Abstract
Objectives
To review the published literature investigating the association between cadmium exposure and osteoporosis.
Methods
A review of published peer-reviewed literature based on a priori criteria was completed. Odds ratios (OR) were abstracted or estimated from observational studies to calculate a pooled OR using inverse variance weighted random effects models.
Results
The review identified seven studies with a pooled OR of OR = 2.22 (95 % CI: 1.16, 4.28) [I 2 = 54.8 % (p < 0.05)] (comparing highest urine cadmium category to lowest). In women over the age of 50 years, the pooled OR was 1.82 (95 % CI: 1.63, 2.02) [I 2 = 73.1 % (p < 0.05)]. A dose response evaluation (six studies) suggested increasing odds for osteoporosis with increasing urine cadmium levels.
Conclusions
This review detected an association between cadmium exposure and the occurrence of osteoporosis in a small number of cross-sectional studies which requires confirmation in using prospective study design.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cadmium is an environmental and occupational contaminant with public health implications first documented in 1942 (U.S. Public health Service 1942), however, it was in 1955 when it was identified as the cause of Itai–Itai disease in a Japanese population that consumed contaminated rice (Hagino and Kono 1955). Environmental exposure to cadmium is mainly through diet in non-occupational, non-smoking populations (Franz et al. 2008; European Food Safety Authority 2009). Cadmium is found in most food through uptake by crops and vegetables consumed by humans or animals. Worldwide, the average person consumes 8–25 μg per day which raises concerns about long-term effects on chronic diseases involving the kidney (Kazantzis 1979; Suwazono et al. 2006), cardiovascular system (Schutte et al. 2008), and the bone and skeletal system (Åkesson et al. 2006; Wallin et al. 2013).
Since the 1950s, findings from observational studies have suggested that cadmium could be associated with adverse effects on bone structures (Kido et al. 1989; Järup et al. 1998; Wang et al. 2003; Järup and Alfvén 2004; Alfvén et al. 2000; Nambunmee et al. 2010; Shin et al. 2011; Thomas et al. 2011; Engström et al. 2012). In conjunction with population-based research, findings from experimental research have presented potential mechanistic pathways to substantiate the possibility that cadmium exposure can have direct and indirect adverse effects on bone structure (Staessen et al. 1999; Brzóska et al. 2005a, b, c; Åkesson et al. 2006; Coonse et al. 2007; Bhattacharyya 2009; Engström et al. 2009). Current proposed mechanisms include (1) disturbance of activation of vitamin D in the kidney (Tsuritani et al. 1992; Chen et al. 2011), (2) interference with calcium absorption in the digestive tract (Nordberg et al. 2007), (3) direct injury on bone metabolism and osteocytes (Bhattacharyya et al. 1988, 1992; Uriu et al. 2000; Brzóska and Moniuszko-Jakoniuk 2005a; Järup and Åkesson 2009), and alteration of osteoblast gene expression (Arbon et al. 2012); however, the exact mechanism is uncertain.
Cadmium is a toxicant with widespread exposure with documented adverse effects on bone, however, the association and dose response curves have yet to be clearly elucidated. This review evaluated the potential relationship between cadmium exposure and osteoporosis in observational studies employing urinary cadmium levels to define exposure and osteoporosis.
Methods
We completed a comprehensive search in the bibliographic databases PubMed (http://www.ncbi.nlm.nih.gov/pubmed/) and TOXLINE (http://toxnet.nlm.nih.gov/cgi-bin/sis/htmlgen?TOXLINE) to identify peer-reviewed articles addressing the relationship between cadmium exposure and osteoporosis. Within the bibliographic databases, the search strategy included a combination of title and topic with the following terms “cadmium”, “bone”, and “osteoporosis”. Boolean operators were used and wild card or truncation symbols were added to select words to account for possible use of similar word forms. The study selection criteria were (1) observational study with urine cadmium measurements and osteoporosis outcome data available; (2) written in the English language.
