Abstract
Salt marshes attenuate waves and thus have an important function for coastal protection. Biophysical properties of salt-marsh plants play a key role in the process of wave attenuation and can be differentiated by morphological properties such as stem density, vegetation height and aboveground biomass as well as by biomechanical properties related to stem flexibility. Numerical or physical scale models predicting wave attenuation over vegetated surfaces need to include biophysical properties. However, few studies have quantified morphological and biomechanical properties of salt-marsh plants and fewer have considered seasonal and within-marsh spatial variability of biomechanical properties. The aim of this study was to quantify biophysical properties of the common salt-marsh grasses Spartina anglica and Elymus athericus, including stem flexibility and density as well as aboveground biomass, temporally and spatially. Samples were collected in spring and in summer 2014 at a study site located in the Northern German Wadden Sea. Aboveground biomass was harvested in plots of 50 × 50 cm, stem density was determined by counting and flexibility of plant stems was determined with three-point bending tests. Biophysical properties of both species varied significantly between seasons with plant stem stiffness being 5.0 (S. anglica) and 2.9 times (E. athericus) higher and aboveground biomass being 2.1 (S. anglica) and 1.3 times (E. athericus) higher in summer than in spring. Small-scale spatial differences for those biophysical plant properties were found for S. anglica with plant stem stiffness being 4.0 (spring) and 2.8 times (summer) higher and aboveground biomass being 1.6 (spring) and 1.5 times (summer) higher in a landward than in a seaward-located zone. Small-scale spatial differences of biophysical properties were not found in E. athericus. We conclude that variability in biophysical properties should be considered in models and experiments especially for S. anglica when predicting and quantifying marsh wave attenuation capacity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Vegetation plays a vital role in the form, functioning and ecosystem service delivery of coastal salt marshes. Many salt-marsh plants act as ecosystem engineers by modifying their physical environment through the reduction of hydrodynamic energy and the enhancement of sediment deposition (Bouma et al. 2005, 2010; Peralta et al. 2008). If sufficient sediment is deposited, marshes can keep pace vertically with rising sea level (Nolte et al. 2013). This ability implies that vegetated salt-marsh surfaces can be an important component of nature-based coastal protection schemes especially in times of climate change, accelerated sea-level rise and increased storm frequency (Koch et al. 2009; Narayan et al. 2016; Sutton-Grier et al. 2018).
Recent studies have shown that biophysical properties of plants, which can be categorized as morphological (e.g. stem density, vegetation height and aboveground biomass) and biomechanical (e.g. stem flexibility), play a key role in the capacity of marshes to dissipate wave height and energy (Möller et al. 2014; Paul et al. 2016; Rupprecht et al. 2017). Wave dissipation is a combined effect of bottom friction and vegetation, which form an obstruction to wave-induced oscillatory flow. Vegetation induced obstruction depends both on standing biomass or stem density and stem flexibility. Vegetation, in turn, experiences drag and re-orientation by wave forces (Mullarney and Henderson 2010). Flexible plants move with the surrounding water and show an avoidance strategy to minimize the risk of folding and breakage under high drag forces. In contrast, stiff plants maximize the resistance to physical damage (tolerance strategy), thus leading to higher drag forces, higher flow resistance and an increased risk of breakage compared to flexible plants (Coops et al. 1994; Puijalon et al. 2011). Apart from stem flexibility, aboveground biomass and stem density also play a crucial role in wave dissipation by vegetation (Bouma et al. 2005, 2010; Widdows et al. 2008; Peralta et al. 2008; Anderson and Smith 2014). For example, species with contrasting biomechanical plant properties can lead to a similar wave dissipation when regarded on a biomass basis (Bouma et al. 2010).
Salt-marsh plants show a wide variability in biophysical properties both within and among species, making their canopies structurally complex (Tempest et al. 2015; Rupprecht et al. 2015a). This structural complexity in combination with the unpredictable nature and high variability of hydrodynamic conditions make field measurements of the interaction between vegetation and hydrodynamics extremely challenging. Hence, many studies rely on numerical or physical modelling approaches (Tempest et al. 2015). A high model quality, however, is often hampered by limited data on biophysical properties of salt-marsh vegetation, especially regarding stem flexibility (Tempest et al. 2015). The majority of numerical wave dissipation models capture vegetation effects in a factor that consists of plant stem height, stem density, stem diameter and an empirical bulk drag coefficient CD (Mendez and Losada 2004; Paul and Amos 2011). Physical models often use plant mimics to simulate the effect of vegetation on currents and waves (e.g. Stewart 2006; Anderson and Smith 2014). However, insufficient data on plant biophysical properties lead to problems in reproducing salt-marsh plants realistically by plant mimics (see Anderson and Smith 2014; Tempest et al. 2015). Consequently, it would be valuable to assess the spatial and temporal variation in biophysical properties of salt-marsh species (Rupprecht et al. 2015a).
