Abstract
Lakeshore residential development (LRD) reduces coarse woody habitat (CWH) in lakes resulting in negative effects on fishes. We tested whether the addition of CWH could reverse those effects. We added CWH to Camp Lake, a lake with CWH abundances similar to developed lakes, following 2 years of study of the fish populations in the reference and treatment basins. Both basins were monitored for 4 years following the manipulation. Specifically, we tested for changes in the population dynamics (densities, size-structure, growth), diet, and behavior (habitat use) of bluegill (Lepomis macrochirus) and largemouth bass (Micropterus salmoides). CWH addition had no discernible effect on fish population dynamics. Diet and behavioral responses were more pronounced in the treatment basin. Prey diversity and availability increased. Piscivory increased, with decreased reliance upon terrestrial prey, for largemouth bass. Habitat use was positively correlated with CWH branching complexity and abundance. Our study suggests that negative effects observed in fish populations through CWH reductions cannot be reversed in the short-term by adding CWH. We recommend that regulations governing the LRD process be protective of CWH.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coarse woody habitat (CWH) is a prominent feature of many lakes and can influence several characteristics of aquatic ecosystems, including fish communities. Loss of CWH from lakes has been well documented and is strongly correlated with lakeshore residential development (LRD). Strong, negative relationships exist between LRD and the amount of littoral CWH found in lakes of northern Wisconsin, the Upper Peninsula of Michigan, and the Pacific Northwest (Christensen et al. 1996; Jennings et al. 2003; Francis and Schindler 2006; Marburg et al. 2006). Reductions in CWH as a consequence of LRD have been shown to reduce organic sediments in the littoral zones of lakes and the densities of shredder, benthic macroinvertebrate taxa (Francis et al. 2007). In the case of fishes, losses of CWH are associated with changes in spatial distributions (Scheuerell and Schindler 2004), slower growth rates (Schindler et al. 2000; Sass et al. 2006a), severe depletions of prey fish populations (Sass et al. 2006a), and altered home range sizes and feeding modes used by fishes (Ahrenstorff et al. 2009). Lake urbanization has also been shown to decrease the abundance of terrestrial insects available to fishes (Francis and Schindler 2009). Therefore, understanding how CWH removals affect aquatic communities in the context of the LRD process and how those effects may be remediated has been an important topic in ecology and fisheries management.
To better understand how CWH loss influenced fish communities, Sass et al. (2006a) conducted a whole-lake removal of CWH from Little Rock Lake in northern Wisconsin, which was physically separated into a reference and treatment basin in a before-after-control-impact experimental design (BACI, Stewart-Oaten et al. 1986; Carpenter 1998). The results of Sass et al. (2006a) showed that the CWH removal caused a severe depletion of the yellow perch (Perca flavescens) population, a switch from a piscivorous to a terrestrially dominated diet in largemouth bass (Micropterus salmoides), and depressed growth rates in largemouth bass in the treatment basin relative to the unmanipulated reference basin.
Following the experiment in Little Rock Lake (Sass et al. 2006a), we questioned whether the effects of CWH removal on fish populations could be remediated by adding CWH to lakes. To address this question, we conducted another ecosystem experiment by adding CWH to a basin of Camp Lake. Camp Lake lies in close proximity to Little Rock Lake (~3.2 km), shares a similar fish community and limnological attributes, is naturally separated into a reference and treatment basin, and naturally maintains low levels of CWH compared to other undeveloped northern Wisconsin lakes. We hypothesized that the CWH addition would reverse the negative effects observed on fish populations and communities with CWH loss. We specifically tested for the effects of the CWH addition on the population dynamics (density, size-structure, growth), diet, and behavior (habitat use) of bluegill (Lepomis macrochirus) and largemouth bass by collecting 2 years of pre-manipulation data and 4 years of post-manipulation data on these attributes of the fish populations.
Methods
Study site
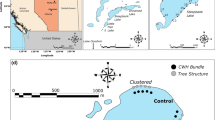
We conducted a whole-lake addition of CWH to Camp Lake, Vilas County, Wisconsin (45°59′58.29″N, 89°43′48.53″W) to test for the influence of littoral habitat additions on the fish populations. Camp Lake is an oligotrophic, seepage lake that has no LRD and receives minimal fishing pressure. Camp Lake consistently maintains Secchi disc transparencies >5 m and has a maximum depth of 10 m. Natural littoral structure (e.g., submersed and emergent aquatic vegetation, CWH) is sparse in Camp Lake. Pre-manipulation CWH abundances in Camp Lake were low (~40 logs km−1 of shoreline) and similar to developed lakes in the Northern Highlands Lake District of Wisconsin (Christensen et al. 1996; Marburg et al. 2006; Sass et al. 2006a) (Fig. 1a). The fish community of Camp Lake is dominated by bluegill and largemouth bass, with small populations of yellow perch, johnny darter (Etheostoma nigrum), and Iowa darter (Etheostoma exile) also present.