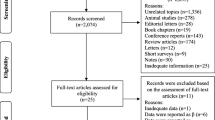
The literature search results yielded 129 articles [PubMed (n = 110); TOXLINE (n = 19)]. Titles and abstracts were first reviewed to determine the relevancy of the document to the study selection criteria listed above excluding a total of 119 documents that were not written in English (n = 11), did not include an osteoporosis outcome (n = 65), government report (n = 6), conference/working group report (n = 4), animal study (n = 29), and non-observational study (n = 4) (Fig. 1). Ten articles underwent a full text review leading to the exclusion of four more (duplicate cohort and outcome definition), however, a bibliographic review for relevant articles not identified in the database search identified one additional article for a total of seven papers for final analysis (Table 1).
A quality assessment was completed for each article using the STROBE checklist (von Elm et al. 2007). The STROBE checklist is a 22-item inventory of key components of observational and/or epidemiology studies used in reviews to assess the quality of the article. STROBE scores are based on the percent of the 22 components within article sections title "Introduction", "Methods", "Results", "Discussion". STROBE scores greater than 61 % are considered substantial for a review article (von Elm et al. 2007).
Odds ratios(OR) for the association between cadmium exposure and osteoporosis were abstracted from five studies (Alfvén et al. 2000; Gallagher et al. 2008; Nawrot et al. 2010; Wu et al. 2010; Engström et al. 2011). Two additional studies (Chen et al. 2009; Jin et al. 2004) had no available measures of association; therefore, unadjusted OR and 95 % confidence intervals were estimated based on the number of cases of osteoporosis and non-cases in each exposure category as defined by urinary cadmium (UCd). For the outcome variable, osteoporosis, there were two diagnostic criteria used in the studies (T and Z-score). The bone mineral density T-score is a comparison of bone density with that of a healthy 30-year old of the same gender whereas a Z-score is a comparison of bone density with a person of the same age and gender adjusted for ethnicity (Hoffman 2013). The T-score <2.5 is used more frequently to diagnose osteoporosis (osteopenia: −2.5 < T-score < −1), however, the Z-score can be used to define the type of osteoporosis (secondary cutoff: Z-score −1) (Swaminathan et al. 2009).
For the exposure categories, four studies had UCd <0.5 μg/g creatinine (Alfvén et al. 2000; Gallagher et al. 2008; Nawrot et al. 2010; Engström et al. 2011) as the lowest exposure group, one study had <1.0 μg/g creatinine (Wu et al. 2010), and two studies had <2.0 μg/g creatinine (Jin et al. 2004; Chen et al. 2009). For the highest category four studies used ≥2.0 μg/g creatinine (Jin et al. 2004; Nawrot et al. 2010; Wu et al. 2010; Chen et al. 2009), one study used >1.0 μg/g creatinine (Gallagher et al. 2008), one study used ≥0.75 μg/g creatinine (Engström et al. 2011), and one study used >3.0 μg/g creatinine (Alfvén et al. 2000). Given that research has shown the benchmark response of 5 % for UCd related to osteoporosis to be 1.88 μg/g creatinine (Chen et al. 2013), we defined the highest and lowest categories as <2.0 and ≥2.0 μg/g creatinine. Three studies do not have those exact cutoffs and therefore used the highest level reported from those studies (Gallagher et al. 2008 UCd >1.0 μg/g creatinine; Alfvén et al. 2000 UCd >2.99 μg/g creatinine; and Engström et al. 2011 UCd >0.75 μg/g creatinine).
Odds ratio estimates comparing osteoporosis risk across UCd groups from each study were pooled to assess heterogeneity based on a calculated I 2 statistic using a fixed and random effects model, where the OR was the difference between the highest and lowest exposure categories reported from the studies. The I 2 statistic is an indication of inconsistency between studies by quantifying how much variance is due to heterogeneity and not chance (Higgins and Thompson 2002). Heterogeneity was also assessed across the two different outcome definitions for osteoporosis (T and Z-score) and in a limited analysis to women only since two study cohorts were all women (Gallagher et al. 2008; Engström et al. 2011). Heterogeneity was also graphically presented in a forest plot. Lastly, a sensitivity assessment was completed to assess the robustness of the findings by assessing the contribution of each study to the heterogeneity. Statistical Analysis System 9.2 (SAS, version 9.2; SAS Institute Inc., Cary, North Carolina) was used for the statistical analyses.
