Abstract
Dry river beds are common worldwide and are rapidly increasing in extent due to the effects of water management and prolonged drought periods due to climate change. While attention has been given to the responses of aquatic invertebrates to drying rivers, few studies exist on the terrestrial invertebrates colonizing dry river beds. Dry river beds are physically harsh and they often differ substantially in substrate, topography, microclimate and inundation frequency from adjacent riparian zones. Given these differences, we predicted that dry river beds provide a unique habitat for terrestrial invertebrates, and that their assemblage composition differs from that in adjacent riparian zones. Dry river beds and riparian zones in Australia and Italy were sampled for terrestrial invertebrates with pitfall traps. Sites differed in substrate type, climate and flow regime. Dry river beds contained diverse invertebrate assemblages and their composition was consistently different from adjacent riparian zones, irrespective of substrate, climate or hydrology. Although some taxa were shared between dry river beds and riparian zones, 66 of 320 taxa occurred only in dry river beds. Differences were due to species turnover, rather than shifts in abundance, indicating that dry river bed assemblages are not simply subsets of riparian assemblages. Some spatial patterns in invertebrate assemblages were associated with environmental variables (irrespective of habitat type), but these associations were statistically weak. We suggest that dry river beds are unique habitats in their own right. We discuss potential human stressors and management issues regarding dry river beds and provide recommendations for future research.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rivers that periodically cease to flow comprise a substantial proportion of the total number, length and discharge of the world’s rivers (Tooth 2000). These ‘temporary’ rivers and streams are found on every continent, and are predicted to increase in their extent and in the duration of their no-flow periods due to the effects of water abstraction for human uses and climate change (Larned et al. 2010). Despite their widespread distribution, temporary rivers and streams remain mostly neglected in water legislation (e.g., EU Water Framework Directive WFD; European Commission 2000).
Temporary rivers are hydrologically dynamic, with aquatic and terrestrial habitats expanding, contracting, and fragmenting through time (Stanley et al. 1997). The responses of aquatic invertebrates to drying is understood for many river systems (e.g., Boulton and Lake 1992; Stanley et al. 1994; Larned et al. 2007). Little attention, however, has been paid to the responses of terrestrial invertebrates to the drying or wetting of their river bed habitat, although drying wetlands have received some attention (Batzer 2004).
The dry beds of temporary rivers and streams can provide habitat for terrestrial invertebrates during times when surface water has contracted or disappeared. They can be sites of high terrestrial invertebrate diversity with ants, beetles and spiders (Formicidae, Coleoptera and Arachnida) recorded as the most abundant groups (Wishart 2000; Larned et al. 2007). For example, a dry river bed recorded the highest abundance, species richness and number of unique species from seven different terrestrial habitats sampled in the Namib Desert in southwest Africa (Lalley et al. 2006).
While riparian zones are well known to link terrestrial and aquatic food-webs along river networks (e.g., Gregory et al. 1991), there is an additional and less well understood link that occurs via the river bed sediments adjacent to flowing rivers. Terrestrial invertebrates such as ground beetles (Carabidae), rove beetles (Staphylinidae) and spiders (Lycosidae) inhabit these sediments and feed predominately on emerging and stranded aquatic invertebrates (Hering and Plachter 1997; Batzer 2004; Paetzold et al. 2005), and some grasshoppers feed on algae at the shoreline (Bastow et al. 2002). However, the feeding strategies and food-web dynamics of terrestrial invertebrates in dry river beds are unknown. Terrestrial invertebrates of dry river beds may provide an important, high quality food source for aquatic biota when the system re-wets (Wishart 2000).
In contrast to permanent rivers, it is the dry phase of the hydrograph that often dominates temporary rivers, with the wet phase being a disturbance to the dry river bed. Compared to adjacent riparian zones, dry river beds can be harsher habitats devoid of vegetation due to flow disturbances in the active channel that mobilize, deposit and scour bed sediments, and they are typically exposed to intense solar radiation and wind. They can also be harsh places for biota due to the high temperatures they experience, with some ground surface temperatures exceeding 60°C (Steward, unpublished data). High temperatures affect biota by denaturing nucleic acid and protein molecules, including the degradation of mitochondrial RNA, and by damaging the membranes of intracellular organelles (Tansey and Brock 1972; Hickey and Singer 2004). The most heat-tolerant eukaryotic organisms have an upper temperature limit of approximately 60°C (Tansey and Brock 1972), with few exceptions (e.g., polychaete worms of hydrothermal vents, Chevaldonné et al. 2000; desert moss, Stark et al. 2009). High temperatures in dry river beds would limit their use by most biota to cooler times of the day (mornings, afternoons, night, cloudy spells, etc.), shaded areas, or cooler spaces within the river bed substrate. Dry river beds also differ from adjacent riparian zones in their substrate composition, topography, microclimate and inundation frequency. Riparian zones are cooler than river beds owing to shading by vegetation, and the absorption and reflection of solar radiation by the canopy. Smaller diel temperature ranges have been recorded from riparian zones than from exposed river bed gravel (Tonolla et al. 2010). Riparian zones are subjected to lower erosive forces during floods, due to increased roughness as a consequence of riparian vegetation, and usually contain finer substrate types than the adjacent river bed (Gregory et al. 1991).
