, screened participants for eligibility, and obtained a written informed consent from them. Patients who were uninterested or ineligible were provided with self-help materials and referred back to their original physician. After recruitment, participants completed a battery of baseline questionnaires including: demographic characteristics, smoking history (e.g., number of cigarettes smoked per day, level of dependence, previous quit attempt, interest in quitting), self-efficacy, stage of change, nicotine withdrawal symptoms, and depression/mood. Participants then were randomized using random permuted blocks stratified by clinic and gender to receive either behavioral counseling + active TN patches (active treatment group) or behavioral counseling + placebo TN patches (control group). The intervention was delivered over 6 weeks, then participants were followed at end of treatment (6 weeks after quit date), and at 6 and 12 months.
Pharmacological Intervention
Patients in the nicotine group received a six-week supply of Nicotinell™ patches, 24-h dose, using a step-down algorithm. Patients who smoked ≥10 cigarettes/day received a 2-week supply of 21-mg patches, then a 2-week supply of 14-mg patches, then a 2-week supply of 7-mg patches. Patients who smoked 5–9 cigarettes per day received a 4-week supply of 14-mg patches, then a 2-week supply of 7-mg patches. Patients in the placebo group received the same step-down algorithm. Placebo patches were provided by a local manufacturer and looked identical to the nicotine patches (Ward et al. 2013).
Behavioral counseling
All patients received a culturally adapted behavioral smoking cessation intervention developed and tested in a pilot trial in the same population (Asfar et al. 2008; Fiore et al. 2008). Participants in both groups received the same intervention that includes three individual in-person sessions (approximately 30 min each) and five brief phone calls (approximately 10 min).
Measures
Baseline variables
Predictors that were assessed at baseline include: socio-demographic characteristics (age, gender, marital status, number of people in the house, years of education and religion); smoking history (number of years as a cigarette smoker, age when daily smoking began, current amount smoked per day), waterpipe use and dose of the treatment for patch (high vs. low dosage scheme).
Assessment of motivation to quit was done using the Readiness to Quit Ladder (Biener and Abrams 1991), higher score represents greater levels of readiness to quit. The scale consists of 10 items and provides a continuous metric of motivation and/or readiness to quit. Confidence in ability to quit was also assessed using a 10-item continuous metric scale.
The Fagerström Test for Nicotine Dependence (FTND) was used for assessing the intensity of physical addiction to nicotine and it contains six items that evaluate the quantity of cigarette consumption, the compulsion to use, and dependence (Heatherton et al. 1991).
Time-varying variables
The Multidimensional Scale of Perceived Social Support (MSPSS) was used to measure social support throughout the study. MSPSS is a brief research tool designed to measure perceptions of support from 3 sources: family, friends, and a significant other (Zimet et al. 1988).
Depressive symptomatology was assessed using the Center for Epidemiologic Studies Depression Scale (CES-D). CES-D scale is a self-report instrument composed of 20 items, and respondents are asked to rate how often they experienced symptoms (Sadness, Dysphoria, Loss of Interest, Appetite, Sleep, Thinking/concentration, Guilt, Fatigued, Agitation and Suicidal ideation) of depression during the past week (Radloff 1977). Participant’s belief whether they had received active nicotine or placebo patches was measured using this question [Which patch do you think you have been using? nicotine or placebo]. Adherence to treatment (pharmacological and behavioral counseling) was assessed throughout the entire period of treatment (6 weeks).
Nicotine withdrawal symptoms
Nicotine withdrawal symptoms were assessed using the Minnesota Nicotine Withdrawal Scale (MNWS) (Hughes and Hatsukami 1986) and was measured at baseline, session 2 (1 week post-quit), session 3 (2 weeks days post-quit), end of treatment (6 weeks post-quit), and at the 6- and 12-month follow-up sessions. The mean of eight scale items (depression/feeling blue, difficulty concentrating, hunger, increased appetite, insomnia, irritability/frustration/anger, restlessness, and anxiety) was calculated to obtain a total withdrawal score (Hughes and Hatsukami 1986, 1998) and was measured on a range of possible values of 0 (not present) to 100 (most severe) for the past week.
