Abstract
Objectives
We compared patterns of care, comorbidity, disability-adjusted life-years (DALYs) and survival in Indigenous and non-Indigenous women with breast cancer in Queensland, Australia (1998–2004).
Methods
A cohort study of Indigenous (n = 110) and non-Indigenous women (n = 105), frequency matched on age and remoteness. We used Pearson’s Chi-squared analysis to compare proportions, hazard models to assess survival differences and calculated disability-adjusted life years (DALYs).
Results
Indigenous women were more likely to be socially disadvantaged (43 vs. 20 %, p < 0.01) have comorbidity (42 vs. 18 % p < 0.01), and have regional spread or distant metastasis (metastasis, 51 vs. 36 %, p = 0.02) than non-Indigenous women; there was no difference in treatment patterns. More Indigenous women died in the follow-up period (p = 0.01). DALY’s were 469 and 665 per 100,000 for Indigenous and non-Indigenous women, respectively, with a larger proportion of the burden attributed to premature death among the former (63 vs. 59 %).
Conclusions
Indigenous women with breast cancer received comparable treatment to their non-Indigenous counterparts. The higher proportion of DALYs related to early death in Indigenous women suggests higher fatality with breast cancer in this group. Later stage at diagnosis and higher comorbidity presence among Indigenous women reinforce the need for early detection and improved management of co-existing disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cancer is the second leading cause of death for Indigenous Australians, who have higher cancer mortality and poorer survival than other Australians (Australian Institute of Health and Welfare 2014). The reasons underlying these disparities are multifactorial and include advanced cancer at diagnosis (Condon 2004; Valery et al. 2006), reduced uptake of/access to treatment (Supramaniam et al. 2014; Valery et al. 2006), treatment delays (Hall and Holman 2003), and higher rates of comorbidities amongst Indigenous patients (Valery et al. 2006). Breast cancer is the most common cancer among Indigenous Australian women (Australian Institute of Health and Welfare and National Breast and Ovarian Cancer Centre 2009); however, diagnostic, treatment and follow-up practices in comparison to non-Indigenous women have been largely unreported. There are approximately 79,000 Indigenous women in the state of Queensland, representing 3.6 % of the state’s female population (Queensland Government 2012) and their life expectancy is 10 years less than that of other Queensland women (Queensland Government 2013). Accurate information on the cause, magnitude and management of health problems such as breast cancer is essential for appropriate health services planning and resource allocation.
In Australia, incidence of breast cancer among Indigenous women is lower, breast cancer mortality similar, and 5-year crude survival worse compared to non-Indigenous women (Australian Institute of Health and Welfare and Cancer Australia 2012), similar to the profile described for Indigenous women in New Zealand, the United States and Canada (Curtis et al. 2005; Jemal et al. 2010; Sheppard et al. 2010). Studies of the reasons for breast cancer survival inequalities, including later cancer stage, greater burden of comorbidity and different patterns of care among Indigenous women are limited (Dasgupta et al. 2012; Roder et al. 2012; Supramaniam et al. 2014). Internationally, lower breast cancer survival has been attributed to economic and treatment disparities among Māori and Pacific Island women in New Zealand (McKenzie and Jeffreys 2009), late stage at diagnosis among American Indian women in Southwest America (Giuliano et al. 1998) and having a pre-existing comorbidity among First Nation women in Ontario, Canada (Sheppard et al. 2011). As Indigenous people have reportedly received less cancer treatment despite similar stage (Moore et al. 2014), and evidence of breast cancer treatment disparities between Indigenous and non-Indigenous women has not been consistent across jurisdictions (Shaw and Elston 2003; Supramaniam et al. 2014), we compared cancer treatment, along with prognostic factors and survival, among Indigenous and non-Indigenous women with breast cancer in the state of Queensland, Australia. Finally, using results of this current study, we calculated the disability-adjusted life years (DALYs), measuring loss of healthy life years among Indigenous and non-Indigenous women with breast cancer.