Nothing is known about the sources of terrestrial invertebrate colonists of dry river beds as surface water disappears. While it is possible that drying river beds could be colonized by terrestrial invertebrates from the riparian zone and thus share common taxa, given their abovementioned harshness and the differences they exhibit in habitat attributes from adjacent riparian zones, we expect that dry river beds support their own specialized terrestrial invertebrate assemblages. Therefore, we predict that assemblages of terrestrial invertebrates sampled from dry river beds will differ in their composition from assemblages in adjacent riparian zones. To test this prediction, and to better understand environmental differences between dry river bed and riparian habitats for terrestrial invertebrates, we addressed the following research questions:
-
1.
Are assemblages of terrestrial invertebrates in dry river bed habitats different in composition from those in adjacent riparian habitats?
-
2.
If so, what taxa of terrestrial invertebrates contribute to this difference?
-
3.
How are the dry river bed and adjacent riparian habitats different in environmental attributes that are relevant to the invertebrate assemblages?
-
4.
Which environmental variables are most strongly associated with patterns in the invertebrate assemblages?
We investigated these questions using samples of terrestrial invertebrates from dry river beds and adjacent riparian zones collected at multiple sites in four Australian river catchments and one Italian river catchment. Catchments with a diversity of different river flow regimes and climate characteristics were chosen for this study to enable us to investigate the geographical and climatic breadth of our prediction that dry river beds harbor unique invertebrate assemblages.
Materials and methods
Defining the habitats
We defined dry river bed habitat as the exposed river bed lacking surface water within a riverine channel. Dry river bed habitat could be located in between patches of surface water, such as isolated pools or waterholes. Dry river bed habitat could also be represented by secondary channels within a braided river network. The dry river beds sampled for this study generally lacked woody vegetation and occasionally contained herbaceous vegetation. We defined riparian habitat as the vegetated banks of rivers and streams but not including the sections of the channel near the low water mark (cf. Naiman and Decamps 1997). Riparian habitat was distinguished from dry river bed habitat by the presence of a distinct woody vegetation type, largely composed of species adapted to such environments (Gregory et al. 1991). Riparian habitat was also distinguished from dry river bed habitat by an abrupt change in slope and substrate type.
River beds that had recently been inundated could potentially be undergoing successional shifts in invertebrate assemblages, from the aquatic phase to the terrestrial phase. We avoided sampling such river beds. This is because we aimed to collect ‘true’ terrestrial invertebrates, rather than semi-terrestrial or aquatic invertebrates that could temporarily resist desiccation. We determined that the dry river beds sampled had not been inundated for weeks to months prior to sampling, based on reference to nearby stream gauge data, local landowner knowledge, the presence of terrestrial herbaceous plants, the absence of aquatic material such as dead aquatic biota or moist algal mats, and the extent of the accumulation of terrestrial organic material such as leaf litter.
Study area
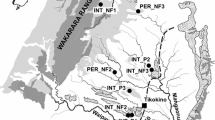
Dry river beds and their adjacent riparian zones were sampled at 22 sites. Eighteen sites were sampled within four river catchments in Australia [Mitchell (six sites), Flinders (six sites), Brisbane (four sites), and Moonie (two sites)], and four sites were sampled within the Tagliamento River catchment in Italy (Table 1; Fig. 1). Catchments were selected to cover different climates, hydrological types and river bed substrate types (Table 1; Figs. 2, 3). Hydrological classification for the Australian rivers was based on Kennard et al. (2010).