Results
Study characteristics
Seven peer-reviewed articles were included in this review of research investigating the association between cadmium exposure as measured in urine and osteoporosis diagnosis (Table 1). The selected studies were cross-sectional and the cohorts ranged in size from 83 (Nawrot et al. 2010) to 10,798 (Wu et al. 2010). Two study cohorts included only women (Gallagher et al. 2008; Engström et al. 2011), one study included only men (Nawrot et al. 2010), two cohorts were population-based but included only older subjects (>50 years) (Gallagher et al. 2008; Engström et al. 2011), and four studies were conducted in a general population (Alfvén et al. 2000; Jin et al. 2004; Wu et al. 2010; Chen et al. 2009). Four studies were completed in a population with high exposure including occupational (Nawrot et al. 2010) and industrial polluted areas (Alfvén et al. 2000; Jin et al. 2004; Chen et al. 2009) and three were completed in a population with general exposure, two in the United States (Gallagher et al. 2008; Wu et al. 2010) and one in Sweden (Engström et al. 2011). In all studies, exposure to cadmium was assessed using the UCd biomarker corrected for creatinine (μg/g) with measurements ranging from 0.007 to 68.0 μg/g creatinine. Osteoporosis diagnosis was based on international guidelines (World Health Organization 1994) with two studies employing a bone mineral density Z-score < −2 criteria (Jin et al. 2004; Chen et al. 2009), one study employing a bone mineral density Z-score < −1 criteria (Alfvén et al. 2000), and four studies employing a bone mineral density with a T-score <2.5 criteria (Gallagher et al. 2008; Wu et al. 2010; Nawrot et al. 2010; Engström et al. 2011). Lastly, the exposure categories for the studies were within a similar range with various cutoffs. Four studies had UCd <0.5 μg/g creatinine (Alfvén et al. 2000; Gallagher et al. 2008; Nawrot et al. 2010; Engström et al. 2011) as the lowest exposure group, one study had <1.0 μg/g creatinine (Wu et al. 2010), and two studies had <2.0 μg/g creatinine (Jin et al. 2004; Chen et al. 2009). For the highest category, four studies used >2.0 μg/g creatinine (Jin et al. 2004; Nawrot et al. 2010; Wu et al. 2010; Chen et al. 2009), one study used >1.0 μg/g creatinine (Gallagher et al. 2008), one study used >0.75 μg/g creatinine (Engström et al. 2011), and one study used >3.0 μg/g creatinine (Alfvén et al. 2000).
Quality assessment
The seven studies meeting the inclusion criteria were assessed for quality in consideration of bias that could be introduced due to limitations in the study design, sample size, research methods, and analytic methods (Table 1). The selected articles underwent a rigorous assessment using the STROBE checklist with scores ranging from 76 % (Nawrot et al. 2010) to 86 % (Gallagher et al. 2008). One study had a sample size significantly smaller than the other studies, 83 subjects compared to the pooled sample size mean of 2,496 subjects (Nawrot et al. 2010). One study presented only osteoporosis prevalence measures and therefore the effect measure (OR) was estimated and unadjusted (Jin et al. 2004). Another study presented OR comparing UCd 10.0 μg/g creatinine to UCd <2.0 μg/g creatinine therefore we estimated OR at UCd >2.0 μg/g creatinine based on prevalence measures presented at that level (Chen et al. 2009). Three studies employed an osteoporosis diagnostic criterion based on a Z-score as compared to a T-score. The difference in the effect measures between the two criteria was assessed in a sensitivity analysis (presented below). Of the five studies that presented effect measures (OR and 95 % confidence limits), all adjusted the OR for age, gender, and smoking. Four studies accounted for weight or body mass index (Alfvén et al. 2000; Gallagher et al. 2008; Wu et al. 2010; Engström et al. 2011) and two studies accounted for dietary factors (Wu et al. 2010; Engström et al. 2011).