Methods
Subjects
The study methods have previously been described (Moore et al. 2014). Briefly, all Indigenous women residing in Queensland and diagnosed with breast cancer during 1998–2004 were identified and matched to a random sample of non-Indigenous women with breast cancer, of corresponding age (within ±5 years) and place of residence (in terms of degree of remoteness from a major centre). For inclusion, all cases had to have been admitted to a public hospital at least once for cancer diagnosis or treatment. Access to free public health care, including cancer treatment, is available to all Australian residents, although those with private insurance, or the means to pay, can also access treatment in the private sector; a previous study reported that only 4 % of Indigenous people with cancer were treated in private hospitals in Queensland between 1997 and 2002 (Valery et al. 2006). Hospital records were reviewed for diagnostic details such as date, histology and method of diagnosis, cancer stage, treatment and presence of comorbidities. Cancer staging was documented by treating doctors as either Tumour, Nodes and Metastasis (TNM) scores, American Joint Committee on Cancer Staging Scores (AJCC) (I–IV) (American Cancer Society 2002) or as localized cancer, regional spread or metastatic disease. For comparability, TNM and AJCC scores were converted to localized/regional/distant spread using commonly accepted cutoff points. Hormone receptor status, specifically oestrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor-2 (HER2: commonly defined as >30 % of cells with intense nuclei staining), was available through Queensland Health laboratories.
Treatment details, including surgery, radiotherapy, chemotherapy and tamoxifen use, as well as treatment intention (any treatment intent, curative intent or intention not known), and date, duration and amount of treatment were collected. A modified Charlson Comorbidity Index Score (‘comorbidity score’) (Charlson et al. 1987) was assigned. The scores (based on severity and number of comorbid conditions) were grouped as: No score (0), 1, 2+. A socioeconomic score was assigned using the Socio-Economic Index For Areas (SEIFA) index (based on geographical areas of residence ranked into quintiles, ranging from 1 as most disadvantage to 5 as most advantaged) (Australian Bureau of Statistics 2003). Date and cause of death were obtained from the Australian National Death Index to 31st December 2006.
Statistical methods
Pearson’s Chi-squared analysis or Fisher’s Exact test was used for categorical data (proportions), t test for normally distributed data (means) and non-parametric tests for non-normally distributed data (medians). All tests were two-tailed, and statistical significance was set at p < 0.05. Crude and adjusted survival analyses were conducted using Cox proportional hazard models. Hazard ratios (HR) were adjusted for stage of cancer at diagnosis, comorbidities, socioeconomic status and cancer treatment. To calculate DALY, we used the incidence and mortality data as reported by the Queensland Cancer Registry, combined with survival and treatment data from the current project. A step-by-step analytical strategy was performed, similar to that previously done by Soerjomataram and colleagues (Soerjomataram et al. 2012). Briefly, years of life lost (YLL) and years of life lived with disability (YLD) were calculated according to Indigenous status and added to derive the DALYs. A standard life table was used to estimate YLL and disability weights to derive the YLD were drawn from previous studies. Finally, numbers of healthy life years lost were adjusted to the population size to derive rates (per 100,000) and age-adjusted to the world standard population. Specifically, the following data from 2001 was used to calculate DALYs: 24 and 2191 new breast cancer cases were reported among Indigenous and non-Indigenous women in Queensland, respectively, corresponding to age-standardized incidence rates of 60 and 89 per 100,000 women, and 9 and 465 women died from breast cancers in each of the respective group (age-standardized mortality rates of 15 and 17 per 100,000). All analyses were calculated using Statistics Package for the Social Sciences (IBM SPSS) for Windows version 18.0-20.0 or STATA for Windows version 12.
Ethical approval was obtained from the Queensland Health Department, all hospitals where data collection took place, and the QIMR Berghofer Institute of Medical Research. An Indigenous Reference Group was established to inform the study investigators about cultural matters and the translation of results to the community.