Camp Lake was an ideal location for this ecosystem experiment because it is naturally separated into a reference and treatment basin by a shallow, narrow channel. We did not attempt to permanently disconnect the basins in this study; inter-basin movement of fishes was negligible based upon a tagging study (<1%, 6 of 820 tagged fish) and a radio telemetry study of largemouth bass (Ahrenstorff et al. 2009). We designated the north basin of Camp Lake as the reference basin (CR, 8.5 ha) and the south basin as the treatment basin (CT, 17.6 ha) (Fig. 1). In the spring of 2004, we added over 300 trees (~1 log10 m−1 of shoreline) to the littoral zone of CT, thus increasing CWH abundances from 41 to 141 logs km−1 of shoreline (Fig. 1). We conducted the CWH addition in late March—early April prior to ice-out. Each tree was placed on the ice perpendicular to the shoreline at 10 m intervals and anchored by five cinder blocks (Fig. 2). During ice-out, all added CWH sank at the designated location. We also constructed two 50 m transects that received double the amount of CWH (2 logs 10 m−1 of shoreline) for a study of fish habitat use described below. Added CWH (>10 cm in diameter) consisted of various deciduous and coniferous trees. We qualitatively scored added CWH for branching complexity on a 0–3 scale with 0 indicating CWH with no branches and three indicating a full crown. Of the added CWH, 8.6% of the CWH scored 0, 20.4% scored 1, 28.4% scored 2, and 42.6% scored 3. The reference basin of Camp Lake remained unchanged (~40 logs km−1 of shoreline) throughout the study (Fig. 1). Our experiment was terminated after the 2007 field season. Drought conditions in the Northern Highlands Lake District had greatly decreased water levels in the region’s seepage lakes and much of our added CWH was out of the water. From 2004 to 2007, all added CWH was submerged and available as structural habitat for fishes. In summer 2008, all cinder blocks were removed from CT and donated to area lake associations to create CWH structures in their lakes.
Photograph of Scott Van Egeren transporting coarse woody habitat (CWH) across the ice with an ATV on the treatment basin of Camp Lake (CT) in the spring of 2004 (a). Photograph of CWH added along the shoreline of CT prior to ice out in the spring of 2004 (b). Photographs courtesy of Michelle Woodford
Population dynamics responses
Pre- and post-CWH addition monitoring of the fish populations occurred in May–September, 2002–2003 and 2004–2007, respectively. We calculated Chapman-modified, continuous Schnabel mark-recapture population estimates annually for bluegill and largemouth bass in CT and CR (Ricker 1975). The Chapman-modified, continuous Schnabel mark-recapture population estimation procedure was given by:
where N was the population estimate, C t was the number of fish captured, M t was the number of marked fish at large, and R was the number of recaptured fish. Ninety-five percent confidence intervals of the population estimate were calculated using a Poisson distribution (Ricker 1975). All largemouth bass were captured by hook-and-line angling because the low conductivity of Camp Lake precluded effective electrofishing. Each captured bass was measured to the nearest mm and received an individually numbered Floy® tag if >150 mm. Bass <150 mm received a basin-specific fin clip. Bluegill were captured by hook-and-line angling and beach seining. All captured bluegill were measured to the nearest mm and given a basin-specific fin clip. All available sizes of bass and bluegill captured were marked and included in our population estimates. To test for effects of the CWH addition on fish abundances, we used linear regression and analyzed whether the 95% confidence intervals of individual population estimates overlapped among basins in a given year. We used the null hypothesis of no change in fish abundances over time at the α = 0.05 level for the regression analyses. In the context of this study, effects of the manipulation were inferred if patterns in CT were different from those observed in CR. For example, a significant effect of the manipulation would be concluded if largemouth bass densities increased in CT after CWH was added, while largemouth bass densities remained the same or declined in CR.
We calculated the proportional size distribution (PSD) for bluegill and largemouth bass annually in each basin based upon the length information of the populations collected during the mark-recapture population estimation procedure (Guy et al. 2007). PSD is a useful assessment tool for monitoring the responses of fish populations because it simplifies size-structure to a single percentage (Gabelhouse 1984). PSD was given by:
where stock and quality length for bluegill and largemouth bass were 80 and 150 mm and 200 and 300 mm, respectively (Gabelhouse 1984). To test for the effects of the CWH addition on PSD, we used simple linear regression. We used the null hypothesis of no change in PSD over time at the α = 0.05 level.
We examined body condition and size-specific growth rates of bluegill and largemouth bass to test for effects of the CWH addition on fish growth. Body condition was calculated for all fish collected for the diet analyses because length and weight were recorded. Body condition was calculated using standard weight (W s) equations developed for bluegill and largemouth bass (Wege and Anderson 1978; Hillman 1982). The minimum total lengths used for the relative weight analyses for bluegill and largemouth bass were 80 and 150 mm, respectively (Wege and Anderson 1978; Hillman 1982). We used t tests (BACI) to determine whether significant differences in body condition for bluegill and largemouth bass were evident among pre- and post-manipulation time periods within and among basins. We used the null hypothesis of no difference in body condition within and among each basin and time period at the α = 0.05 level.
Fish scales were used to determine size-specific growth rates for bluegill and largemouth bass. Our methods for determining size-specific growth rates and statistical analyses can be found in Schindler et al. (2000). Size-specific growth rates have greater statistical power than other indicators to detect effects of habitat manipulations (Carpenter et al. 1995). Annually, we collected scales from behind the pectoral fin from 5 individual fish of each species for every available 10 mm increment of length (e.g. 100–109, 110–119 mm, etc.) captured. Bluegill and largemouth bass scales were pressed between glass slides and photographed with a Polaroid DMC-2 digital camera. Scales were read using a Fishomatic optical imaging system developed by the Center for Limnology at the University of Wisconsin-Madison to determine an individual’s growth rate in the previous year. Growth was determined by the Fraser-Lee method of back-calculating the length of the previous year. We then regressed loge growth rate (mm year−1, dependent variable) on fish size (mm, independent variable) for each species in each year of the study to determine mean growth rates for four common size classes of bluegill (60, 100, 140, 180 mm total length) and largemouth bass (100, 200, 300, 400 mm total length) (Carlander 1982; Schindler et al. 2000). Only one size-specific growth rate was calculated from each individual fish. We tested for effects of the CWH addition on bluegill and largemouth bass size-specific growth rates by identifying patterns that opposed one another before and after the manipulation. For example, a manipulation effect on fish growth would be inferred if size-specific growth rates of the 100 mm length class of largemouth bass increased after the CWH addition in CT, but did not change or decreased for the same length class in CR.