Effect measures
In this review, four of the seven studies identified an increased risk for osteoporosis with higher cadmium exposure as indicated by UCd (Gallagher et al. 2008; Wu et al. 2010; Nawrot et al. 2010; Engström et al. 2011) and three studies reported a suggested increased risk for osteoporosis with higher cadmium exposure (Alfvén et al. 2000; Jin et al. 2004; Chen et al. 2009). Among the studies located in high exposure populations, the OR estimates comparing highest to lowest cadmium exposure groups ranged from OR = 1.7 (95 % CI: 0.56, 5.78) in a study located in China (Jin et al. 2004) to OR = 9.9 (95 % CI: 1.8, 55.2) in an occupational cohort (Nawrot et al. 2010) whereas the three studies completed in general populations reported OR ranging from 1.43 (95 % CI: 1.02, 2.00) (Gallagher et al. 2008) to 3.8 (95 % CI: 2.36, 6.14) (Wu et al. 2010) both studies using the National Health and Nutrition Examination Survey (both 1988–1994 and Gallagher et al. 2008 also 1999–2004) cohort in the United States. Three sets of studies were from similar cohorts with each set producing similar results. Two studies from the NHANES cohort found similar OR when limited to only women, OR = 1.41 (95 % CI: 1.13, 1.75) (Wu et al. 2010) and OR = 1.43 (95 % CI: 1.02, 2.00) (Gallagher et al. 2008). Two studies based on Swedish cohorts found similar OR when limited to women only OR = 1.80 (95 % CI: 0.65, 5.30) (Alfvén et al. 2000) and OR = 1.95 (95 % CI: 1.30, 2.93) (Engström et al. 2011). Lastly, the two studies completed in a polluted area in China had similar OR estimated for UCd >2.0 μg/g creatinine with OR ~ 1.73 (95 % CI: 0.30, 5.87) (Chen et al. 2009) and OR ~ 1.83 (95 % CI: 0.60, 6.27) (Jin et al. 2004).
Heterogeneity was visually assessed using a forest plot (Fig. 2). The pooled effect measure (OR) for osteoporosis comparing the highest and lowest UCd (exposure) in the seven studies reviewed was OR = 2.22 (95 % CI: 1.16, 4.28) with an I 2 of 54.8 % (p < 0.05). When the occupational based study with a small sample size was eliminated, the corresponding pooled OR was 2.10 (95 % CI: 1.80, 2.93) with an I 2 of 61.2 % (p < 0.05). When the analysis is limited to effects on only women the pooled OR was 1.82 (95 % CI: 1.63, 2.02) with an I 2 of 73.1 % (p < 0.05). In an analysis stratifying the studies by outcome measure (T versus Z-score), the pooled OR for studies employing a T-score was OR = 2.25 (95 % CI: 1.83, 3.15) with an I 2 of 77.4 % (p < 0.05) and the pooled OR for studies employing a Z-score was OR = 2.30 (95 % CI: 1.58, 2.88) with an I 2 of 8.8 % (p < 0.05).
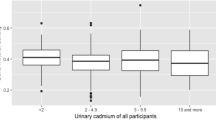
The dose response relationship between UCd and osteoporosis was assessed (Fig. 3). Compared with the referent group, all but one study suggests a dose response relationship. Three studies suggest a steeper dose response curve (Nawrot et al. 2010; Wu et al. 2010; Engström et al. 2011); however, the remaining four studies are more suggestive of dose response curve with a smaller slope (Alfvén et al. 2000; Jin et al. 2004; Gallagher et al. 2008; Chen et al. 2009).