Results
The study included 110 Indigenous and 105 non-Indigenous women with breast cancer after exclusion of 28 women (11 Indigenous and 17 non-Indigenous), who did not meet the inclusion criteria. Clinical data were abstracted from records at 23 Queensland public hospitals, with additional data abstracted from secondary hospitals for 34 cases.
Patient and clinical characteristics
Mean age was the same for both Indigenous and non-Indigenous women (54 years) and matching resulted in no difference in remoteness of residence (Table 1). Indigenous women were more likely to be disadvantaged compared to their non-Indigenous counterparts (43 vs. 20 %, p < 0.01) and more likely to be diagnosed with advanced cancer (regional spread or distant metastasis, 51 vs. 36 %, p = 0.02). The median number of days from presentation to diagnosis was similar and there was no difference in the histological types of breast tumours between the women (p = 0.46).
When compared to non-Indigenous women, Indigenous women were significantly (p < 0.01) less likely to have a comorbidity score of zero (58 % vs. 82 %), more likely to have a score of 2 or more (16 vs. 9 %) and had a higher prevalence of diabetes (26 % vs. 11 %) and serious diseases of the circulatory system (e.g. stroke, myocardial infarction, chronic heart failure) (23 % vs. 8 %) (Table 1). Indigenous women with regional spread or metastatic disease were significantly more likely to have a comorbidity score greater than zero (45 vs. 19 %, p < 0.01) than non-Indigenous women.
Cancer treatment (any treatment vs. no treatment) was given to 96 % Indigenous and 98 % non-Indigenous women (p = 0.12) (Table 1). Regarding mode of treatment, fewer Indigenous women underwent a lumpectomy and more underwent a mastectomy (56 % vs. 66 % and 44 % vs. 34 %, respectively, p = 0.17) compared to their counterparts. Time from diagnosis to treatment was available for 96 % Indigenous and 99 % non-Indigenous women; there was no significant difference in time from diagnosis to treatment (p = 0.26) or completion rates of treatment (p > 0.05) between groups, nor in number of cycles of chemotherapy (p = 0.55) or dose of radiotherapy (p = 0.81).
There was no difference in oestrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER2) expression between Indigenous and non-Indigenous women, for the small number of cases with available results. Rates of tamoxifen use, the hormone treatment generally prescribed for women with oestrogen receptive tumours (National Health and Medical Research Centre 2001), were similar for those with data recorded (80 % in each group, p = 0.17).
Breast cancer recurrence and survival
There was no difference in the percentage of Indigenous and non-Indigenous women who had a recurrence of their breast cancer recorded (25 % vs. 21 %, p = 0.61). Significantly, more Indigenous women were deceased by the end of the follow-up period (32 % vs. 18 %, p = 0.01,) although they tended to die of causes other than breast cancer than non-Indigenous women (26 % vs. 16 %, not statistically significant) (Table 1). The majority of Indigenous women who died survived for up to 2 years after diagnosis. To explore why more Indigenous women had died overall, the characteristics of deceased Indigenous women were compared to those of deceased non-Indigenous women. There was no significant difference in age, cancer stage at diagnosis, or remoteness between the women who had died at the end of the follow-up period; however, from the highest two socioeconomic strata, six Indigenous women were deceased compared to no non-Indigenous women (p = 0.01). Furthermore, deceased Indigenous women were more likely to have a comorbidity than non-Indigenous women who were also deceased (60 % vs. 30 %, p < 0.01).
The crude HR of all-cause mortality among Indigenous women as compared to non-Indigenous was 2.09 (95 % CI 1.18, 3.70). After adjustment for stage, prevalence of comorbidities and any treatment, the risk of dying was not significantly different (HR 1.56 95 % CI 0.83, 2.90) (Table 2). Similarly, there was no significant difference in death rates between Indigenous and non-Indigenous women with localized cancer or advanced cancer at diagnosis. The crude risk of dying from breast cancer was marginally higher for Indigenous women (HR 1.88, 95 % CI 1.00, 3.56) but when adjusted for stage, comorbidities and any treatment, there was no significant survival difference (HR 1.39, 95 % CI 0.71, 2.76).