Diet responses
We collected diet information from bluegill and largemouth bass at biweekly intervals during May–September, 2002–2007 in CT and CR. All fish used for diet analyses were collected by hook-and-line angling. We determined diet composition by performing gastric lavage on at least 10 bluegill and largemouth bass (range 10–20) on each sampling occasion in CT and CR (Seaburg 1957; Hodgson and Kitchell 1987). We recorded length to the nearest mm and weight to the nearest g for all fish sampled for diet contents. Diet items were preserved in 95% ethanol and later analyzed in the laboratory. Diet items were separated into major taxonomic categories (e.g., Amphipoda, Coleoptera, Diptera, fish, Odonata, terrestrial invertebrate, terrestrial vertebrate, Trichoptera), enumerated, and dried to determine the dry mass proportion of each prey item in the diet. An index of absolute importance (IAI) was calculated for each prey item and was given by:
where %N was the percentage of the total number of food items represented by food type a, %W the percentage weight (grams of dry biomass) of each food item a of the total weight of foods eaten, and FO was the frequency of occurrence of each food type a (the percentage of fish that eat that food type) (George and Hadley 1979). We then used IAI values to calculate an index of relative importance (IRI) for each prey item:
where a is the specific food item and the summation in the denominator is taken over all food items. The range of IRI values for any diet was 0–100 (Hodgson and Kitchell 1987). We calculated diet breadth (B) for bluegill and largemouth bass in each basin following Levins (1968):
where pi is the fraction of total diet mass represented by item i and the summation is over 1. This index is minimized at 1.0 when only one prey type is found in the diet and is maximized at n, where n is the total number of prey types, each representing an equal proportion of the diet (Schindler et al. 1997). We tested for differences in the diet metrics of bluegill and largemouth bass in response to the CWH addition by comparing pre- and post-dry mass diet proportions in each basin. We also tested for patterns in the metrics of diet breadth, the percentage of empty stomachs, and average dry mass per individual fish diet among basins and time periods. Opposing patterns in the diet metrics among basins after the CWH addition in CT would indicate a manipulation effect.
Behavioral responses
We used snorkeling surveys to test for a relationship among bluegill and largemouth bass habitat use along a gradient of CWH branching complexities (surveyed in 2004) and abundances (surveyed in 2005). Both tests were conducted post-CWH addition. An individual snorkeler surveyed each piece of new CWH biweekly from June to August, 2004 and recorded the number of bluegill and largemouth bass observed on each piece of structure. We used regression to test for a relationship among the average number of fish observed and CWH branching complexity with the null hypothesis of no change in fish use with increasing levels of CWH branching complexity at the α = 0.05 level. We also designated ten 50 m transects that varied in CWH abundances to test for habitat use patterns by bluegill and largemouth bass. Four transects had 0 logs 50 m−1 (CR), four had 5 logs 50 m−1 (CT), and two had 10 logs 50 m−1 (CT) of shoreline. Each transect was snorkeled by three people at separate depths (shoreline, 0.5 m, and 1 m) biweekly during June–August, 2005. During snorkeling surveys, each person recorded the number and species of fish observed in each transect. We used a one-way ANOVA to test for differences in the average number of bluegill and largemouth bass observed in each of the three treatment transects (0 CWH 50 m−1, 5 CWH 50 m−1, 10 CWH 50 m−1) with the null hypothesis of no difference in the number of fish observed among transects at the α = 0.05 level.
Results
Population dynamics responses
Bluegill densities in each basin of Camp Lake increased coherently from 2002 to 2007 (Fig. 3). Largemouth bass densities increased, but did not differ significantly among basins or over time within basins (Fig. 3). Bluegill densities were significantly greater in CR compared to CT in 2004, while largemouth bass densities were not significantly different among basins in any year based upon overlap of the 95% confidence intervals of the density estimates (Fig. 3). Increases in bluegill densities were statistically significant within basins over time [CR; bluegill density (no. ha−1) = 1,322 × year − 0.000003; n = 5; df = 1, 3; f = 18; p = 0.024; CT; bluegill density (no. ha−1) = 1973.4 × year − 0.000004; n = 5; df = 1, 3; f = 18.4, p = 0.023]. The year effect explained 86% of the variability in bluegill densities over time in each basin. Largemouth bass densities in each basin did not change over time (p > 0.05).
Population estimates (no. ha−1) and 95% confidence intervals for largemouth bass (Micropterus salmoides) (LMB) and bluegill (Lepomis macrochirus) (BGL) in the treatment (closed circles) and reference basins (open circles) of Camp Lake during 2002–2007. The coarse woody habitat addition to CT was completed prior to the 2004 field season. Please note that some upper 95% confidence intervals exceed the limits of the y axis
PSD for bluegill declined, while largemouth bass PSD declined and then increased in each basin over time (Fig. 4). No statistically significant change was detected for bluegill and largemouth bass PSD in each basin over time (p > 0.05). In CR, average bluegill and largemouth bass PSD decreased from 59.5 to 53% and 46.5 to 36.75%, respectively, prior to and following the whole-lake addition of CWH to CT. Mean bluegill PSD decreased from 62.5 to 45.5% and mean largemouth bass PSD declined from 57 to 39% in CT.