Graphical presentation of the dose relationship between urinary cadmium levels and the associated odds for osteoporosis in seven reviewed studies (total N = 20,339; total cases = 2,032 in four countries, China, United States, Japan, Sweden). *Y-axis truncated therefore not showing the data point OR=9.9 at urinary cadmium=2.0 µg/g from Nawrot et al. (2010)
Discussion
We present a review on the association between UCd and osteoporosis representing heterogeneous populations, outcome measures, and time period. The association between UCd and osteoporosis was present in the pooled OR coupled with an I 2 index of 54.8 % indicating that most of the total variability among effect sizes is not caused by sampling error but actual heterogeneity. This association was present in studies that employed either a T-score or a Z-score to classify a subject as an osteoporotic case and in studies that were located in a high exposure population or the general population. The association between UCd and osteoporosis remained when the review was limited to female subjects only. There is evidence of a dose response relationship; however, the shape of the dose response curve still requires more research. The understanding of the association between UCd and osteoporosis is limited by the small number of studies included in this review, the between-study heterogeneity, and the lack of prospective study designs. In addition, conclusive evidence was limited by inconsistent disease status definitions and inconsistent adjustment for relevant confounders. This review presents evidence suggestive of an association between cadmium exposure as represented by UCd, however, any etiologic inference would require more research especially prospective in design.
Four of the seven studies selected for this review had consistent OR (Alfvén et al. 2000; Gallagher et al. 2008; Engström et al. 2011; Wu et al. 2010) while adjusting for major risk factors in a large sample. One study was not consistent with the other studies (Nawrot et al. 2010) with an OR in working men of 9.9, which is about three times higher than the other studies with effect measures at similar exposure categories (Alfvén et al. 2000; Jin et al. 2004; Wu et al. 2010; Chen et al. 2009). This difference can be explained by the small sample size (n = 83) and male only cohort, however, when Nawrot et al. (2010) is excluded from the heterogeneity analysis there is a little change in the new pooled OR and the I 2 = 61.2 %. The other two studies that were divergent in results (Jin et al. 2004; Chen et al. 2009) had smaller sample sizes and OR, however, had similar findings in high exposure populations in China. This consistency is also found in the two other sets of studies completed in similar datasets (Gallagher et al. 2008; Wu et al. 2010) or similar location (Alfvén et al. 2000; Engström et al. 2011).
Three of the seven studies employed a case criterion based on a Z-score of bone mineral density and four employed a case criterion based on a T-score. When heterogeneity was assessed by diagnostic criteria, the pooled OR for T-score was not significantly different from the pooled OR from the total group of studies (OR = 2.25 versus 2.30); however, the I 2 indices were different suggesting that variation in outcome definition can influence heterogeneity. The above findings suggest that the results from this meta-analysis are robust to the exclusion of the Nawrot et al. (2010) study and differences in diagnostic criteria in three of the studies.
Even though the findings from this meta-analysis have shown to be robust, caution should be heeded in making causal inferences specific to cadmium exposure and osteoporosis. All of the reviewed studies employed a cross-sectional design which is unable to establish exposure prior to osteoporosis diagnosis. Also, potential effect modifiers and confounders relevant to the association between osteoporosis and cadmium exposure such as hormone replacement therapy, micronutrient intake (calcium or vitamin D), family history, co-exposures, and physical activity were not included universally across studies. Two studies did not adjust for any factors (Jin et al. 2004; Chen et al. 2009) and the remaining studies adjusted for factors with available data, but no study examined all relevant factors. The inconsistency in adjustment for confounders across all seven studies is a limitation in this meta-analysis that cannot be quantified. Nonetheless, the relatively consistent results across the studies, adds weight to the findings of an association between urine cadmium levels and osteoporosis independent of other risk factors.
Osteoporosis risk is higher in women than men, therefore, heterogeneity was assessed limiting analysis to the female sample of each study (where information available). Three studies limited their cohort to women (Jin et al. 2004; Gallagher et al. 2008; Engström et al. 2011) and three others stratified their analysis by gender. The pooled OR for female only was 1.82 (95 % CI: 1.63, 2.02), however, the I 2 index improved to 73.1 % suggesting that variability among effect sizes is caused by true heterogeneity between studies and less due to sampling error as when all the studies (including males) are pooled. This improvement in heterogeneity based on female cohorts given that osteoporosis is more of a health concern for women than men. The focus on female cohorts could represent a possible publication bias where null findings involving all male or general populations may not have been published. The Nawrot et al. 2010 is the only all-male cohort identified in this review with most studies containing predominantly female populations.