Morphological properties of salt-marsh plants have been examined (e.g. Morris and Haskin 1990; Möller and Spencer 2002; Neumeier 2005; Foster-Martinez et al. 2018), however, those concerned with biomechanical properties focused predominantly on freshwater plants (Ostendorp 1995; Coops and van der Velde 1996; Miler et al. 2012, 2014), brackish plants (Heuner et al. 2015; Carus et al. 2016; Silinski et al. 2015, 2018), macroalgae (Harder et al. 2006; Paul et al. 2014) or seagrass (Patterson et al. 2001; Fonseca et al. 2007; Luhar and Nepf 2011; Paul and Amos 2011). Studies of salt marshes are scarce (but see Feagin et al. 2011; Rupprecht et al. 2015a). Biomechanical properties of salt-marsh plants are likely to be affected by seasonal climatic variation in temperate zones as previously found for helophytes (Coops and van der Velde 1996) or lake and river plants (Miler et al. 2014).
Recently, the importance of considering seasonal variability in vegetative and biomechanical properties of salt marshes for estimates of wave attenuation over salt marshes was addressed by van Loon-Steensma et al. (2016). In order to generate reliable predictions of the marsh wave attenuation capacity and successfully incorporate marshes in coastal protection schemes, both seasonal and spatial variability in biomechanical and morphological vegetation properties need to be integrated in numerical and physical scale models (van der Meer 2002; Smith et al. 2016).
The aim of this study is to quantify stem flexibility, stem density and aboveground biomass of salt-marsh plants seasonally and spatially between seaward and landward-located zones. Data were collected for two perennial grasses that are widely spread in salt marshes of NW Europe (Spartina anglica and Elymus athericus) to answer the following questions: (I) how do biophysical properties of the salt-marsh grasses S. anglica and E. athericus differ between spring and summer?; and (II) how do biophysical properties of S. anglica and E. athericus differ between seaward and landward-located zones?
Methods
Species
Spartina anglica
The perennial grass S. anglica (hereafter referred to as Spartina) typically occurs in the salt-marsh pioneer zone (below mean high tide level) and the low marsh, where it can form monospecific stands (Nehring and Adsersen 2006). In late fall, shoots die but largely remain as dead vegetation canopies while rhizome development increases (Nehring and Adsersen 2006). Throughout the last century, Spartina has spread from the south coast of the UK to salt marshes all over Europe, both naturally and by deliberate transplantations (Nehring and Adsersen 2006; Nehring and Hesse 2008). A reason for deliberate transplantations was its function to act as an ecosystem engineer by enhancing sedimentation through dense aboveground canopies and a dense root system (Chung 1993; Bouma et al. 2005, 2010; Van Hulzen et al. 2007).
Elymus athericus
The perennial grass E. athericus (hereafter referred to as Elymus) occurs in European salt marshes from Northern Portugal to Southern Denmark and at the southeastern coast of the British Isles (Veeneklaas et al. 2013). Elymus is sensitive to grazing and relies on aerated soils (Bockelmann and Neuhaus 1999). In salt marshes of the Wadden Sea, it forms monospecific dense stands mainly in the high marshes, and it is also increasingly establishing at lower elevations (Bockelmann and Neuhaus 1999; Valéry et al. 2004). In the recent decades, spreading of Elymus has been observed, which is caused by the abandonment of grazing, an increasing marsh age and the ability to reproduce by rhizomes, which survive the winter season (Rupprecht et al. 2015b). The shoots die off over the winter season but largely remain withered on the marsh platform.
Study site
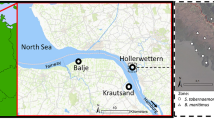
Plant samples were obtained from a salt marsh on the mainland coast of Northern Frisia, German Wadden Sea (54.62°N, 8.84°E; Fig. 1A). The studied salt marsh developed after the embankment of the adjacent Sönke-Nissen-Koog (SNK) polder and subsequent constructions of sedimentation fields in front of the dike (Kunz and Panten 1997; Mueller et al. 2019). As a salt marsh of anthropogenic origin with a thick clayish sediment layer and a regular system of creeks and drainage ditches, it can be considered representative of many salt marshes of North-West Europe. The tidal range is 3.4 m, the mean high tide is + 1.59 m NHN (Normalhöhennull, which is comparable to mean sea level). Elevations within the salt marsh range from 0.9 to 2.6 m NHN with a mean elevation of 2 m NHN (Müller et al. 2013). The marsh stretches from the dike over 700 m to the tidal flats (Fig. 1B) and is predominantly covered by Elymus in the high marsh (Mueller et al. 2017) and by Spartina in the low marsh (personal observations and the Trilateral Monitoring and Assessment Program, TMAP; Petersen et al. 2013).
A Location of the study site in the Wadden Sea National Park Schleswig–Holstein at the German North Sea coast. The black rectangle shows the position of the area in Europe. B Satellite image of the study site with the sampling zones. Shown are the seaward and landward located Elymus (ESZ, ELZ; vertically hatched) and Spartina (SSZ, SLZ; diagonally hatched) sampling zones with respective mean elevations above NHN. The map was created using a base map in ArcGIS © Desktop: Release 10, ESRI 2014, Redlands, CA: Environmental Systems Research Institute
Sampling design
Two sampling zones were chosen in the low marsh (dominated by Spartina) and in the high marsh (dominated by Elymus). One of the two sampling zones per vegetation type was set closer to the seaward marsh edge (‘seawards’), and one closer to the landward marsh edge (‘landwards’; Fig. 1B). The seaward-located Spartina zone (hereafter referred to as SSZ; inundation frequency: 182 times per year; total inundation time: 557.76 h/year; Müller unpublished data) stretches 40 m perpendicular along the marsh towards the landward-located zone (hereafter referred to as SLZ; inundation frequency: 156 times per year; total inundation time: 452.88 h/year). For Elymus, one zone was chosen towards the low marsh (ESZ; inundation frequency: 23 times per year; total inundation time: 62.64 h/year) and one zone was chosen closer to the dike (ELZ; inundation frequency: 23 times per year; total inundation time: 64.32 h/year). An area-based stratified random design was applied with 40 random sampling points (20 points for flexibility measurements; 20 points for aboveground biomass and stem density measurements) generated within each sampling zone of the Spartina and Elymus vegetation type using a random point tool of QGIS 2.0.1 Dufour (QGIS Development Team 2014). The elevation of each point was assessed using a Trimble LL500 precision laser and a Trimble HL 700 receiver as a levelling instrument (2.0 mm accuracy) and a known closely located benchmark. Data were used to calculate mean elevation per zone (Fig. 1B).