Statistical analysis
Descriptive statistics were used to examine participants’ demographic and smoking history characteristics at baseline. The generalized estimating equation (GEE) procedure was used to assess the longitudinal effects of baseline and time-varying variables on the total nicotine withdrawal score throughout the entire period of the study (from baseline through 12 months follow-up). A separate model was built to assess early withdrawal symptoms (from baseline through 6 weeks post-quit day). GEE allows to estimate the parameters of the generalized linear model with a possible unknown correlation between outcomes, accounts for time-dependent covariates, and allows for specifying the random and fixed effect (Zeger et al. 1988). We used a model that considered all variables of interest (complete model). Social support, depression and the perception of treatment allocation were modeled in GEE as time-varying predictors (i.e., repeated measures analysis). All analyses were conducted using SPSS version 21 (SPSS Inc., Chicago, IL, USA) and a p value < 0.05 was considered statistically significant.
Results
Males comprised 78 % of the sample. The mean age of study participants was 39.9 years [standard deviation (SD) = 11.4], with a mean of 10.2 years of education (SD = 4.0). The mean number of cigarettes smoked per day was 27.7 (SD = 12.7), while the mean age for starting daily smoking was 18.6 years (SD = 5.3). The mean of Fagerström nicotine dependence score was 5.7 (SD = 2.2), while the mean for the total withdrawal score at baseline was 28.9 (SD = 18.6). The two treatment groups (nicotine vs. placebo) did not differ significantly on any of the baseline characteristics (Table 1).
Finding from the GEE linear regression, indicated that throughout the entire study period, lower total withdrawal score was associated with greater years of education [β = −0.008 (95 % CI = −0.016, −0.001), p = 0.044], older age of smoking initiation [β = −0.006 (95 % CI = −0.012, −0.001), p = 0.017], greater adherence to patch [β = −0.090 (95 % CI = −0.169, −0.011), p = 0.026], smoking waterpipe at baseline [β = −0.080 (95 % CI = −0.596, −0.001), p = 0.047] and higher confidence in ability to quit smoking [β = −0.015 (95 % CI = −0.027, −0.002), p = 0.020]. On the other hand, higher total withdrawal score was associated with greater baseline nicotine dependence [β = 0.021 (95 % CI = 0.003, 0.038), p = 0.024], greater self-reported depression [β = 0.013 (95 % CI = 0.011, 0.016), p < 0.001] and the belief that one had received placebo (not nicotine) patches [β = 0.065 (95 % CI = 0.015, 0.115), p = 0.011] (Table 2). To assess predictors of early withdrawal severity during the duration of the treatment (6 weeks) we ran a secondary GEE analysis. Similar to the primary model, adherence to patch use [β = −5.543 (95 % CI = −9.817,−1.269), p = 0.011], and greater self-reported depression [β = 0.401 (95 % CI = 0.211, 0.592), p < 0.001] was associated with higher withdrawal severity (Table 3).
Discussion
This is the first study to examine predictors of nicotine withdrawal severity during smoking cessation trial in a low-income country. Throughout the study, lower total withdrawal score was associated with lower baseline nicotine dependence, higher confidence in ability to quit, lower depression, belief that one had received nicotine patch rather that placebo, greater years of education, older age of smoking initiation, greater adherence to patch, and waterpipe use. Our findings provide insight into factors that influence nicotine withdrawal symptomology among smokers undergoing cessation trial in a low-income country setting. The results of this study offer an important guide on the pattern and the severity of nicotine withdrawal symptoms and deliver valuable information for clinicians and researchers in designing tailored and effective cessation interventions in low-income countries.
Throughout the study, participants who reported higher confidence in the ability to quit at baseline experienced less severe nicotine withdrawal symptoms. Likely, this is because those who manifest higher confidence in ability to quit were more successful in maintaining abstinence and, therefore, experience less severe withdrawal. This finding emphasizes the role of building confidence in self-ability to quit and strengthening self-efficacy prior to quit attempt to improve cessation outcomes (Nides et al. 1995).
It is also noteworthy that waterpipe smoking at baseline was associated with less severe withdrawal symptoms throughout the study. It is possible that some smokers switch to waterpipe as substitute to cigarette during their quit attempt, which was also observed in a previous pilot behavioral cessation RCT from our team publication of our trial (Asfar et al. 2008). Additionally, qualitative evidence from the same population has shown that some cigarette smokers intentionally switch to waterpipe after quitting cigarettes to satisfy their craving for nicotine (Hammal et al. 2008). This highlights the fact that waterpipe smoking can serve as a barrier to smoking cessation efforts in countries where its use is highly prevalent.