The mechanistic pathway for the association between cadmium exposure and osteoporosis is complex and has yet to be clearly defined. There are several proposed pathways including: (1) disturbance of the renal normal activation process of vitamin D as a secondary effect of renal tubular injury (Järup et al. 1998; Chen et al. 2011), (2) interference with calcium absorption in the digestive tract (Nordberg et al. 2007), and (3) direct injury on bone metabolism and osteocytes (Bhattacharyya 2009; Uriu et al. 2000; Brzóska and Moniuszko-Jakoniuk 2005a; Arbon et al. 2012). Observational research has examined the association between bone mineral density measurements and UCd to determine whether cadmium exposure is associated with bone demineralization leading to osteoporosis with inconclusive results (Staessen et al. 1999; Horiguchi et al. 2005; Trzcinka-Ochocka et al. 2010; Youness et al. 2012). Therefore, additional research with a prospective design incorporating clinical markers for kidney and bone disease is needed to determine whether cadmium is an independent and direct toxicant on bones and thereafter osteoporosis.
Conclusions
This is the first review and meta-analysis evaluating the relationship between cadmium exposure and osteoporosis. A positive association between elevated UCd and the occurrence of osteoporosis was identified; however, the inference of causation is ambiguous because of the small number of studies in this review, the cross-sectional designs, and other methodological limitations. Prospective cohort studies conducted in general populations with variable exposure and strong assessment of osteoporosis, major confounders, and effect measure modifiers such as consistent clinical measures [bone mineral density from the same bone, using the same device, and same measurement (T versus Z-score)], gender, smoking history, diet, micronutrient intake, family history, and hormone therapy are needed to better characterize the relationship between cadmium and bone disease. Cadmium is a universal toxicant that plagues all humans regardless of location or diet and research has shown that adverse effects may be permanent and cannot be reversed (Chen et al. 2013); therefore even a modest osteopathic effect could have substantial public health impact. Future research needs to evaluate the shape of the dose response especially in susceptible populations such as post-menopausal females and children (Sughis et al. 2011), and means for reduction in exposure should be a worldwide public health priority.
References
Åkesson A, Bjellerup P, Lundh T, Lidfeldt J, Nerbrand C, Samsioe G, Skerfving S, Vahter M (2006) Cadmium-induced effects on bone in a population-based study of women. Environ Health Persp 114:830–834
Alfvén T, Elinder CG, Carlsson MD, Grubb A, Hellström L, Persson B, Pettersson C, Spang G, Schutz A, Järup L (2000) Low-level cadmium exposure and osteoporosis. J B Miner Res 15:1579–1586
Arbon KS, Christensen CM, Harvey WA, Heggland SJ (2012) Cadmium exposure activates the ERK signaling pathway leading to altered osteoblast gene expression and apoptotic death in Saos-2 cells. Food Chem Toxicol 50(2):198–205
Bhattacharyya MH (2009) Cadmium osteotoxicity in experimental animals: mechanisms and relationship to human exposures. Toxicol Appl Pharmacol 3(238):258–265
Bhattacharyya MH, Whelton BD, Stern PH, Petrson PD (1988) Cadmium accelerates bone loss in ovariectomized mice and fetal rat limb bones in culture. Proc Natl Acad Sci USA 85:8761–8765
Bhattacharyya MH, Sacco-Gibson NA, Peterson DP (1992) Cadmium-induced bone loss: increased susceptibility in female beagles after ovariectomy. IARC Sci Publ 118:279–286
Brzóska MM, Moniuszko-Jakoniuk J (2005a) Disorders in bone metabolism of female rats chronically exposed to cadmium. Toxicol Appl Pharmacol 202:68–83