Measurements of plant stem flexibility
Three-point bending tests were performed to quantify plant stem flexibility under bending forces orthogonal to the plants stem. Plant samples were collected both in mid-March (before the onset of plant growth) and in late August. In the field, samples were excavated as small marsh blocks with a dimension of 10 × 10 × 10 cm and were packed in plastic bags to conduct measurements on fresh material. From each marsh block, a single adult and undamaged plant stem was chosen randomly and the stem length up to the inflorescence was measured and divided in four equal parts. A test section was defined as the beginning of the second quarter starting from the bottom end of the stem and was cut out with a razor blade. Test sections were consistently cylindrical. To minimize the effect of shear stress in bending tests, a stem diameter to stem length ratio (here stem length means the horizontal span of the tested stem section between the two metal support bars, see Fig. 2) of 1:15 was chosen (see also Miler et al. 2012, 2014; Rupprecht et al. 2015a). The bending tests were performed with a Zwick/Roell testing machine (Type 1120.25, Nominal Force: max. 1 kN, using a 10 N load cell; initial load 0.01 N; Zwick GmbH & Co. KG, Ulm, Germany).
For the measurements, a metal bar was lowered with a displacement rate of 10 mm min−1. Then, the vertical deflection of the tested stem section and the applied force were recorded (see also Miler et al. 2012, 2014; Rupprecht et al. 2015a; Silinski et al. 2015, 2018). The slope was determined from the most linear part of the force–deflection curve. Furthermore, the diameter and the span of the stem between the two metal support bars were used to determine the following mechanical properties following Rupprecht et al. (2015a): (1) the second moment of area (I given in m4) which describes the effect of stem morphology (considering stem diameter) on flexibility; (2) the Young’s modulus (E given in Pa) which here describes the flexibility of the plant stem tissue without considering stem morphology; (3) the flexural rigidity (EI given in Nm2) which describes the overall stem flexibility considering stem tissue and morphological parameters. In this study, results on the Young’s modulus and flexural rigidity are presented.
Biomass and stem density measurements
Aboveground biomass (hereafter referred to as biomass) was harvested twice in 2014; in early April and in mid-August in order to identify differences in morphological properties between spring and summer. All plants rooting inside a 50 × 50 cm frame were cut at the soil surface. Summer sampling was carried out within 1 m distance of the spring plots. Samples were dried for 48 h at 65 °C to determine the dry biomass. Stem density was measured after the removal of litter by counting only the remaining stems that were still connected to a root. For Elymus, stem density was quantified on a 20 × 20 cm subplot due to large numbers of stems per area.
Statistical analysis
To analyze differences in biophysical parameters between the seasons and zones within one species, two-way analysis of variance (ANOVA) were performed. If necessary, data were log transformed prior to ANOVA to meet normality assumptions and to improve homogeneity of variances. Levene’s test was used to test for homogeneity of variances, while Kolmogorov–Smirnov test was used to test the normal distribution of the data. Equal sample sizes assured robustness of parametric testing (McGuinness 2002). As a post hoc test, Tukey’s-HSD (honest significant difference) test was applied to determine pairwise differences. To assess the relationship between plant stem diameter and flexural rigidity, linear and non-linear regressions were used. Statistical analyses were conducted with STATISTICA 10 (StatSoft Inc.).
Results
Flexural rigidity
Flexural rigidity of Spartina differed significantly between seasons and zones (Fig. 3A, Table 1). However, the interaction between season and zone was also significant. Flexural rigidity was 5.0 times higher in summer compared to spring. In spring, Spartina stems of the SLZ were 4.0 times more rigid compared to the stems of the SSZ. In summer, stems of the SLZ showed a 2.8 times higher value compared to stems of the SSZ.
Flexural rigidity (A), biomass (B) and stem density (C) of Spartina and Elymus in spring and summer, respectively. Light bars show the zone directed seawards while dark bars show the zone directed landwards. Each bar represents 20 samples. Presented are mean values ± standard deviations. Different lowercase letters indicate significant differences among the zones in both seasons. Interspecific differences have not been assessed
For Elymus, flexural rigidity significantly differed between seasons (Fig. 3A, Table 1). Stems were 2.9 times more rigid in summer compared to spring. In both seasons, stems of the ESZ slightly, but not significantly, exceeded the rigidity of stems of the ELZ with a factor of 1.34 in spring and 1.14 in summer.