Disability-adjusted life years
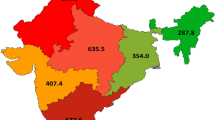
For DALYs, we noted a larger loss of healthy life years in non-Indigenous women with breast cancer as compared with Indigenous women (Fig. 1: 665 and 469 per 100,000, respectively). In addition to estimates of DALYs and its components, we calculated YLLs as a proportion of total DALYs, to illustrate which component of the DALYs was more important. In both Indigenous and non-Indigenous women, premature mortality contributed to more than half of the total burden, though among Indigenous women the proportion was relatively larger (YLL/DALY: 63 vs. 59 %).
Discussion
This study found that Indigenous women with breast cancer were more likely to be socially disadvantaged, have serious comorbidity and more advanced disease at diagnosis than non-Indigenous women. Indigenous women had no treatment delays compared to others in this study, and the mode of treatment as well as completion rates was similar between groups. There was no difference in breast cancer survival outcomes between Indigenous women and other women treated in Queensland public hospitals, after adjusting for stage, comorbidity and treatment.
The finding that Australian Indigenous women had more advanced breast cancer at diagnosis than non-Indigenous women has been reported previously (Condon et al. 2005; Valery et al. 2006). A range of Indigenous cultural beliefs and understanding about cancer have been reported as possible factors inhibiting an early cancer diagnosis (Shahid and Thompson 2009), as has lower rates of participation in breast screening programs compared to non-Indigenous women (54 % compared to 36 %). A greater understanding of these beliefs would inform policy and practice for screening programs. Community education strategies are also required to increase Indigenous women’s understanding about breast cancer and encourage health care-seeking behaviour among women.
Compared to non-Indigenous women, Indigenous women scored significantly higher comorbidity scores overall, and those with later stage cancer were over twice as likely to have a comorbidity, in particular diabetes, which has been shown in a meta-analysis to increase the risk of breast cancer and the risk of death from breast cancer (Larsson et al. 2007). A greater burden of comorbidity has been associated with later stage at diagnosis of breast cancer in another study; competing demand for other health care may override screening and earlier diagnosis (Gonzalez et al. 2001). Although women with serious comorbidities reported receiving less breast cancer treatment when compared to those without comorbidity (Louwman et al. 2005), our study found Indigenous women were as likely to receive curative cancer treatment as other women.
Whilst acknowledging the small numbers of cases with available ER, PR and HER2 receptor status, in view of a lack of similar data for Indigenous Australian women, this data is important to report. We found the majority of women in both groups were diagnosed with hormone receptor-positive tumours, in contrast to studies among Indigenous women in New Zealand (McKenzie et al. 2008) and West Africa (Gukas et al. 2008; Huo et al. 2009) which reported a predominance of hormone receptor-negative breast cancers. However, the response of Indigenous women to tamoxifen compared to non-Indigenous women is not known. A higher proportion of non-Indigenous women received breast conserving therapy (lumpectomy with or without chemotherapy/radiotherapy) while a large proportion of Indigenous women received radical surgery (mastectomy with or without chemotherapy/radiotherapy), however, the difference was not significant. Similar rates of breast conserving therapy between Indigenous and non-Indigenous women were reported in a surgical review in far north Queensland (Shaw and Elston 2003), and a Western Australian study (Hall et al. 2004) but higher rates of mastectomy have been reported in New South Wales (Supramaniam et al. 2014) and South Australia (Roder 2007).