Hydrographs of sites in each catchment for 1/01/2007–1/01/2010, displayed as discharge (m3/s) for a the Flinders River, b the Moonie River, c the Walsh River in the Mitchell River catchment, and d Purga Creek in the Brisbane River catchment; and as stage (cm) for e the Tagliamento River (upstream of the section which dries completely). Arrows indicate the sampling date, except for the Tagliamento River catchment as the hydrological data for this sampling period was unavailable (September 2010). Note that the vertical axes have different scales. Seasons are shown, with S summer, A autumn, W winter, Sp spring
The Mitchell and Flinders River catchments (Figs. 1, 3) in the Australian wet-dry tropics are monsoonal with peak discharge in the austral summer, resulting in high predictability of the annual wet and dry phases (Fig. 2). Both of these rivers flow into the Gulf of Carpentaria in northern Queensland, Australia. During the dry season, surface water in the Flinders River catchment is largely confined to a series of isolated waterholes, whereas the main channel of the Mitchell River catchment contracts to a sinuous, low flow channel with multiple secondary channels, and the location of the main channel is highly dynamic (Brooks et al. 2009). Large, dry, secondary channels were sampled if surface water was present in the main channel. These secondary channels carry water less often than the primary channel of the Mitchell, but more often than primary channels sampled in some other catchments. The Mitchell River experiences large floods every year (every ‘wet’ season) that inundate these channels, resulting in a single, large macro-channel (Brooks et al. 2009). Dry river beds were typically wider than 100 m in the Mitchell River catchment, and wider than 50 m in the Flinders River catchment. Both the Mitchell and Flinders were dominated by fine substrate types (Fig. 3).
The Brisbane and Moonie River catchments are located in south-east Queensland, Australia (Fig. 1). The Brisbane River flows east into Moreton Bay, while the Moonie River is part of the upper Murray-Darling Basin and flows south to the sea in South Australia. In both catchments, rainfall is mostly associated with subtropical lows and storms resulting in an unpredictable flow regime (Fig. 2). Rivers and streams in these catchments have dried for months, or even years, at a time. The dry river beds sampled in the Brisbane and Moonie River catchments were less than 10 m wide. Substrate varied from fine to coarse in the Brisbane River catchment, with cracking clay substrates being typical of the Moonie River catchment (Fig. 3).
The Tagliamento River catchment was selected for sampling in addition to the Australian river catchments to extend the global relevance of the study. There are no rivers with its type of hydrological regime in Australia (Kennard et al. 2010). The Tagliamento River (Figs. 1, 2, 3) has a flashy flow regime with discharge peaks in spring and autumn, although flow, flood pulses and dry spells may occur at any time of the year (Tockner et al. 2003; Döring et al. 2007). The Tagliamento River is one of the last morphologically intact rivers in the European Alps, containing up to 11 individual channels in the braided middle reaches (Ward et al. 1999). These channels can be dry at times and a section of the entire channel network up to 20 km long can lose all surface water during low flow conditions (Döring et al. 2007). The width of the active channel containing dry river beds was up to 1 km wide and substrate was coarse (Fig. 3).
Data collection
To determine whether the terrestrial invertebrate assemblage composition from dry river bed and riparian habitats was different, we sampled both habitats at each site using pitfall traps. The traps consisted of 250-mL plastic jars, 77 mm high and 67 mm in diameter, filled with 70% ethanol and glycerol as per Wishart (2000). The ethanol acted as a killing agent and preservative, and a drop of detergent was added to break the surface tension, preventing captured invertebrates from escaping. This method collected invertebrates that were potentially attracted to ethanol, or at least were not repelled by it. A plastic cover was positioned approximately 100 mm over each pitfall trap to prevent rain, leaf litter and other debris from blocking the trap and reducing its efficiency (Williams 1959). Six replicate pitfall traps were randomly positioned in each habitat type (dry river bed or riparian zone) at each site and set for approximately 24 h. Environmental data were visually estimated from a 1-m diameter area surrounding each pitfall trap (Table 2). Environmental variables were chosen that were expected to influence terrestrial invertebrates. Substrate particle sizes were recorded as a percentage of the area, and defined as follows: silt/clay <0.05 mm, sand 0.05–2 mm, gravel 2–4 mm, pebble 4–64 mm, cobble 64–256 mm, bedrock >256 mm (Cummins 1962). The following substrate cover variables were recorded as a percentage of the 1-m diameter area: bare ground, detritus, ground vegetation, sticks, branches and logs. Canopy cover (%) above each pitfall trap was also recorded.