Influences of the CWH addition on bluegill and largemouth bass body condition and size-specific growth rates were variable (Table 1). No statistically significant differences were observed for bluegill body condition within each basin before or after the manipulation (p > 0.05) or among basins in each time period (p > 0.05). Largemouth bass body condition was significantly greater in CT compared to CR after the CWH addition (df = 339, t = 4.62, p < 0.001). Average body condition of largemouth bass was 6% greater in CT compared to CR in the post-manipulation time period. Body condition of largemouth bass decreased significantly in CR among time periods (78 to 73%) (df = 264, t = 2.71, p = 0.007). No difference in body condition of largemouth bass was observed among time periods in CT (p > 0.05). Trends in size-specific growth rates of bluegill were consistent among basins before and after the CWH addition to CT (Table 1). Opposing trends in size-specific growth rates for the 100 and 200 mm length classes of largemouth bass were observed among basins over time (Table 1). Size-specific growth rates for the smallest length classes of largemouth bass declined in CT following the CWH addition, while growth rates for the same length classes increased in CR.
Diet responses
Changes in bluegill dry mass diet proportions were similar among basins before and after the CWH addition to CT (Fig. 5). Bluegill diets were dominated by odonates, with several other invertebrate taxa comprising the rest of the diet throughout the study. Zooplankton disappeared from bluegill diets in both basins after the manipulation to CT. Fish dominated the dry mass proportion of largemouth bass diets in each basin throughout the study (Fig. 6). A greater proportion of fish were consumed in CT after the CWH addition, with a concurrent decline in terrestrial prey (invertebrates and vertebrates) within the bass diets. Fish consumption increased slightly in CR after the CWH addition to CT; however, the terrestrial proportion of the diet remained relatively consistent among time periods. Opposing patterns were observed among basins in bluegill diet breadth and the percentage of empty stomachs after the manipulation, while mass per diet changed similarly among basins (Table 2). Bluegill diet breadth increased in CT after the CWH addition, but decreased in CR. No change was observed in the percentage of empty bluegill stomachs in CT before and after the manipulation, while the percentage of empty stomachs increased in CR among time periods. Dry mass per bluegill diet decreased among time periods in both basins. Similar patterns in diet breadth, the percentage of empty stomachs, and mass per diet were observed for largemouth bass (Table 2). Largemouth bass diet breadth increased from 1.65 to 1.87 in CT among time periods, while diet breadth decreased from 2.27 to 1.94 in CR. The percentage of empty largemouth bass stomachs in CT decreased by about 10% among time periods. Over the same time periods, the percentage of empty largemouth bass stomachs increased by about 6% in CR. Dry mass per largemouth bass diet decreased among time periods in both basins.
Average dry mass diet proportions for bluegill (Lepomis macrochirus) in the treatment (CT) and reference basins (CR) of Camp Lake prior to (PRE) and after (POST) the coarse woody habitat addition to CT in the spring of 2004. Lepidop. Lepidoptera, T. Inv. terrestrial invertebrate, Trich. Trichoptera, Zoop. zooplankton
Average dry mass diet proportions for largemouth bass (Micropterus salmoides) in the treatment (CT) and reference basins (CR) of Camp Lake prior to (PRE) and after (POST) the coarse woody habitat addition to CT in the spring of 2004. Trich. Trichoptera, T. Inv. terrestrial invertebrate, T Vert. terrestrial vertebrate
Behavioral responses
Spatial distribution patterns along the branching complexity gradient of CWH added to CT were different for bluegill and largemouth bass. The relationship among the mean number of bluegill observations and CWH branching complexity was parabolic with greater numbers of bluegill observed at the lowest and highest CWH branching complexities. The relationship for bluegill observations and CWH branching complexity was best explained by a quadratic model (mean bluegill observations (no.) = 0.7288 × CWH branching complexity2 − 1.6714 × CWH branching complexity + 2.6081, r 2 = 0.99). Mean bluegill observations were about 2.6 at the lowest, 1.8–2.2 at intermediate, and 4.3 bluegill CWH−1 at the greatest branching complexities. The high proportion of variability explained by this model was likely related to the low number of data points used in the analysis (n = 4). Mean largemouth bass observations were positively correlated with CWH branching complexity (mean largemouth bass observations (no.) = 0.203 × CWH branching complexity + 0.302, n = 294, df = 1, 292, f = 17.01, p < 0.001, r 2 = 0.05); however, CWH branching complexity only explained 5% of the variability in the number of largemouth bass observations. Mean largemouth bass observations increased from about 0.2 at the lowest branching complexities to 1.0 largemouth bass CWH−1 at the greatest branching complexities.
More bluegill and largemouth bass were observed in 50 m shoreline transects with greater abundances of CWH, although the greater number of observations of bluegill with increasing densities of CWH was not statistically significant (p > 0.05). Observations of largemouth bass were significantly greater in the 2 logs 10 m−1 transects compared to the 0 and 1 log 10 m−1 transects (ANOVA, 0 vs. 2 logs 10 m−1, df = 1, 52, f = 10.67, p = 0.002; 1 vs. 2 logs 10 m−1, df = 1, 52, f = 6.61, p = 0.01). No difference was found among observations of largemouth bass in the 0 and 1 logs 10 m−1 transects (p > 0.05).