Brzóska MM, Moniuszko-Jakoniuk J (2005b) Bone metabolism of male rats chronically exposed to cadmium. Toxicol Appl Pharmacol 207:195–211
Brzóska MM, Majewska K, Moniuszko-Jakoniuk J (2005) Bone mineral density, chemical composition and biomechanical properties of the tibia of female rats exposed to cadmium since weaning up to skeletal maturity. Food Chem Toxicol 43:1507–1519
Chen A, Zhu G, Jin T, Akesson A, Bergdahl IA, Lei L, Weng S, Liang Y (2009) Changes in bone mineral density 10 years after marked reduction of cadmium exposure in a Chinese population. Environ Res 109:874–879
Chen X, Zhu G, Jin T, Lei L, Liang Y (2011) Bone mineral density is related with previous renal dysfunction caused by cadmium exposure. Environ Toxicol Pharmacol 32(1):46–53
Chen X, Gan C, Zhu G, Jin T (2013) Benchmark dose for estimation of cadmium reference level for osteoporosis in a Chinese female population. Food Chem Toxicol 55:592–595
Coonse KG, Coonts AJ, Morrison EV, Heggland SJ (2007) Cadmium induces apoptosis in the human osteoblast-like cell line Saos-2. J Toxicol Environ Health A 70(7):575–581
Engström A, Skerving S, Lidfeldt J, Burgaz A, Lundh T, Samsioe G, Vahter M, Akesson A (2009) Cadmium-induced bone effect is not mediated via low serum 1,25-dihydroxy vitamin D. Environ Res 109:188–192
Engström A, Michaëlsson K, Vahter M, Julin B, Wolk A, Åkesson A (2011) Long-term cadmium exposure and the association with bone mineral density and fractures in a population-based study among women. J B Miner Res 26(3):486–495
Engström A, Michaëlsson K, Vahter M, Julin B, Wolk A, Åkesson A (2012) Associations between dietary cadmium exposure and bone mineral density and risk of osteoporosis and fractures among women. Bone 50(6):1372–1378
European Food Safety Authority (2009) Cadmium in food: scientific opinion of the panel on contaminants in the food chain (question no EFSA-Q-2007-138). EFSA J 980:1–139
Franz E, Römkens P, van Raamsdonk L, van der Fels-Klerx I (2008) A chain modeling approach to estimate the impact of soil cadmium pollution on human dietary exposure. J Food Prot 71(12):2504–2513
Gallagher CM, Kovach JS, Meliker JR (2008) Urinary cadmium and osteoporosis in U.S. women > or = 50 years of age: nHANES 1988–1994 and 1999–2004. Environ Health Persp 116:1338–1343
Hagino N, Kono, M (1955) A study on the cause of Itai–itai disease In: Proc 17th Mtg Japanese Soc of Clinical Surgeons (in Japanese)
Higgins JPT, Thompson SG (2002) Quantifying heterogeneity in a meta-analysis. Statist Med 21:1539–1558
Hoffman, Matthew http://www.webmd.com/osteoporosis/living-with-osteoporosis-7/tests. (Accessed March, 2013)
Horiguchi H, Oguma E, Sasaki S, Miyamato K, Ikeda Y, Machida M, Kayama F (2005) Environmental exposure to cadmium at a level insufficient to induce renal tubular dysfunction does not affect bone density among female Japanese farmers. Environ Res 97:83–93
Järup L, Åkesson A (2009) Current status of cadmium as an environmental health problem. Toxicol Appl Pharmacol 238:201–208
Järup L, Alfvén T (2004) Low level cadmium exposure, renal and bone effects–the OSCAR study. Biometals 17(5):505–509
Järup L, Alfven T, Persson B, Toss G, Elinder CG (1998) Cadmium may be a risk factor for osteoporosis. Occup Environ Med 55:435–439
Jin T, Nordberg G, Ye T, Bo M, Wang H, Zhu G, Kong Q, Bernard A (2004) Osteoporosis and renal dysfunction in a general population exposed to cadmium in China. Environ Res 96(3):353–359
Kazantzis G (1979) Renal tubular dysfunction and abnormalities of calcium metabolism in cadmium workers. Environ Health Persp 28:155–159
Kido T, Nogawa K, Yamada Y et al (1989) Osteopenia in inhabitants with renal dysfunction induced by exposure to environmental cadmium. Int Arch Occup Environ Health 61:271–276
Nambunmee K, Honda R, Nishijo M, Swaddiwudhipong W, Nakagawa H, Ruangyuttikarn W (2010) Bone resorption acceleration and calcium reabsorption impairment in a Thai population with high cadmium exposure. Toxicol Mech Methods 20(1):7–13