Terrestrial invertebrates collected in the pitfall traps were identified to family level where possible, then grouped according to morphospecies based on guidelines from the literature (Beattie and Oliver 1994; Oliver and Beattie 1996) and counted. Morphospecies are ‘taxa readily separable by morphological differences that are obvious to individuals without extensive taxonomic training’ (Oliver and Beattie 1996). Estimates of richness of terrestrial invertebrates from pitfall samples have been shown to vary little between morphospecies identified by non-specialists and species identified by specialists (Oliver and Beattie 1996). Species level spatial patterns in invertebrate data can be similar at lower levels of taxonomic resolution, such as genus level (Pik et al. 1999; Cardoso et al. 2004) and family level (Marshall et al. 2006).
All sampling took place between August 2009 and September 2010 during the ‘dry’ phase. Different rivers dried at different times of the year, and as a result, different seasons were sampled in this study. Sites were sampled during the austral spring (October 2009) in the Mitchell and Flinders River catchments, in the austral winter (August 2009) in the Moonie River catchment, in the austral summer (December 2009) in the Brisbane River catchment, and in the boreal autumn (September 2010) in the Tagliamento River catchment.
To determine that our sampling effort was sufficient to define habitat richness and abundance at each site, we generated randomised taxon accumulation curves (with 50 randomisations) for dry river bed and riparian replicates within each habitat, site and catchment using the EstimateS software program (Colwell 2006). We found that our sampling design was adequate as habitat-specific estimates of both taxon richness and abundance stabilized with five to six replicate samples (Table 3).
Statistical analyses
All multivariate analyses were conducted in the PRIMER version 6.1.10 software program (Clarke and Gorley 2007). To determine whether the terrestrial invertebrate assemblage composition was different between dry river bed and riparian habitats at each site within each catchment, we used a two-way crossed analysis of similarity (ANOSIM) with 9999 permutations based on a Bray–Curtis association matrix between samples characterised by taxa. In these analyses, we tested for differences between habitats (dry river bed and riparian zone), allowing for differences between sites within each catchment. This allowed us to investigate our prediction that the assemblages would differ between adjacent habitats, and to consider the generality of this result across multiple catchments with varying hydrology and climate. The two-way crossed ANOSIM design applied to individual catchments was considered the most suitable (as opposed, for instance, to a nested analysis) because it accounted for two factors, site and catchment, that we a priori assumed to be major sources of variability not directly related to our research questions, allowing the results to focus on our interest in differences between dry river beds and adjacent riparian zones. Whilst a standard significance threshold of p < 0.05 was used to determine if there were differences, pair-wise R values were used to indicate the magnitude of differences between habitats based on the ‘rule of thumb’ provided by Clarke and Gorley (2006), where R > 0.75 indicates groups are well separated, R = 0.50–0.75 indicates overlapping groups that are clearly different, R = 0.25–0.50 indicates groups with considerable overlap and R < 0.25 indicates groups that are barely separable. We used Non-metric Multi-Dimensional Scaling (nMDS) to graphically display the ANOSIM results.
Rare taxa were removed prior to analysis because they were considered to be inadequately sampled for us to be confident in our representation of their distributions and thus their inclusion would distort assemblage differences. They were defined as those taxa contributing less than 1% of the total number of individuals in the catchment-level dataset (i.e., all samples from all sites in a catchment) and contributing less than 5% of the total number of individuals in their specific sample. The abundance data were log10 (x + 1) transformed to down-weight the influence of highly abundant taxa on the assemblage patterns. After down-weighting in this way, association measures between samples better reflect differences in the overall assemblage composition (Clarke and Warwick 1994). An additional dataset was created with abundance data transformed to presence/absence (again following removal of rare taxa). Contrasting results of the analyses of the abundance and presence/absence datasets allowed interpretation of the relative contributions of abundance and composition in generating differences between dry river bed and adjacent riparian invertebrate assemblages.
To identify what types of invertebrates contributed to differences between dry river bed and riparian habitats for significant ANOSIM tests, we calculated similarity percentages using SIMPER.
Differences between dry river bed and adjacent riparian habitats in environmental variables were assessed using a two-way crossed ANOSIM with 9999 permutations based on a normalised Euclidean distance association matrix between samples characterised by their environmental attributes. This tested for differences between habitat types allowing for differences between sites and was repeated for each catchment. SIMPER was again used to identify which variables contributed most to the significant differences between the habitat types.