Discussion
Population dynamics responses
Increases in bluegill and largemouth bass densities and declines in PSD for each species were parallel over time in the two basins suggesting no effect of the CWH addition on these characteristics of the centrarchid populations. Adding woody structure to the littoral zones of lakes and reservoirs has been shown to increase black bass (Micropterus spp.) nest densities (Vogele and Rainwater 1975; Hoff 1991; Hunt and Annett 2002; Hunt et al. 2002). If CWH addition to CT directly improved largemouth bass nesting success, as suggested by increases in density and decreases in PSD, we would have expected no change or declines in density and no change or increases in PSD within CR. Because largemouth bass are one of the more ubiquitous freshwater fishes in North America, we were not surprised that CWH was unrelated to increases in density. Increases in largemouth bass densities were more likely related to other abiotic and biotic factors that may have influenced largemouth bass recruitment equivalently among basins. Further, cannibalism within the largemouth bass populations in each basin was rarely observed. Largemouth bass densities in the two basins of Camp Lake were similar to those found in Little Rock Lake (Sass et al. 2006a; Ahrenstorff et al. 2009). Relationships among bluegill spawning habitat, colony site selection, nesting success, and CWH abundances are poorly understood (Spotte 2007). Our results suggest that the CWH addition did not have a direct positive effect on bluegill abundances in CT because responses by the bluegill population in CR were similar.
Declines in PSD of the bluegill and largemouth bass populations over time in both basins suggest that the CWH addition did not have a direct effect on size-structure. Because Camp Lake does receive some fishing pressure, it is more plausible that selective harvest of larger individuals, senescence of stronger and older bluegill and largemouth bass cohorts, and recruitment of juveniles caused the decline in PSD. In many minimally harvested fish populations, size-structure is often dominated by strong cohorts (Warner and Chesson 1985). In the case of Camp Lake, it appears that the larger and more abundant individuals within the bluegill and largemouth bass populations were lost over the course of the study and replaced by juveniles.
Effects of the CWH addition on fish growth showed no clear pattern among basins, with the exception of a significant increase in largemouth bass body condition and slight decreases in size-specific growth rates for the smallest length classes of largemouth bass (100, 200 mm) in CT relative to CR. The increase in body condition of largemouth bass in CT may have resulted from increased consumption of more energetically favorable fish prey. We expected that individual growth rates would be positively correlated with consumption of more energetically favorable prey for largemouth bass. For example, declines in fish consumption by largemouth bass in a CWH removal experiment resulted in decreased individual growth rates (Sass et al. 2006a). Improvements in largemouth bass growth rates were not observed with the addition of CWH to CT, despite increases in fish consumption and a less energetically costly sit-and-wait foraging strategy (Ahrenstorff et al. 2009). Slight decreases in the size-specific growth rates for the smallest length classes of largemouth bass were observed after the CWH addition, which contrasted patterns observed in CR. According to Gaeta et al. (2011), growth rates of largemouth bass <210 mm were positively correlated with LRD. Thus, small largemouth bass growth rates would be negatively correlated with littoral CWH abundance, which supports our observation. Decreased size-specific growth rates of small largemouth bass in our study may have been density-dependent (Gaeta et al. 2011). Our results suggest that factors other than the CWH addition may be more responsible for the patterns observed in fish growth in Camp Lake.
Our growth results contrast those found for bluegill and largemouth bass along a LRD and CWH gradient of lakes by Schindler et al. (2000), for large bass in Gaeta et al. (2011), and for observations reported by Sass et al. (2006a) from a whole-lake CWH removal study. Schindler et al. (2000) showed that growth rates of bluegill and largemouth bass were greater in undeveloped lakes with greater abundances of CWH. The findings of Gaeta et al. (2011) support the observations of Schindler et al. (2000) for the largest size classes of bass examined. Our results may have contrasted with those of Schindler et al. (2000) and Gaeta et al. (2011) because we conducted an experiment testing for short-term responses, whereas their comparative studies revealed patterns that may have been attained after many years. Sass et al. (2006a) reported that growth rates of largemouth bass declined when LRD was simulated by removing CWH from a lake. Our results suggest that the presence/absence of CWH is not solely responsible for explaining patterns in fish growth in Camp Lake, which is not surprising. Short-term fish growth responses to CWH additions may occur more slowly than growth responses to removals. Although the presence/absence of CWH may influence fish growth rates as suggested in previous studies, other factors such as fishing, harvest and length restrictions, the presence/absence of submersed aquatic vegetation, lake productivity, and food web complexity are also likely important.
Diet responses
Dry mass diet proportions of bluegill changed similarly among basins and time periods. Odonates dominated the diets of bluegill in both basins over time. In CT, mollusks, annelids, and zooplankton were replaced in diets by lepidopterans, dipterans, and trichopterans. Because diet breadth of bluegill in CT increased, prey availability and diversity may have improved as a result of the new CWH. The presence of CWH, particularly highly decayed wood, may directly increase macroinvertebrate densities by providing a structural habitat and refuge and indirectly by retaining organic sediments in the littoral zones of lakes (Smokorowski et al. 2006; Francis et al. 2007). In contrast to CT, bluegill consumption of odonates slightly decreased in CR and trichopteran consumption was replaced by terrestrial invertebrates. Bluegill diet breadth declined and the percentage of empty stomachs increased in CR. Therefore, the CWH addition may have increased prey availability and diversity for bluegill in CT without resulting in a decline in the percentage of empty stomachs, despite increases in bluegill densities. The increase in the percentage of empty stomachs observed in bluegill in CR may be a density-dependent response. Such a response may have been dampened in CT by an increase in prey availability and diversity.