For Spartina, a second order polynomial regression was found to best represent the positive relationship between stem diameter and flexural rigidity. For Elymus, we found a linear regression to best represent the positive relationship between stem diameter and flexural rigidity (Fig. 4).
Aboveground biomass
Biomass of Spartina differed significantly between spring and summer and between SSZ and SLZ (Fig. 3B, Table 1). Additionally, a significant interaction between season and zone was found. Compared to spring, biomass was 2.1 times higher in summer. SLZ exhibited 1.6 times more biomass compared to SSZ in spring and 1.5 times more biomass in summer. For Elymus, significant differences in biomass were only found between the seasons but not between the zones (Fig. 3B, Table 1). Elymus biomass was 1.3 times higher in summer compared to spring.
Stem density
Stem density of Spartina significantly differed both between seasons and zones (Fig. 3C, Table 1). Furthermore, a significant interaction between season and zone was found. Stem density was 1.7 times higher in summer than in spring. While in spring no difference was detected between the two zones, in summer stem density was 1.4 times higher in SSZ than in SLZ. Stem densities in Elymus differed between seasons, but not between zones (Fig. 3C, Table 1). Stem density in spring was 1.4 times greater than in summer.
Stem length, stem diameter, Young’s Modulus
Spartina and Elymus stems were significantly longer in summer compared to spring in both zones (Fig. 5, Table 1). Furthermore, Spartina stems were significantly longer in the SLZ than in the SSZ in either season, whereas for Elymus no spatial differences were detected. Stem diameters show the same pattern with higher values in summer compared to spring for both species, and higher values in the landward zone only for Spartina. The least variability between the seasons and zones was detected for Young’s modulus. Here, only Spartina stems showed slightly, but not significantly, higher values in summer compared to spring and in the SLZ compared to the SSZ in either season. No differences for Young’s modulus were detected in Elymus stems.
Stem length (A), stem diameter (B) and Young’s modulus (C) of Spartina and Elymus in spring and summer, respectively. Light bars show the zone directed seawards while dark bars show the zone directed landwards. Each bar represents 20 samples. Presented are mean values ± standard deviations. Different lowercase letters indicate significant differences among the zones in both seasons. Interspecific differences have not been assessed
Discussion
Stem flexibility
Seasonal variability in stem flexibility was detected for both species with significantly higher values for flexural rigidity during summer. These results indicate the importance of considering plant morphology (here diameter) when describing plant stem flexibility. According to the regression analyses, more than 70% and 80% (R2 values) of the variability in stem flexibility was explained by the variability in stem diameter of Elymus and Spartina stems, respectively. The increase of stem diameter by approximately 30% from spring to summer for both species explains the increase of the flexural rigidity, whereas plant tissue properties (characterized by the Young’s modulus) did not vary significantly between spring and summer. As flexible stems avoid high drag forces by reconfiguration and movement with the wave-induced oscillatory flow (Bouma et al. 2005; Paul et al. 2014), the lower resistance of plant stems to wave forces in spring should result in a lower wave dissipation capacity of vegetation compared to summer.
The small-scale spatial differences with smaller diameters and hence higher flexibility of Spartina stems in the SSZ, which stretches 40 m from the seaward marsh edge towards the SLZ, can be interpreted as a response to physical stress by higher hydrodynamic forcing close to the seaward marsh edge. Möller and Spencer (2002) found that most wave energy is attenuated in the first 38 m on a vegetated marsh while Silinski et al. (2018) found high wave attenuation rates on a 12 m transect and Ysebaert et al. (2011) for a distance up to 50 m. Similar to our results, Heuner et al. (2015) found a pattern with more flexible plants and lower biomass amounts at the marsh in the Elbe estuary for Schoenoplectus tabernaemontani. In accordance, Silinski et al. (2018) found an increase of stiffness in Bolboschoenus maritimus stems from the marsh edge towards the higher zones of an elevational gradient.
In contrast, Carus et al. (2016) found the opposite pattern for stems of Bolboschoenus maritimus, a typical species in the pioneer zone of European freshwater and brackish marshes along shorelines of estuaries where ship and wave induced wave forcing occurs. These findings suggest that species growing under harsh hydrodynamic conditions may develop different biomechanical properties to either minimize physical stress (avoidance strategy; i.e. flexible stems, low flexural rigidity) from waves and currents or to withstand these mechanical forces (tolerance strategy; i.e. stiff stems, high flexural rigidity). Our results show an avoidance strategy of Spartina to increasing hydrodynamic forces and drag forces lower in the elevational gradient in salt marshes, as individuals in the SSZ were significantly smaller, thinner and more flexible than in the SLZ in both seasons. These characteristics should minimize the impact of hydrodynamic forces and the risk of plant breakage. However, it may also be possible that stem development in the SSZ is inhibited by constant wave action leading to thinner, smaller and more flexible stems.
Small-scale spatial variability of stem flexibility in Elymus was minor compared to Spartina. One reason for that may be that Elymus is growing in the high marsh and is exposed to more stable environmental conditions facing wave forcing only during extreme storm surge events. Furthermore, inundation frequency and time in the ESZ were similar to those in the ELZ as the difference in elevation between the two zones was only one centimeter. Therefore, the spatial signal was comparatively low.