On the other hand, more severe nicotine withdrawal symptoms were associated with younger age of smoking initiation, higher level of nicotine dependence at baseline and fewer years of education. However, there was no association between withdrawal severity and daily cigarette smoking at baseline. One might expect that heavier smokers at baseline would experience greater nicotine withdrawal severity throughout the study. Nevertheless, it should be noticed that although withdrawal discomfort is related to loss of nicotine, it would not necessarily follow that it is also closely associated with the amount of daily cigarette consumption at baseline. This could be explained by the fact that levels of nicotine intake actually depend more on how the cigarettes are smoked than on the quantity of cigarettes being smoked. It is well known that smokers vary significantly in the amount they puff and inhale from their cigarettes; therefore, the measure of daily cigarette consumption at best only offers a crude guide to levels of nicotine intake (West and Russell 1985).
During the study, adherence to patch, regardless of whether it contained active nicotine or placebo, predicted less withdrawal. Similarly, the belief that one was assigned to active nicotine patch, regardless of actual assignment, predicted less withdrawal symptoms. In contrast, actual assignment to nicotine patch had no effect on withdrawal. Effects of expectancies appear to be specific to pharmacological treatment, because withdrawal severity was not predicted by adherence to behavioral counseling. Thus, expectancies about medication effects, rather than actual pharmacologic effect, seem to be a key mechanism in nicotine withdrawal relief in this sample. This has also been shown in previous studies, where expectations about receiving active nicotine rather than placebo was associated with less withdrawal symptoms, greater adherence to cessation treatment (Dar et al. 2005; Darredeau and Barrett 2011; Ben Taleb et al. 2015).
Our results lend further support to findings by Ward et al. (2013) that in a “real life” (primary care) setting in a low-income country, unlike most results from highly controlled cessation trials in high-income countries, nicotine patch may not offer much benefit for reducing withdrawal or promoting long-term abstinence. However, the role of pharmacological therapy in decreasing withdrawal symptoms should not be underestimated. Certainly, the results of this trial need to be followed up in other populations and settings, to determine whether better control of withdrawal symptoms is possible pharmacologically. However, if this finding of limited utility for pharmacotherapy is confirmed in other studies, it would indicate the need to culturally adapt behavioral strategies to control withdrawal, such as coping skill training and educating quitters about the time course and cognitive vs. pharmacological determinants of withdrawal symptoms. A challenge to such an approach in countries such as Syria is the strong belief that medical consultations should result in a prescription for medication, together with the lack of familiarity/belief in behavioral treatment, and the belief that using medication is the most effective way to quit smoking (Asfar et al. 2008).
Another important finding of this study was the positive association between depression and withdrawal symptoms. This is in accordance with previous studies in high-income countries, which showed that depressed smokers appear to experience more severe withdrawal symptoms while quitting (Covey et al. 1990; Hall et al. 1992; Morrell et al. 2008) and, therefore, they are less likely to be successful at their quitting attempts. Our results emphasize the role of depressed mood in predicting the intensity of the withdrawal syndrome in a low-income country setting, and the need to incorporate treatment for depression in smoking cessation programs (e.g., Shiffman et al. 2000).
This study comes with a few limitations. This is the first prospective study of nicotine withdrawal symptoms in a low-income country, but results may not generalize to other countries and treatment settings. Nonetheless, the similarity of many predictors of withdrawal between our study and others conducted in high-income countries supports the robustness and generalizability of our findings. Another point of consideration is that our results are based on self-reports of withdrawal symptoms; however, we employed a widely accepted and reliable instrument for the assessment of nicotine withdrawal symptoms (Shiffman et al. 2004). Lastly, we did not collect information regarding passive smoking exposure at home or at work place. Therefore, we were not able to assess how it may have influenced the severity of nicotine withdrawal symptoms in our study.
In summary, our study shows that in a real-world setting in a low-income country, the expectancy of an effect from patch, rather than the pharmacological effect of nicotine replacement per se, mediates the effect on withdrawal symptoms severity. Furthermore, greater confidence in ability to quit and waterpipe use at baseline were associated with less severe withdrawal symptoms. Additionally, similar to findings from high-income countries, more severe withdrawal symptoms were associated with higher nicotine dependence, younger age of initiation, lower education and greater self-reported depression.