To calculate how much of the overall faunal variation in each catchment was associated with environmental variables we used the BIO-ENV routine in PRIMER. The BIO-ENV analyses used Bray–Curtis similarity matrices of the invertebrate data, and a Spearman Rank correlation of environmental variables with normalized Euclidean distance measures.
Results
Terrestrial invertebrate assemblage composition
We collected a total of 22,150 invertebrates from 256 pitfall samples from dry river bed and riparian habitats across the five catchments, representing 320 invertebrate morphospecies from 24 orders (Table 4).
There was a significant difference in the composition of terrestrial invertebrate assemblages between dry river bed and adjacent riparian habitats in all 5 catchments (in all cases p < 0.0001; Table 5; Fig. 4). Applying Clarke and Gorley’s (2006) rule of thumb for interpreting ANOSIM results, dry river bed and adjacent riparian assemblages were ‘clearly different’ when using abundance data in most catchments; however there was ‘some overlap’ in invertebrate composition in the Mitchell and Moonie River catchments (Table 5; Fig. 4). Likewise, with presence/absence data, there was a significant difference between dry river bed and adjacent riparian habitats in all five catchments, and the magnitudes of the differences were comparable to those from the abundance data results (Table 5).
Terrestrial invertebrate assemblage composition (abundance data) from dry river bed (open circles) and riparian (closed triangles) habitats for sites in a Mitchell River catchment, b Flinders River catchment, c Brisbane River catchment, d Moonie River catchment, e Tagliamento River catchment. Each point represents the mean x and y 2-dimensional nMDS coordinate for each habitat at each site (a–e) with ±1 standard error as error bars. Stress is shown. See Table 1 for site codes
Total invertebrate abundances were higher in dry river beds than in riparian habitats in the Mitchell and Flinders River catchments, and higher in riparian habitats than in dry river beds in the remaining catchments (Table 4). More taxa were recorded from riparian than dry river bed habitats. Sixty-six morphospecies (20% of total) were unique to dry river beds and from the following groups: Coleoptera (35 morphospecies), Formicidae (12), Acarina (3), Diptera (3), Hymenoptera (3), Dermaptera (2), Hemiptera (2), Lepidoptera (2), Orthoptera (2), Collembola (1), and Isoptera (1). Approximately 50% of all morphospecies recorded in each Australian catchment were shared between dry river bed and riparian habitats, but this was even lower in the Tagliamento catchment (31% shared taxa) (Table 4).
Across all catchments, the results from the SIMPER analyses were consistently similar for abundance and presence/absence data, with the top five morphospecies associated with 21–38% of the differences between dry river bed and riparian habitats (Table 6). In the Mitchell and Flinders River catchments, the top five most important morphospecies associated with these differences belonged to Formicidae, Coleoptera and Diptera, with Hemipteran morphospecies also explaining some of the presence/absence differences in the Flinders (Table 6). In the Brisbane and Moonie River catchments, the top five most important morphospecies associated with the differences between the habitat types belonged to the Formicidae, Collembola and Acarina groups, with Hemipteran morphospecies also explaining some of the presence/absence differences (Table 6). Finally, in the Tagliamento River catchment, the top five most important morphospecies associated with the differences between the habitat types belonged to the Formicidae, Coleoptera, Collembola and Arionoidea groups, with Lycosidae morphospecies also associated with the presence/absence differences (Table 6).
Environmental variation
Environmental variables of dry river beds and riparian zones were significantly different (p < 0.0001) in all catchments and the magnitudes were classified as ‘clearly different’ (Table 5). Large proportions (44–88%) of these differences were explained by variation in substrate composition and bare ground in all catchments, ground vegetation cover in all except the Mitchell, and detritus cover in all but the Brisbane (Table 6).
Despite these environmental differences, little of the overall biological patterns were associated with the environmental variation in the BIO-ENV analyses, as indicated by their relatively small R statistics (Table 7). Canopy cover was associated with some of the biological variation in the Mitchell River catchment (R = 0.344, p = 0.001), whereas silt/clay, sand and detritus were associated with some of the variation in the Flinders River catchment (R = 0.247, p < 0.001). Silt/clay, sand, cobble, and detritus were associated with some of the variation in the Brisbane River catchment (R = 0.371, p = 0.001), whereas sticks, branches and logs were associated with a higher proportion of the faunal variation in the Moonie River catchment (R = 0.602, p = 0.001). In the Tagliamento River catchment, bare ground and ground vegetation were associated with some of the variation (R = 0.39, p = 0.001).