Biomass
For both Spartina and Elymus, seasonal differences with higher biomass in summer than in spring were found which can be explained with the breakdown of canopies during the winter season in temperate zones (Bellis and Gaither 1985; Morris and Haskin 1990; Koch et al. 2009). In Spartina, we found up to two times higher biomass in summer compared to spring. Seasonal biomass changes in temperate zones have been found to affect wave dissipation in seagrass beds (Chen et al. 2007; Paul and Amos 2011), brackish marshes (Silinski et al. 2018; Schoutens et al. 2019) and salt marshes (Möller and Spencer 2002; Möller 2006). Accordingly, seasonal variability in Spartina biomass, as in our study, can be expected to affect wave dissipation capacity of the marsh with a higher contribution of vegetation to wave dissipation in summer than in winter and spring (see Foster-Martinez et al. 2018). Elymus, by contrast, shows minor although significant seasonal differences in biomass, which suggests a more continuous contribution of Elymus biomass to wave dissipation throughout the year. Overall, wave attenuation and resulting coastal protection should be highest when the biomass of biotic structures is at its maximum (Coops et al. 1996; Chen et al. 2007; Koch et al. 2009).
Spatial variability in Spartina biomass between the SSZ and the SLZ shows the same pattern as for stem flexibility with lower values for the SSZ than the SLZ in both seasons. Coops et al. (1994) found similar results with lower biomass in an exposed site compared to a sheltered site for two helophytes. Furthermore, a biomass decrease down an elevational gradient was observed. We assume that higher wave action and higher physiological stress due to salinity and longer inundation time in the SSZ compared to the SLZ explain the significantly lower biomass in Spartina (see also Huckle et al. 2000). The lower biomass amounts in the SSZ zone seem to correlate with a decrease in stem diameter and length accompanied by a higher flexibility in this zone compared to the SLZ. Stem length of different Spartina populations were studied previously by Thompson (1990), where plants sampled from the pioneer populations had significantly smaller inflorescence sizes and vegetative statures in comparison with plants from higher marsh elevations which is consistent with our results. In contrast to Spartina, we found no spatial variability in Elymus biomass. This implies a spatially stable contribution of the Elymus canopy to wave dissipation.
Stem density
Significant seasonal differences in stem density were found for Spartina and Elymus. Spartina stem densities were higher in summer than in spring, whereas Elymus showed higher stem densities in spring compared to summer. The high stem densities in Spartina during summer correlate with high biomass amounts in summer. This pattern in Spartina biomass and stem density confirms results of Hill (1984) and Neumeier (2005). Carus et al. (2016) found lower stem densities at the marsh edge for B. maritimus, which underpins the previously discussed strategies of plants in coastal habitats to cope with mechanical stress induced by hydrodynamic forces. In contrast, high stem densities in Elymus in spring seem to be negatively correlated with biomass. Similar patterns have been reported by Morris and Haskin (1990) for Spartina alterniflora. Numerous studies report that variation in plant stem density affects flow velocity and wave dissipation (Bouma et al. 2005; Widdows et al. 2008; Peralta et al. 2008; Anderson and Smith 2014). Paul and Amos (2011) found highest wave dissipation in seagrass beds in summer, when stem density was high. Increasing stem densities in Spartina tussocks with decreasing elevations, as found in our study, were previously observed for Spartina densiflora and S. anglica (Nieva et al. 2005; Van Hulzen et al. 2007). Variability in stem density affects hydrodynamic energy within the Spartina canopy (Neumeier and Ciavola 2004; Bouma et al. 2005). Van Hulzen et al. (2007) suggest that high stem densities at lower elevations may thus enhance sediment accretion within the canopy. In turn, high accretion rates can enhance growth of Spartina (Hemminga et al. 1998), but it is still not resolved which factor induces the increased stem densities at lower elevations (Van Hulzen et al. 2007).
Implications of seasonal and spatial variability in biophysical properties
The data presented here show that biophysical properties of salt-marsh plants may differ between seasons and change over small spatial scales, which is probably related to the strength of hydrodynamic forcing, inundation frequency, sedimentation rates and soil properties. Our results support the assumption of seasonal and spatial non-linearity in the delivery of ecosystem services such as coastal protection by vegetation (Koch et al. 2009). This finding has to be taken into account when regarding the coastal protection potential of salt-marsh vegetation. Furthermore, the data provided can be used to incorporate salt-marsh plants, entire canopies and plant surrogates more realistically in numerical and physical models describing the interaction between vegetation and hydrodynamics. Models and flume experiments should incorporate seasonal variability in plant biophysical properties, especially when simulating storm surge conditions that occur in the winter season when vegetation is degenerated. Future research should provide measurements of biophysical plant properties over the course of the year to get a better overall picture of the change of these properties.
Furthermore, spatial variability in biophysical properties within the pioneer and low marsh zone (e.g. lower biomass, lower flexural rigidity but higher stem density in Spartina growing at the marsh edge compared to Spartina growing more landwards) should be considered and incorporated in models predicting wave attenuation. High marshes by contrast, show spatially more homogenous biophysical properties and can therefore be represented as one coherent zone. When data on stem flexibility are needed, stem diameter can be used as a proxy for flexibility as bending measurements are often time consuming. Whether this is appropriate for other species than Spartina and Elymus needs to be tested in further studies.