This knowledge will help in advancing the treatment of smoking dependence by informing intervention schemes so it targets important sources of withdrawal phenomena, and those likely to experience more withdrawal in low-income countries. Our findings deliver a guide on the pattern and the severity of nicotine withdrawal symptoms and provide valuable information for clinicians and researchers in designing tailored and effective cessation interventions in low-income countries.
References
Allen SS, Bade T, Hatsukami D, Center B (2008) Craving, withdrawal, and smoking urges on days immediately prior to smoking relapse. Nicotine Tob Res 10:35–45. doi:10.1080/14622200701705076
Asfar T, Vander Weg MW, Maziak W, Hammal F, Eissenberg T, Ward KD (2008) Outcomes and adherence in Syria’s first smoking cessation trial. Am J Health Behav 32:146–156. doi:10.5993/AJHB.32.2.4
Ben Taleb Z, Ward KD, Asfar T, Bahelah R, Maziak W (2015) Predictors of adherence to pharmacological and behavioral treatment in a cessation trial among smokers in Aleppo, Syria. Drug Alcohol Depend 153:167–172
Bidwell LC, Leventhal AM, Tidey JW, Brazil L, Niaura RS, Colby SM (2013) Effects of abstinence in adolescent tobacco smokers: withdrawal symptoms, urge, affect, and cue reactivity. Nicotine Tob Res 15:457–464. doi:10.1093/ntr/nts155
Biener L, Abrams DB (1991) The contemplation ladder: validation of a measure of readiness to consider smoking cessation. Health Psychol 10:360–365. doi:10.1037//0278-6133.10.5.360
Covey LS, Glassman AH, Stetner F (1990) Depression and depressive symptoms in smoking cessation. Compr Psychiatry 31:350–354. doi:10.1016/0010-440X(90)90042-Q
Dar R, Stronguin F, Etter JF (2005) Assigned versus perceived placebo effects in nicotine replacement therapy for smoking reduction in Swiss smokers. J Consult Clin Psychol 73:350. doi:10.1037/0022-006X.73.2.350
Darredeau C, Barrett SP (2011) The role of nicotine content information in smokers’ subjective responses to nicotine and placebo inhalers. Hum Psychopharmacol 25:577–581. doi:10.1002/hup.1159
Fiore MC, Jaen CR, Baker TB et al (2008) A clinical practice guideline for treating tobacco use and dependence: 2008 update. A US Public Health Service report. Am J Prev Med 35:158–176
Gritz ER, Carr CR, Marcus AC (1991) The tobacco withdrawal syndrome in unaided quitters. Br J Addict 86:57–69. doi:10.1111/j.1360-0443.1991.tb02629.x
Hall SM, Muñoz RF, Reus VI, Sees KL (1992) Nicotine, negative affect, and depression. J Consult Clin Psychol 61:761. doi:10.1037/0022-006X.61.5.761
Hammal F, Mock J, Ward KD, Eissenberg T, Maziak W (2008) A pleasure among friends: how narghile (waterpipe) smoking differs from cigarette smoking in Syria. Tob Control 17:e3
Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO (1991) The Fagerstrom test for nicotine dependence: a revision of the Fagerstrom tolerance questionnaire. Br J Addict 86:1119–1127. doi:10.1111/j.1360-0443.1991.tb01879.x
Hendricks PS, Leventhal AM (2013) Abstinence-related expectancies predict smoking withdrawal effects: implications for possible causal mechanisms. Psychopharmacology 230:363–373. doi:10.1007/s00213-013-3169-7
Hendricks PS, Delucchi KL, Benowitz NL, Hall SM (2014) Clinical Significance of early Smoking Withdrawal effects and their relationships With Nicotine Metabolism: preliminary results from a pilot Study. Nicotine Tob Res 16:615–620. doi:10.1093/ntr/ntt204
Hughes JR (2007) Effects of abstinence from tobacco: valid symptoms and time course. Nicotine Tob Res 2007(9):315–327. doi:10.1080/14622200701188919
Hughes JR, Hatsukami D (1986) Signs and symptoms of tobacco withdrawal. Arch Gen Psychiatry 43:289–294. doi:10.1001/archpsyc.1986.01800030107013
Hughes J, Hatsukami D (1998) Errors in using tobacco withdrawal scale. Tob Control 7:92. doi:10.1136/tc.7.1.92a