There was no significant difference in the percentage of women who had recurrent breast cancer recorded on the medical chart. Numbers in both groups may not be complete as some of those with recurrence may have been lost to follow-up, may not have been biopsied, or may have presented to a private hospital with their recurrence; however, there is no reason to assume that incomplete information on recurrence was differentially biased. Although not significantly different, more Indigenous than non-Indigenous women had died by the end of the follow-up period, and that was more likely to be a non-cancer death. Other studies have reported lower 5-year survival for Indigenous women in comparison to other Australian women, from all causes (Australian Institute of Health and Welfare and Cancer Australia 2012) and also from breast cancer (Chong and Roder 2010). A study of First Nation women in Ontario, Canada with early stage breast cancer, reported that the existence of a pre-existing health condition accounted for the survival disparity (Sheppard et al. 2011), a factor which may also have contributed to poorer overall survival among Indigenous women in our cohort.
Overall survival among Indigenous women was half that of non-Indigenous, although survival disparities diminished after adjustment for stage, comorbidities and treatment. This contrasts with the survival differential reported for Indigenous and non-Indigenous women with breast cancer in the Northern Territory (NT, Relative Risk adjusted for age and stage 2.4, 95 % CI 1.1, 5.2) (Condon et al. 2005). However, the NT study results were not adjusted for comorbidity and they compared Indigenous women with all women in the territory with breast cancer, whereas our study compared only those treated primarily in public hospitals, where most Indigenous people receive their cancer care. We acknowledge that the small numbers of Indigenous women with breast cancer in our Queensland sample may have resulted in non-significant results and that a larger study is required for their confirmation. However, based on a 2010 report (Moore et al. 2010), we would have expected approximately 152 Indigenous women to be diagnosed with breast cancer and treated in Queensland public hospitals during 1998–2004, for whom 72 % of records were ascertained.
Overall DALYs were less for Indigenous compared to non-Indigenous women with breast cancer. For both groups more than half of the burden was due to premature mortality, yet this was proportionally larger among the Indigenous women (63 vs. 59 %), reflecting higher fatality rates or lower survival.
In Australia, ethnicity is defined by self-assessment (Robertson et al. 1995) and therefore not all Indigenous women with cancer may have been identified by the Registry. There is also the potential for misclassification. However, we believe the Indigenous to non-Indigenous comparison is internally valid, with little, if any, misclassification as medical charts were carefully reviewed to verify Indigenous status. Information about treatment and stage was obtained retrospectively from the medical charts and therefore subject to coding and interpretative uncertainties, but were probably not differentially biased. Our study of survival was also limited by small numbers of breast cancer deaths in the cohort.
Conclusion
In summary, Australian Indigenous women received comparable breast cancer treatment to non-Indigenous women treated in public hospitals in Queensland. Differences in comorbidity and stage at diagnosis between the two groups reinforce the need for early detection and improved management of co-existing disease in Indigenous women with breast cancer.
References
American Cancer Society (2002) Cancer facts and figures 2002. In: Society American Cancer (ed) Cancer facts and figures. American Cancer Society, Atlanta
Australian Bureau of Statistics (2003) Information Paper: Socio-Economic Indexes for Areas (SEIFA). Information paper, vol, vol 2039. Australian Bureau of Statistics, Canberra
Australian Institute of Health and Welfare (2014) Mortality and life expectancy of Indigenous Australian’s 2008–2012. AIHW, Canberra