Nawrot T, Geusens P, Nulens TS, Nemery B (2010) Occupational cadmium exposure and calcium excretion, bone density, and osteoporosis in men. J B Miner Res 25(6):1441–1445
Nordberg GF, Nogawa K, Nordberg M, Friberg LT (2007) Cadmium. In: Nordberg GF, Fowler BA, Nordberg M, Friberg LT (eds) Handbook on the toxicology of metals. Third edition. Academic Press, New York, pp 446–486
Schutte R, Nawrot TS, Richart T, Thijs L, Vanderschueren D, Kuznetsova T et al (2008) Bone resorption and environmental exposure to cadmium in women: a population study. Environ Health Persp 116:777–783
Shin M, Paek D, Yoon C (2011) The relationship between the bone mineral density and urinary cadmium concentration of residents in an industrial complex. Environ Res 111(1):101–109
Staessen JA, Roels HA, Emelianov D, Kuznetsova T, Thijs L, Vangronsveld J, Fagard R (1999) Environmental exposure to cadmium, forearm bone density, and risk of fractures: prospective population study. Lancet 353:1140–1144
Sughis M, Penders J, Haufroid V, Nemery B, Nawrot TS (2011) Bone resorption and environmental exposure to cadmium in children: a cross–sectional study. Environ Health 8(10):104
Suwazono Y, Sand S, Vahter M, Filipsson AF, Skerfving S, Lidfeldt J et al (2006) Benchmark dose for cadmium-induced renal effects in humans. Environ Health Persp 114:1072–1076
Swaminathan K, Flynn R, Garton M, Paterson C, Leese G (2009) Search for secondary osteoporosis: are Z scores useful predictors? Postgrad Med J 85(999):38–39
Thomas LDK, Michaëlsson K, Julin B, Wolk A, Åkesson A (2011) Dietary cadmium exposure and fracture incidence among men: a population-based prospective cohort study. J B Miner Res 26:1601–1608
Trzcinka-Ochocka M, Jakubowski M, Szymczak W, Janasik B, Brodzka R (2010) The effects of low environmental cadmium exposure on bone density. Environ Res 110(3):286–293
Tsuritani I, Honda R, Ishizaki M et al (1992) Impairment of vitamin D metabolism due to environmental cadmium exposure, and possible relevance to sex-related differences in vulnerability to the bone damage. J Toxicol Environ Health 37:519–533
Uriu K, Morimoto I, Kai K, Okazaki Y, Okada Y, Qie YL, Okimoto N, Kaizu K, Nakamura T, Eto S (2000) Uncoupling between bone formation and resorption in ovariectomized rats with chronic cadmium exposure. Toxicol Appl Pharmacol 164:264–272
U.S. Public health Service (1942) Public health report, cadmium poisoning. Division industrial hygiene. Nation Inst Health 57:601–612
von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP (2007) The strengthening the reporting of observational studies in epidemiology (STROBE) statement: guidelines for reporting observational studies. STROBE initiative. Lancet 370(9596):1453–1457
Wallin M, Sallsten G, Fabricius-Lagging E, Öhrn C, Lundh T, Barregard L (2013) Kidney cadmium levels and associations with urinary calcium and bone mineral density: a cross-sectional study in Sweden. Environ Health 12:22
Wang H, Zhu G, Shi Y, Weng S, Jin T, Kong Q, Nordberg GF (2003) Influence of environmental cadmium exposure on forearm bone density. J B Miner Res 18:553–560
World Health Organization (1994) Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. WHO (WHO Technical Report Series 843) Geneva
Wu Q, Magnus JH, Hentz JG (2010) Urinary cadmium, osteopenia, and osteoporosis in the US population. Osteoporos Int 21(8):1449–1454
Youness ER, Mohammed NA, Morsy FA (2012) Toxicol Mech Methods 22(7):560–567
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the special issue: “Environment and Health Reviews”.
Rights and permissions
About this article
Cite this article
James, K.A., Meliker, J.R. Environmental cadmium exposure and osteoporosis: a review. Int J Public Health 58, 737–745 (2013). https://doi.org/10.1007/s00038-013-0488-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00038-013-0488-8