The addition of CWH may have decreased the reliance of fishes upon terrestrial prey by increasing the relative abundance of benthic prey. According to Francis and Schindler (2009), increased LRD decreased the terrestrial portion of fish diets. In our experiment, the addition of CWH may have supported increased abundances and diversity of benthic invertebrates to counteract the loss of terrestrial prey; however, this was not tested for specifically. Although Helmus and Sass (2008) did not observe a rapid change in the macroinvertebrate community following CWH removal in Little Rock Lake, several studies suggest that CWH is important for directly or indirectly supporting increased benthic invertebrate abundances and diversity in lakes, rivers, and streams (Benke and Wallace 2003; Smokorowski et al. 2006; Francis et al. 2007). Sass et al. (2006a) found that largemouth bass diets were dominated by terrestrial prey when the majority of CWH was removed from an undeveloped lake. Bluegill reliance on terrestrial prey remained low in CT after the CWH addition, but increased in CR. Therefore, the loss of terrestrial prey associated with LRD and its effects on fishes may be dampened by the addition of CWH (Roth et al. 2007).
Largemouth bass dry mass diet proportions were dominated by fish among basins and over time periods. The magnitude of the increase in fish consumption was greater in CT after the CWH addition compared to CR. Terrestrial prey in largemouth bass diets decreased in CT after the CWH addition relative to CR. The decline in terrestrial prey reliance observed in CT was reciprocal of the response noted by Sass et al. (2006a). Plausible mechanisms leading to an increase in terrestrial prey reliance with CWH loss and a decrease in terrestrial prey reliance with CWH addition are likely different. With CWH removal, terrestrial prey was preferred when yellow perch were reduced in abundance (Sass et al. 2006a). In our CWH addition, the fish forage base was not limiting because bluegill densities increased over time. Decreased reliance upon terrestrial prey was more likely due to a change in largemouth bass foraging behavior (Hodgson and Kitchell 1987; Sass et al. 2006b). Home range sizes of largemouth bass in CT were smaller than CR suggesting the preference of a sit-and-wait foraging strategy (Ahrenstorff et al. 2009). The CWH addition to CT may not only have increased foraging efficiencies for largemouth bass, but also increased overall prey availability and diversity. In contrast to CR, largemouth bass diet breadth increased and the percentage of empty stomachs decreased despite increases in largemouth bass densities. Our results suggest that the CWH addition nullified some of the potential density-dependent constraints observed on the diets and foraging behavior of bluegill and largemouth bass in CR, where CWH abundances were unaltered and low.
Behavioral responses
The number of bluegill and largemouth bass observed in association with littoral CWH was positively correlated with CWH density and branching complexity. Littoral transects containing greater abundances of CWH concentrated more fish to those areas in Camp Lake. Therefore, spatial arrangement of CWH in the littoral zones of lakes may be as, or more important, than the total abundance of CWH in a given lake for fish habitat. Similar to other studies, fish species diversity and the number of fish using structural habitats were positively correlated with CWH branching complexity (Everett and Ruiz 1993; Newbrey et al. 2005). Patterns of habitat use with CWH branching complexity were fish species-specific in CT. More bluegill were observed using the least and most complex CWH. Because largemouth bass use was positively correlated with CWH complexity, the patterns observed for bluegill are likely due to predation risk. Bluegill appeared to prefer less complex structure that largemouth were not using or very complex structure, which may have reduced predation risk (Werner et al. 1977). Selection of habitats that reduce predation risk has been shown in aquatic macrophyte and CWH structural refuges (Savino and Stein 1989; Eklov 1997; Sass et al. 2006b).
We hypothesized that a whole-lake CWH addition would reverse the negative effects associated with CWH loss on fish populations in north temperate lakes. Our hypothesis was supported by reciprocal diet and behavioral responses, but not supported by population dynamics responses. Several limitations of this study may have precluded the detection of CWH addition influences on fish population dynamics. First, we question whether our manipulation added enough CWH to observe responses in fish population dynamics. The manipulated CWH density of CT was still low at 141 logs km−1 of shoreline compared to other undeveloped lakes in the region (Marburg et al. 2006; Sass et al. 2006a). It is possible that we did not add enough CWH to observe responses in fish population dynamics. Nevertheless, our CWH additions may be near the maximum that is feasible within constraints of funding and public tolerance of added trees. It is important to note that branching complexity of the added CWH was likely much greater than that observed in other undeveloped lakes in the region. Second, we acknowledge that this was a short-term study and that effects on the dynamics of fish populations may take longer than 4 years of post-manipulation monitoring to manifest themselves. Unfortunately, drought conditions in northern Wisconsin severely decreased water levels and terminated our experiment early by stranding added CWH. Massive CWH additions and longer studies may result in more discernible population dynamics responses.
Management implications
Ecosystem experiments allow scientists and managers to manipulate a potentially important factor (in this case, CWH abundance) to test whether it influences some aspect(s) of the system in the context of larger-scale ecosystem processes. Our results suggest that other ecosystem processes, besides the addition of CWH, may be more important drivers of Camp Lake fish population dynamics and/or we did not maintain the experiment long enough to observe responses. CWH removal did not affect largemouth bass densities, but had a negative effect on yellow perch abundances (Sass et al. 2006a). Therefore, it is likely that CWH removal or addition will influence fish abundances differently depending upon the fish species assemblage present within the lake. Density-dependent effects may have masked any growth responses that could have been attributed to the CWH addition (Schindler et al. 2000; Sass et al. 2006a; Gaeta et al. 2011). However, it appears more plausible that other ecosystem processes played a larger role in influencing short-term patterns of fish growth in this study. It is likely that the influences of CWH removal and addition on fish growth involve very different processes and subsequent responses.