References
Anderson ME, Smith JM (2014) Wave attenuation by flexible, idealized salt marsh vegetation. Coast Eng 83:82–92. https://doi.org/10.1016/j.coastaleng.2013.10.004
Bellis VJ, Gaither CA (1985) Seasonality of aboveground and belowground biomass for six salt marsh plant species. J Elisha Mitchell Sci Soc 101:95–109
Bockelmann AC, Neuhaus R (1999) Competitive exclusion of Elymus athericus from a high-stress habitat in a European salt marsh. J Ecol 87:503–5013. https://doi.org/10.1046/j.1365-2745.1999.00368.x
Bouma TJ, De Vries MB, Low E et al (2005) Trade-offs related to ecosystem engineering: a case study on stiffness of emerging macrophytes. Ecology 86:2187–2199. https://doi.org/10.1890/04-1588
Bouma TJ, De Vries MB, Herman PMJ (2010) Comparing ecosystem engineering efficiency of two plant species with contrasting growth strategies. Ecology 91:2696–2704. https://doi.org/10.1890/09-0690.1
Carus J, Paul M, Schröder B (2016) Vegetation as self-adaptive coastal protection: reduction of current velocity and morphologic plasticity of a brackish marsh pioneer. Ecol Evol 6:1579–1589. https://doi.org/10.1002/ece3.1904
Chen S-N, Sanford LP, Koch EW et al (2007) A nearshore model to investigate the effects of seagrass bed geometry on wave attenuation and suspended sediment transport. Estuaries Coasts 30:296–310
Chung C-H (1993) Thirty years of ecological engineering with Spartina plantations in China. Ecol Eng 2:261–289. https://doi.org/10.1016/0925-8574(93)90019-C
Coops HG, van der Velde G (1996) Effects of waves on helophyte stands: mechanical characteristics of stems of Phragmites australis and Scirpus lacustris. Aquat Bot 53:175–185. https://doi.org/10.1016/0304-3770(96)01026-1
Coops HN, Geilen N, van der Velde G (1994) Distribution and growth of the helophyte species Phragmites australis and Scirpus lacustris in water depth gradients in relation to wave exposure. Aquat Bot 48:273–284. https://doi.org/10.1016/0304-3770(94)90020-5
Coops H, Geilen N, Verheij HJ et al (1996) Interactions between waves, bank erosion and emergent vegetation: an experimental study in a wave tank. Aquat Bot 53:187–198. https://doi.org/10.1016/0304-3770(96)01027-3
Feagin RA, Irish JL, Möller I et al (2011) Short communication: engineering properties of wetland plants with application to wave attenuation. Coast Eng 58:251–255. https://doi.org/10.1016/j.coastaleng.2010.10.003
Fonseca MS, Koehl MAR, Kopp BS (2007) Biomechanical factors contributing to self-organization in seagrass landscapes. J Exp Mar Bio Ecol 340:227–246. https://doi.org/10.1016/j.jembe.2006.09.015
Foster-Martinez MR, Lacy JR, Ferner MC, Variano EA (2018) Wave attenuation across a tidal marsh in San Francisco Bay. Coast Eng 136:26–40. https://doi.org/10.1016/j.coastaleng.2018.02.001
Harder DL, Hurd CL, Speck T (2006) Comparison of mechanical properties of four large, wave-exposed seaweeds. Am J Bot 93:1426–1432. https://doi.org/10.3732/ajb.93.10.1426
Hemminga MA, van Soelen J, Maas YEM (1998) Biomass production in pioneer Spartina anglica patches: evidence for the importance of seston particle deposition. Estuar Coast Shelf Sci 47:797–805. https://doi.org/10.1006/ecss.1998.0388
Heuner M, Silinski A, Schoelynck J et al (2015) Ecosystem engineering by plants on wave-exposed intertidal flats is governed by relationships between effect and response traits. PLoS One 10:e0138086. https://doi.org/10.1371/journal.pone.0138086
Hill MI (1984) Population studies on the Dee estuary. In: Doody P (ed) Spartina anglica in Great Britain, report no. 5. Nature Conservancy Council, Peterborough, pp 53–58
Huckle JM, Potter JA, Marrs RH (2000) Influence of environmental factors on the growth and interactions between salt marsh plants: effects of salinity, sediment and waterlogging. J Ecol 88:492–505. https://doi.org/10.1046/j.1365-2745.2000.00464.x
Koch EW, Barbier EB, Silliman BR et al (2009) Non-linearity in ecosystem services: temporal and spatial variability in coastal protection. Front Ecol Environ 7:29–37. https://doi.org/10.1890/080126
Kunz H, Panten A (1997) Die Köge Nordfrieslands. Nordfriisk Instituut, Bredstedt
Luhar M, Nepf HM (2011) Flow-induced reconfiguration of buoyant and flexible aquatic vegetation. Limnol Oceanogr 56:2003–2017. https://doi.org/10.4319/lo.2011.56.6.2003
McGuinness KA (2002) Of rowing boats, ocean liners and tests of the ANOVA homogeneity of variance assumption. Austral Ecol 27:681–688. https://doi.org/10.1046/j.1442-9993.2002.01233.x
Mendez FJ, Losada IJ (2004) An empirical model to estimate the propagation of random breaking and nonbreaking waves over vegetation fields. Coast Eng 51:103–118. https://doi.org/10.1016/j.coastaleng.2003.11.003