Australian Institute of Health and Welfare and Cancer Australia (2012) Breast cancer in Australia: an overview. In: Australian Institute of Health and Welfare (ed). vol AIHW cat. No. CAN 67 Cancer Series Number 71, Canberra
Australian Institute of Health and Welfare and National Breast and Ovarian Cancer Centre (2009) Breast cancer in Australia: an overview, 2009. Vol Cancer series no. 50. Cat.no. CAN 46. AIHW, Canberra
Charlson ME, Pompei P, Ales KL, MacKenzie CR (1987) A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40(5):373–383
Chong A, Roder D (2010) Exploring differences in survival from cancer among Indigenous and non-indigenous Australians: implications for health service delivery and research. Asian Pac J Cancer Prev 11(4):953–961
Condon J (2004) Cancer and Indigenous Australians in the Northern Territory (Thesis). Charles Darwin University, Darwin, N.T. Australia
Condon JR, Barnes T, Armstrong BK, Selva-Nayagam S, Elwood JM (2005) Stage at diagnosis and cancer survival for Indigenous Australians in the Northern Territory. Med J Aust 182(6):277–280
Curtis E, Wright C, Wall M (2005) The epidemiology of breast cancer in Maori women in Aotearoa New Zealand: implications for ethnicity data analysis. N Z Med J 118(1209):U1298
Dasgupta P, Baade PD, Aitken JF, Turrell G (2012) Multilevel determinants of breast cancer survival: association with geographic remoteness and area-level socioeconomic disadvantage. Breast Cancer Res Treat 132(2):701–710
Giuliano A, Papenfuss M, de Guernsey de Zapien J, Tilousi S, Nuvayestewa S (1998) Breast cancer screening among southwest American Indian women living on-reservation. Prev Med 27(1):135–143
Gonzalez EC, Ferrante JM, Van Durme DJ, Pal N, Roetzheim RG (2001) Comorbid illness and the early detection of cancer. South Med J 94(9):913–920
Gukas ID, Girling AC, Mandong BM, Prime W, Jennings BA, Leinster SJ (2008) A comparison of clinicopathological features and molecular markers in british and nigerian women with breast cancer. Clin Med Oncol 2:347–351
Hall SE, Holman CDJ (2003) Inequalities in breast cancer reconstructive surgery according to social and locational status in Western Australia. Eur J Surg Oncol 29(6):519–525 (see comment)
Hall SE, Bulsara CE, Bulsara MK, Leahy TG, Culbong MR, Hendrie D, Holman CD (2004) Treatment patterns for cancer in Western Australia: does being Indigenous make a difference? Med J Aust 181(4):191–194
Huo D, Ikpatt F, Khramtsov A, Dangou JM, Nanda R, Dignam J, Zhang B, Grushko T, Zhang C, Oluwasola O, Malaka D, Malami S, Odetunde A, Adeoye AO, Iyare F, Falusi A, Perou CM, Olopade OI (2009) Population differences in breast cancer: survey in indigenous African women reveals over-representation of triple-negative breast cancer. J Clin Oncol 27(27):4515–4521
Jemal A, Siegel R, Xu J, Ward E (2010) Cancer Statistics, 2010. CA Cancer J Clin 60(5):277–300
Larsson SC, Mantzoros CS, Wolk A (2007) Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer 121(4):856–862
Louwman WJ, Janssen-Heijnen ML, Houterman S, Voogd AC, van der Sangen MJ, Nieuwenhuijzen GA, Coebergh JW (2005) Less extensive treatment and inferior prognosis for breast cancer patient with comorbidity: a population-based study. Eur J Cancer 41(5):779–785
McKenzie F, Jeffreys M (2009) Do lifestyle or social factors explain ethnic/racial inequalities in breast cancer survival? Epidemiol Rev 31:52–66
McKenzie F, Jeffreys M, Mannetje A, Pearce N (2008) Prognostic factors in women with breast cancer: inequalities by ethnicity and socioeconomic position in New Zealand. Cancer Causes Control 19(4):403–411
Moore SP, O’Rourke PK, Mallitt KA, Garvey G, Green AC, Coory MD, Valery PC (2010) Cancer incidence and mortality in Indigenous Australians in Queensland, 1997-2006. Med J Aust 193(10):590–593
Moore SP, Green AC, Bray F, Garvey G, Coory M, Martin J, Valery PC (2014) Survival disparities in Australia: an analysis of patterns of care and comorbidities among indigenous and non-indigenous cancer patients. BMC Cancer 14:517
National Health and Medical Research Centre (2001) Clinical practice guidelines for the management of early breast cancer: 2nd edition. In: Prepared by the iSource National Breast Cancer Centre (ed). National Health and Medical Research Council, Canberra