The results of our study suggest that short-term responses of fish populations to CWH additions may be manifested more in behavioral changes (diet, habitat use) compared to changes in longer-term population dynamics (fish abundance, size-structure, growth). The addition of CWH appeared to increase prey diversity and availability, and foraging efficiency of the fishes examined in this study. Therefore, the addition of CWH may improve fish production by increasing available resources, particularly in oligotrophic systems such as the lake studied here. Because LRD has been shown to decrease terrestrial insect availability to fishes (Francis and Schindler 2009) and CWH abundances in lakes (Christensen et al. 1996; Francis and Schindler 2006; Marburg et al. 2006), additions of CWH may offset the effects of losing terrestrial sources of prey to fishes. CWH additions may also provide structural refuges to sustain forage fish populations and increase foraging efficiency of piscivores. Habitat use by fishes in this study were clearly linked to the spatial distribution, abundance, and branching complexity of CWH. We recommend that these factors be considered when implementing projects using CWH to improve fish habitat. It is possible that tree drops (i.e., felling trees into the littoral zone from intact riparian forests without the use of cinder blocks for anchoring) more naturally simulate additions of CWH to lakes and provide greater CWH complexities than what we were able to achieve in this study.
CWH additions to lakes do not appear to completely remediate the effects on fishes that losses of CWH associated with LRD may cause, at least in the short term. The processes that change fish communities through CWH removal are likely different than the processes necessary for reversing those changes. For example, a fish species extirpated as a consequence of CWH removal cannot simply be replaced by adding CWH. While species extirpations may be the most extreme example, continual thinning of riparian forests and physical removals of CWH by humans may result in adverse effects that may not be fixed by simply adding CWH back to lakes. Although our CWH addition did not remediate all of the negative influences of CWH removals on fish communities, it is important to note that CWH is a natural feature of many lakes and fish and other organisms have evolved in the presence of CWH. Because natural inputs of CWH are being altered by LRD (e.g., riparian forest thinning) (Roth et al. 2007; Marburg et al. 2006), we suggest that intact riparian forests be conserved in order to contribute natural CWH inputs to lakes.
References
Ahrenstorff TD, Sass GG, Helmus MR (2009) The influence of littoral zone coarse woody habitat on home range size, spatial distribution, and feeding ecology of largemouth bass (Micropterus salmoides). Hydrobiologia 623:223–233
Benke AC, Wallace JB (2003) Influence of wood on invertebrate communities in streams and rivers. In: Gregory S, Boyer K, Gurnell A (eds) The ecology and management of wood in world rivers. American Fisheries Society, Bethesda, pp 149–177
Carlander KD (1982) Standard intercepts for calculating lengths from scale measurements for some centrarchid and percid fishes. Trans Am Fish Soc 111:332–336
Carpenter SR (1998) The need for large-scale experiments to assess and predict the response of ecosystems to perturbation. In: Pace ML, Groffman PM (eds) Successes, limitations and frontiers in ecosystem science. Springer, New York, pp 287–312
Carpenter SR, Cunningham P, Gafny S, Munoz del Rio A, Nibbelink N, Olson M, Pellet T, Storlie C, Trebitz A (1995) Responses of bluegill to habitat manipulations: power to detect effects. N Am J Fish Manage 15:519–527
Christensen DL, Herwig BR, Schindler DE, Carpenter SR (1996) Impacts of lakeshore development on coarse woody debris in north temperate lakes. Ecol Appl 6:1143–1149
Eklov P (1997) Effects of habitat complexity and prey abundance on the spatial and temporal distributions of perch (Perca fluviatilis) and pike (Esox lucius). Can J Fish Aquat Sci 54:1520–1531
Everett RA, Ruiz GM (1993) Coarse woody debris as a refuge from predation in aquatic communities—an experimental test. Oecologia 93:475–486
Francis TB, Schindler DE (2006) Degradation of littoral habitats by residential development: woody debris in lakes of the Pacific Northwest and Midwest. United States Ambio 35:274–280
Francis TB, Schindler DE (2009) Shoreline urbanization reduces terrestrial insect subsidies to fishes in North American lakes. Oikos 118:1872–1882
Francis TB, Schindler DE, Fox JM, Seminet-Reneau E (2007) Effects of urbanization on the dynamics of organic sediments in temperate lakes. Ecosystems 10:1057–1068
Gabelhouse DW (1984) A length-categorization system to assess fish stocks. N Am J Fish Manage 4:273–285
Gaeta JW, Guarascio MJ, Sass GG, Carpenter SR (2011) Lakeshore residential development and growth of largemouth bass (Micropterus salmoides): a cross-lakes comparison. Ecol Freshwater Fish 20:92–101
George EL, Hadley WF (1979) Food and habitat partitioning between rock bass (Ambloplites rupestris) and smallmouth bass (Micropterus dolomieui) young of the year. Trans Am Fish Soc 108:253–261
Guy CS, Neumann RM, Willis DW, Anderson RO (2007) Proportional size distribution (PSD): a further refinement of population size structure index terminology. Fisheries 32:348
Helmus MR, Sass GG (2008) The rapid effects of a whole-lake reduction of coarse woody debris on fish and benthic macroinvertebrates. Freshwater Biol 53:1423–1433