Miler O, Albayrak I, Nikora V, O’Hare M (2012) Biomechanical properties of aquatic plants and their effects on plant–flow interactions in streams and rivers. Aquat Sci 74:31–44. https://doi.org/10.1007/s00027-011-0188-5
Miler O, Albayrak I, Nikora V, O’Hare M (2014) Biomechanical properties and morphological characteristics of lake and river plants: implications for adaptations to flow conditions. Aquat Sci 76(465–481):1–17. https://doi.org/10.1007/s00027-014-0347-6
Möller I (2006) Quantifying saltmarsh vegetation and its effect on wave height dissipation: results from a UK East coast saltmarsh. Estuar Coast Shelf Sci 69:337–351. https://doi.org/10.1016/j.ecss.2006.05.003
Möller I, Spencer T (2002) Wave dissipation over macro-tidal saltmarshes: effects of marsh edge typology and vegetation change. J Coast Res 36:506–521
Möller I, Kudella M, Rupprecht F et al (2014) Wave attenuation over coastal salt marshes under storm surge conditions. Nat Geosci 7:727–731. https://doi.org/10.1038/ngeo2251
Morris JT, Haskin B (1990) A 5-year record of aerial primary production and stand characteristics of Spartina alterniflora. Ecology 71:2209–2217
Mueller P, Granse D, Nolte S et al (2017) Top-down control of carbon sequestration: grazing affects microbial structure and function in salt marsh soils: Grazing. Ecol Appl 27:1435–1450. https://doi.org/10.1002/eap.1534
Mueller P, Ladiges N, Jack A et al (2019) Assessing the long-term carbon-sequestration potential of the semi-natural salt marshes in the European Wadden Sea. Ecosphere 10(1):e02556. https://doi.org/10.1002/ecs2.2556
Mullarney JC, Henderson SM (2010) Wave-forced motion of submerged single-stem vegetation. J Geophys Res Ocean 115:C12061. https://doi.org/10.1029/2010JC006448
Müller F, Struyf E, Hartmann J et al (2013) A comprehensive study of silica pools and fluxes in wadden sea salt marshes. Estuaries Coasts 36:1150–1164. https://doi.org/10.1007/s12237-013-9621-4
Narayan S, Beck MW, Reguero BG et al (2016) The effectiveness, costs and coastal protection benefits of natural and nature-based defences. PLoS One 11(5):e0154735. https://doi.org/10.1371/journal.pone.0154735
Nehring S, Adsersen H (2006) NOBANIS—invasive alien species fact sheet Spartina anglica. From online database eur netw invasive alien species—NOBANIS. www.nobanis.org. Accessed 10/01/2015
Nehring S, Hesse K-J (2008) Invasive alien plants in marine protected areas: the Spartina anglica affair in the European Wadden Sea. Biol Invasions 10:937–950. https://doi.org/10.1007/s10530-008-9244-z
Neumeier U (2005) Quantification of vertical density variations of salt-marsh vegetation. Estuar Coast Shelf Sci 63:489–496. https://doi.org/10.1016/j.ecss.2004.12.009
Neumeier U, Ciavola P (2004) Flow resistance and associated sedimentary processes in a Spartina maritima salt-marsh. J Coast Conserv 20:435–447. https://doi.org/10.2112/1551-5036(2004)020%5b0435:fraasp%5d2.0.co;2
Nieva FJJ, Castellanos EM, Castillo JM, Enrique Figueroa M (2005) Clonal growth and tiller demography of the invader cordgrass Spartina densiflora Brongn. At two contrasting habitats in SW European salt marshes. Wetlands 25:122–129. https://doi.org/10.1672/0277-5212(2005)025%5b0122:cgatdo%5d2.0.co;2
Nolte S, Koppenaal EC, Esselink P et al (2013) Measuring sedimentation in tidal marshes: a review on methods and their applicability in biogeomorphological studies. J Coast Conserv 17:301. https://doi.org/10.1007/s11852-013-0238-3
Ostendorp W (1995) Estimation of mechanical resistance of lakeside Phragmites stands. Aquat Bot 51:87–101. https://doi.org/10.1016/0304-3770(95)00470-K
Patterson MR, Harwell MC, Orth LM, Orth RJ (2001) Biomechanical properties of the reproductive shoots of eelgrass. Aquat Bot 69:27–40
Paul M, Amos CL (2011) Spatial and seasonal variation in wave attenuation over Zostera noltii. J Geophys Res 116:C08019. https://doi.org/10.1029/2010JC006797
Paul M, Henry P-YT, Thomas RE (2014) Geometrical and mechanical properties of four species of northern European brown macroalgae. Coast Eng 84:73–80. https://doi.org/10.1016/j.coastaleng.2013.11.007
Paul M, Rupprecht F, Möller I et al (2016) Plant stiffness and biomass as drivers for drag forces under extreme wave loading: a flume study on mimics. Coast Eng 117:70–78. https://doi.org/10.1016/j.coastaleng.2016.07.004
Peralta G, van Duren LA, Morris EP, Bouma TJ (2008) Consequences of shoot density and stiffness for ecosystem engineering by benthic macrophytes in flow dominated areas: a hydrodynamic flume study. Mar Ecol Prog Ser 368:103–115. https://doi.org/10.3354/meps07574
Petersen J, Kers B, Stock M (2013) TMAP—typology of coastal vegetation in the Wadden Sea area. CommonWadden Sea Secretariat, Wilhelmshaven
Puijalon S, Bouma TJ, Douady CJ et al (2011) Plant resistance to mechanical stress: evidence of an avoidance-tolerance trade-off. New Phytol 191:1141–1149. https://doi.org/10.1111/j.1469-8137.2011.03763.x