Hughes JR, Higgins ST, Hatsukami DK (1990) Effects of abstinence from tobacco: a critical review. In: Kozlowski LT, Annis HM, Cappell HD, et al. (Eds.) Research advances in alcohol and drug problems, pp 317–398. doi:10.1007/978-1-4899-1669-3_10
Maziak W, Eissenberg T, Klesges RC, Keil U, Ward KD (2004) Adapting smoking cessation interventions for developing countries: a model for the Middle East. Int J Tuberc Lung Dis 8(4):403–413
Maziak W, Nakkash R, Bahelah R, Husseini A, Fanous N, Eissenberg T (2014) Tobacco in the Arab world: old and new epidemics amidst policy paralysis. Health policy plan 29:784–794. doi:10.1093/heapol/czt055
Morrell HE, Cohen LM, Al’Absi M (2008) Physiological and psychological symptoms and predictors in early nicotine withdrawal. Pharmacol Biochem Behav 89:272–278. doi:10.1016/j.pbb.2007.12.020
Neiro BP, Durán AL, Del Río EF, Pradeda ÚM, Brandon TH, Iglesias EB (2014) Craving and nicotine withdrawal in a Spanish smoking cessation sample. Adicciones 26:230–237
Nides MA, Rakos RF, Gonzales D, Murray RP, Tashkin DP, Bjornson-Benson WM et al (1995) Predictors of initial smoking cessation and relapse through the first 2 years of the Lung Health Study. J Consult Clin Psychol 63:60–69
Piasecki TM, Jorenby DE, Smith SS, Fiore MC, Baker TB (2003) Smoking withdrawal dynamics: iI. Improved tests of withdrawal-relapse relations. J Abnorm Psychol 112:14. doi:10.1037/0021-843X.112.1.14
Radloff LD (1977) The CES-D: a self-report depression scale for research in the general population. Appl Psychol Meas 1:385–401. doi:10.1177/014662167700100306
Ray R, Schnoll RA, Lerman C (2009) Nicotine dependence: biology, behavior, and treatment. Annu Rev Med 60:247–260. doi:10.1146/annurev.med.60.041707.160511
Shiffman S, Johnston JA, Khayrallah M et al (2000) The effect of bupropion on nicotine craving and withdrawal. Psychopharmacology 148:33–40. doi:10.1007/s002130050022
Shiffman S, West RJ, Gilbert DG (2004) Recommendation for the assessment of tobacco craving and withdrawal in smoking cessation trials. Nicotine Tob Res 6:599–614. doi:10.1080/14622200410001734067
Ward KD, Eissenberg T, Rastam S et al (2006) The tobacco epidemic in Syria. Tob Control 15:i24–i29. doi:10.1136/tc.2005.014860
Ward KD, Asfar T, Al Ali R et al (2013) Randomized trial of the effectiveness of combined behavioral/pharmacological smoking cessation treatment in Syrian primary care clinics. Addiction 108:394–403. doi:10.1111/j.1360-0443.2012.04048.x
West RJ, Russell MA (1985) Pre-abstinence smoke intake and smoking motivation as predictors of severity of cigarette withdrawal symptoms. Psychopharmacology 87:334–336. doi:10.1007/BF00432717
West RJ, Hajek P, Belcher M (1989) Severity of withdrawal symptoms as a predictor of outcome of an attempt to quit smoking. Psychol Med 19(04):981–985. doi:10.1017/S0033291700005705
West R, Baker CL, Cappelleri JC, Bushmakin AG (2008) Effect of varenicline and bupropion SR on craving, nicotine withdrawal symptoms, and rewarding effects of smoking during a quit attempt. Psychopharmacology 197(3):371–377. doi:10.1007/s00213-007-1041-3
World Health Organization (2011) WHO Report on the Global Tobacco Epidemic: Warning about the Dangers of Tobacco. Geneva. http://www.who.int/tobacco/global_report/2011/en/. Accessed March 12, 2015
Zeger SL, Liang KY, Albert PS (1988) Models for longitudinal data: a generalized estimating equation approach. Biometrics 44:1049–1060. doi:10.2307/2531734
Zimet GD, Dahlem NW, Zimet SG, Farley GK (1988) The multidimensional scale of perceived social support. J Pers Assess 52:30–41. doi:10.1207/s15327752jpa5201_2
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ben Taleb, Z., Ward, K.D., Asfar, T. et al. Predictors of nicotine withdrawal symptoms: findings from the first randomized smoking cessation trial in a low-income country setting. Int J Public Health 61, 701–708 (2016). https://doi.org/10.1007/s00038-016-0818-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00038-016-0818-8