Hillman WP (1982) Structure and dynamics of unique bluegill populations. M.Sc. Thesis, The University of Missouri, Columbia, Missouri, USA
Hodgson JR, Kitchell JF (1987) Opportunistic foraging by largemouth bass (Micropterus salmoides). Am Midl Nat 118:323–336
Hoff MH (1991) Effects of increased nesting cover on nesting and reproduction of smallmouth bass in northern Wisconsin. In: Jackson DC (ed) Proceedings of the first international smallmouth bass symposium. Mississippi Agricultural and Forestry Experiment Station, Mississippi State University, pp 39–43
Hunt J, Annett CA (2002) Effects of habitat manipulation on reproductive success of individual largemouth bass in an Ozark reservoir. N Am J Fish Manage 22:1201–1208
Hunt J, Bacheler N, Wilson D, Videan E, Annett CA (2002) Enhancing largemouth bass spawning: behavioral and habitat considerations. Am Fish Soc Symp 31:277–290
Jennings MJ, Emmons EE, Hatzenbeler GR, Edwards C, Bozek MA (2003) Is littoral habitat affected by residential development and land use in watersheds of Wisconsin lakes? Lake Reservoir Manage 19:272–279
Levins R (1968) Evolution in changing environments: some theoretical explorations. Princeton University Press, Princeton
Marburg AE, Turner MG, Kratz TK (2006) Natural and anthropogenic variation in coarse wood among and within lakes. J Ecol 94:558–568
Newbrey MG, Bozek MA, Jennings MJ, Cook JE (2005) Branching complexity and morphological characteristics of coarse woody structure as lacustrine fish habitat. Can J Fish Aquat Sci 62:2110–2123
Ricker WE (1975) Computation and interpretation of biological statistics of fish populations. Bulletian Fish Research, Board Can
Roth BM, Kaplan IC, Sass GG, Johnson PT, Marburg AE, Yannarell AC, Havlicek TD, Willis TV, Turner MG, Carpenter SR (2007) Linking terrestrial and aquatic ecosystems: the role of woody habitat in lake food webs. Ecol Model 203:439–452
Sass GG, Kitchell JF, Carpenter SR, Hrabik TR, Marburg AE, Turner MG (2006a) Fish community and food web responses to a whole-lake removal of coarse woody habitat. Fisheries 31:321–330
Sass GG, Gille CM, Hinke JT, Kitchell JF (2006b) Whole-lake influences of littoral structural complexity and prey body morphology on fish predator–prey interactions. Ecol Freshwater Fish 15:301–308
Savino JF, Stein RA (1989) Behavior of fish predators and their prey—habitat choice between open water and dense vegetation. Environ Biol Fish 24:287–293
Scheuerell MD, Schindler DE (2004) Changes in the spatial distribution of fishes in lakes along a residential development gradient. Ecosystems 7:98–106
Schindler DE, Hodgson JR, Kitchell JF (1997) Density-dependent changes in individual foraging specialization of largemouth bass. Oecologia 110:592–600
Schindler DE, Geib SI, Williams MR (2000) Patterns of fish growth along a residential development gradient in north temperate lakes. Ecosystems 3:229–237
Seaburg KG (1957) A stomach sampler for live fish. Progress Fish-Cult 19:137–139
Smokorowski KE, Pratt TC, Cole WG, McEachern LJ, Mallory EC (2006) Effects on periphyton and macroinvertebrates from removal of submerged wood in three Ontario lakes. Can J Fish Aquatic Sci 63:2038–2049
Spotte S (2007) Bluegills: biology and behavior. Am Fish Soc, Bethesda
Stewart-Oaten A, Murdoch WW, Parker KR (1986) Environmental impact assessment: Pseudoreplication in time? Ecology 67:929–940
Vogele CE, Rainwater WE (1975) Use of brush shelters as cover by spawning black basses (Micropterus) in Bull Shoals Reservoir. Trans Am Fish Soc 104:264–269
Warner RR, Chesson PL (1985) Coexistence mediated by recruitment fluctuations: a field guide to the storage effect. Am Nat 125:769–787
Wege GJ, Anderson RO (1978) Relative weight (Wr): a new index of condition for largemouth bass. In: Novinger GD, Dillard JG (eds) New approaches to the management of small impoundments. Am. Fish. Soc., North Central Division, Special Publication 5. Bethesda, Maryland, pp 79–91
Werner EE, Hall DJ, Laughlin DR, Wagner DJ, Wilsmann LA, Funk FC (1977) Habitat partitioning in a freshwater fish community. J Fish Res Board Can 34:360–370
Acknowledgments
We thank Jeff Bierman, Justin Fox, Matt Guarascio, Jon Hansen, James Jenders, Adam Kautza, Zach Lawson, Michelle Nault, Steve Reinhardt, Matt Rounds, and J.J. Weis for field assistance. We are particularly indebted to those that helped with the CWH addition; Darren Bade, Chad Harvey, Matt Helmus, Susan Knight, Tim Kratz, Norman Mercado-Silva, Brian Roth, Chris Solomon, Scott Van Egeren, and Michelle Woodford. Jeff Bode, Steve Gilbert, and Jayne Wade of the Wisconsin Department of Natural Resources were instrumental for acquiring the permitting to conduct this research on Camp Lake. This research was funded by a National Science Foundation Integrated Graduate Education and Research Traineeship (IGERT) awarded to G.G. Sass, the North Temperate Lakes LTER, a Graduate Engineering Research Scholars (GERS) Fellowship awarded to J.W. Gaeta, and an NSF biocomplexity grant (DEB-0083545) awarded to S.R. Carpenter. Equipment was provided by the Anna Grant Birge Memorial fund to G.G. Sass. The University of Wisconsin-Madison, Center for Limnology, Trout Lake Station provided housing and support for this project.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sass, G.G., Carpenter, S.R., Gaeta, J.W. et al. Whole-lake addition of coarse woody habitat: response of fish populations. Aquat Sci 74, 255–266 (2012). https://doi.org/10.1007/s00027-011-0219-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00027-011-0219-2