QGIS Development Team (2014) QGIS Geographic Information System. Open Source Geospatial Foundation Project
Rupprecht F, Möller I, Evans B et al (2015a) Biophysical properties of salt marsh canopies—quantifying plant stem flexibility and above ground biomass. Coast Eng 100:48–57. https://doi.org/10.1016/j.coastaleng.2015.03.009
Rupprecht F, Wanner A, Stock M, Jensen K (2015b) Succession in salt marshes—large-scale and long-term patterns after abandonment of grazing and drainage. Appl Veg Sci 18:86–98. https://doi.org/10.1111/avsc.12126
Rupprecht F, Möller I, Paul M et al (2017) Vegetation-wave interactions in salt marshes under storm surge conditions. Ecol Eng 100:301–315. https://doi.org/10.1016/j.ecoleng.2016.12.030
Schoutens K, Heuner M, Minden V et al (2019) How effective are tidal marshes as nature-based shoreline protection throughout seasons? Limnol Oceanogr. https://doi.org/10.1002/lno.11149
Silinski A, Heuner M, Schoelynck J et al (2015) Effects of wind waves versus ship waves on tidal marsh plants: a flume study on different life stages of Scirpus maritimus. PLoS One 10:e0118687. https://doi.org/10.1371/journal.pone.0118687
Silinski A, Schoutens K, Puijalon S et al (2018) Coping with waves: plasticity in tidal marsh plants as self-adapting coastal ecosystem engineers. Limnol Oceanogr 63:799–815. https://doi.org/10.1002/lno.10671
Smith JM, Bryant MA, Wamsley TV (2016) Wetland Buffers 854:847–854. https://doi.org/10.1002/esp.3904
Stewart HL (2006) Hydrodynamic consequences of flexural stiffness and buoyancy for seaweeds: a study using physical models. J Exp Biol 209:2170–2181. https://doi.org/10.1242/jeb.02254
Sutton-Grier AE, Gittman RK, Arkema KK et al (2018) Investing in natural and nature-based infrastructure: building better along our coasts. Sustainability 10(2):523. https://doi.org/10.3390/su10020523
Tempest JA, Möller I, Spencer T (2015) A review of plant-flow interactions on salt marshes: the importance of vegetation structure and plant mechanical characteristics: salt marsh plant-flow interactions. Wiley Interdiscip Rev: Water 2(6):669–681. https://doi.org/10.1002/wat2.1103
Thompson JD (1990) Morphological variation among natural populations of Spartina anglica. In: Gray AJ, Benham PEM (ed) Spartina anglica – a Research Review. Institute of Terrestrial Ecology, Wareham, England, pp 26–33
Valéry L, Bouchard V, Lefeuvre J-C (2004) Impact of the invasive native species Elymus athericus on carbon pools in a salt marsh. Wetlands 24:268–276
van der Meer JW (2002) Technisch rapport Golfoploop en Golfoverslag bij Dijken. Technische Adviescommissie voor de Waterkeringen, Delft, p 44
van Hulzen JB, Van Soelen J, Bouma TJ (2007) Morphological variation and habitat modification are strongly correlated for the autogenic ecosystem engineer Spartina anglica (common cordgrass). Estuaries Coasts 30:3–11. https://doi.org/10.1007/BF02782962
van Loon-Steensma JM, Van HuZ, Slim PA (2016) Modelled impact of vegetation heterogeneity and salt-marsh zonation on wave damping. J Coastal Res 32:241–252. https://doi.org/10.2112/JCOASTRES-D-15-00095.1
Veeneklaas RM, Dijkema KS, Hecker N, Bakker JP (2013) Spatio-temporal dynamics of the invasive plant species Elytrigia atherica on natural salt marshes. Appl Veg Sci 16:205–216. https://doi.org/10.1111/j.1654-109X.2012.01228.x
Widdows J, Pope ND, Brinsley MD (2008) Effect of Spartina anglica stems on near-bed hydrodynamics, sediment erodability and morphological changes on an intertidal mudflat. Mar Ecol Prog Ser 362:45–57. https://doi.org/10.3354/meps07448
Ysebaert T, Yang SL, Zhang L et al (2011) Wave attenuation by two contrasting ecosystem engineering salt marsh macrophytes in the intertidal pioneer zone. Wetlands 31:1043–1054. https://doi.org/10.1007/s13157-011-0240-1
Acknowledgements
The authors would like to thank Annika Krull, Julian Gührs and Kay Sellenschloh, Technical University of Hamburg-Harburg, for providing a beam loading device and for giving helpful advice on measurements of plant stem flexibility. We thank Peter Mueller for valuable comments on our manuscript and Svenja Reents for fieldwork support. We further thank the Wadden Sea National Park Schleswig–Holstein for cooperation and two anonymous reviewers for their valuable comments to improve the overall quality of this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Schulze, D., Rupprecht, F., Nolte, S. et al. Seasonal and spatial within-marsh differences of biophysical plant properties: implications for wave attenuation capacity of salt marshes. Aquat Sci 81, 65 (2019). https://doi.org/10.1007/s00027-019-0660-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00027-019-0660-1