Queensland Government (2012) Census 2011: Women in Queensland In: Queensland GovernmentTreasury and Trade: Office of Economic and Statistical Research (ed). Brisbane, p 1–4
Queensland Government (2013) Queensland women 2013—a statistical snapshot. In: Queensland Government: Department of Communities Child Safety and Disability Services (ed). Brisbane, p 1–6
Robertson H, Lumley J, Berg S (1995) How midwives identify women as aboriginal or Torres Strait Islanders. Aust Coll Midwives Inc J 8(3):26–29
Roder D (2007) Epidemiology of cancer in indigenous Australians: implications for service delivery. Cancer Forum 31(2):85–90
Roder D, Webster F, Zorbas H, Sinclair S (2012) Breast screening and breast cancer survival in Aboriginal and Torres Strait Islander women of Australia. Asian Pac J Cancer Prev 13(1):147–155
Shahid S, Thompson SC (2009) An overview of cancer and beliefs about the disease in Indigenous people of Australia, Canada, New Zealand and the US. Aust NZ J Public Health 33(2):109–118
Shaw IM, Elston TJ (2003) Retrospective, 5 year surgical audit comparing breast cancer in indigenous and non-indigenous women in Far North Queensland. Aust NZ J Surg 73(9):758–760
Sheppard AJ, Chiarelli AM, Marrett LD, Mirea L, Nishri ED, Trudeau ME, Aboriginal Breast Cancer Study G (2010) Detection of later stage breast cancer in First Nations women in Ontario. Canada. Can J Public Health 101(1):101–105
Sheppard AJ, Chiarelli AM, Marrett LD, Nishri ED, Trudeau ME (2011) Stage at diagnosis and comorbidity influence breast cancer survival in first nations women in Ontario. Canada, Cancer Epidemiol Biomarkers Prev
Soerjomataram I, Lortet-Tieulent J, Ferlay J, Forman D, Mathers C, Parkin DM, Bray F (2012) Estimating and validating disability-adjusted life years at the global level: a methodological framework for cancer. BMC Med Res Methodol 12:125
Supramaniam R, Gibberd A, Dillon A, Goldsbury D, O’Connell D (2014) Increasing rates of surgical treatment and preventing comorbidities may increase breast cancer survival for Aboriginal women. BMC Cancer 14(1):163
Valery PC, Coory M, Stirling J, Green AC (2006) Cancer diagnosis, treatment, and survival in Indigenous and non-indigenous Australians: a matched cohort study. Lancet 367(9525):1842–1848
Acknowledgments
S. Moore was supported by a National Health and Medical Research Council (NHMRC) Training Scholarship for Indigenous Australian Health Research (No. 389935) and a Postdoctoral Fellowship from the International Agency for Research on Cancer/Cancer Australia. The NHMRC Project Grant (No. 1004643) partly funded this project. S Moore and P Valery were also supported by the former Australian Centre for International and Tropical Health, UQ. PC Valery was supported by an Australian Research Council Future Fellowship (No. 100100511). This work was produced as part of the In-Kind activities of the Lowitja Institute incorporating the Cooperative Research Centre for Aboriginal and Torres Strait Islander Health. The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Authors’ contributions
SP Moore participated in the conception, design, analyses of the data, interpretation of results, writing and editing the manuscript. I Soerjomataram conducted analysis of DALYS, interpretation of results, and editing of the manuscript. A Green participated in the conception, design, analyses of the data, interpretation of results and editing the manuscript. G Garvey participated in the interpretation of results and editing the manuscript. J Martin participated in the interpretation of results and editing the manuscript. P Valery participated in the conception, design, analyses of the data, interpretation of results and editing the manuscript. We confirm that all authors have seen and approved its final version.
Rights and permissions
About this article
Cite this article
Moore, S.P., Soerjomataram, I., Green, A.C. et al. Breast cancer diagnosis, patterns of care and burden of disease in Queensland, Australia (1998–2004): does being Indigenous make a difference?. Int J Public Health 61, 435–442 (2016). https://doi.org/10.1007/s00038-015-0739-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00038-015-0739-y