Discussion
Terrestrial invertebrates of dry river beds and riparian habitats
In every catchment, the terrestrial invertebrate assemblage composition of dry river beds was significantly different from that in adjacent riparian habitats, as we predicted. These differences were not simply due to abundances of taxa, but also the presence and absence of taxa. The fact that dry river bed and riparian habitats were significantly different shows that there was sufficient power to detect a difference, even with only two sites from the Moonie River catchment.
The dry river bed habitats sampled contained a diverse terrestrial invertebrate assemblage that was dominated by ants (Formicidae) in every catchment but also beetles (Coleoptera) in the Mitchell, Flinders and Tagliamento River catchments and springtails (Collembola) in the Brisbane, Moonie and Tagliamento River catchments, with mites (Acarina), flies (Diptera), bugs (Hemiptera), cockroaches (Blattodea) and spiders (Lycosidae) also abundant in some catchments (Table 6; Fig. 5). Similar patterns have been found in dry river beds elsewhere, with high abundances of ants and springtails in New Zealand (Larned et al. 2007), and high abundance of ants, beetles and spiders in South Africa (Wishart 2000) and Namibia (Lalley et al. 2006).
Riparian habitat taxon richness was higher in all catchments, although dry river bed habitats contained more individuals in the Mitchell and Flinders River catchments. Up to half of the taxa were shared between dry river beds and riparian habitats, and 66 out of a total of 320 taxa occurred only in dry river beds. The dry river bed invertebrate assemblages sampled in this study were not simply subsets of adjacent riparian assemblages differing in taxon abundance. Habitat partitioning amongst taxa appeared to be occurring, with some habitat generalist taxa, some riparian habitat specialists, and some dry river bed habitat specialists. This general pattern has been observed in Lycosid spiders (Moring and Stewart 1994), where overall abundances were higher in exposed cobble streamside habitats than in adjacent grassy riparian zones and some individual species were confined to only one of these habitats or the other, with other species common to both. Dry river beds may contain specialist terrestrial invertebrates with ‘inundation-resistant’ stages evolved for wet times, much like aquatic invertebrates with desiccation-resistant stages evolved for dry times. This is the case for some terrestrial invertebrates in the flooded forests of the Amazon, which are regularly flooded for up to 6 months of the year. Some invertebrates in these forests have inundation-resistant eggs, and some have physiological adaptations allowing the adults to survive underwater (Adis 1986, 1992; Adis and Junk 2002).
Based on our results, we propose that dry river beds represent habitat for a unique invertebrate assemblage. Our repetition of these results across five different catchments with different zoogeographic histories, hydrology, substrate and climate reinforces the generality of these findings. The differences between dry river bed and riparian invertebrate assemblages can be large, as in the Tagliamento River catchment where the assemblages were clearly different, but the magnitude varied between catchments, with the smallest differences in the Moonie River catchment where the assemblages were different but had considerable overlap.
Environmental differences
Environmental differences between dry river bed and adjacent riparian habitats in each catchment were consistently greater than or equal to differences in the invertebrate assemblages. Despite this, the overall patterns in invertebrate assemblage composition were not strongly associated with the environmental variability in any catchment. This indicates that our results did not simply reflect a gradient response of the invertebrates to variability in the environment. If such a gradient response existed, it would suggest that assemblage composition was tracking environmental variation and that samples with similar environmental attributes would share similar invertebrate assemblages whether they were from the riparian zone or the dry river bed. The absence of such a gradient response in combination with the consistent faunal difference between habitats further supports our conclusion that dry river beds represent a different habitat in their own right.
Canopy cover was weakly associated with patterns in the invertebrate assemblages of the Mitchell River catchment. Some of the dry river beds in the Mitchell were extremely wide, up to 500 m, meaning that most of the dry river bed surfaces were not shaded by riparian vegetation, resulting in a hotter habitat than the adjacent shaded riparian habitat. These river beds resembled hot, sandy deserts by day, but cooled considerably by night. Invertebrate activity in these river beds could well be limited to night time, or else displayed by invertebrates tolerant of extreme temperatures. In the Flinders River catchment, patterns in the invertebrate assemblages were associated with silt/clay, sand and detritus, but again the statistical association was weak. The dry river bed habitats were predominantly sand, and the riparian habitats were predominately silt/clay, with more detritus on average found in the riparian habitats than in the dry river beds. This was consistent with the Brisbane River catchment, with patterns in the invertebrate assemblages weakly associated with sand and detritus, and also bare ground and cobble, with these substrates mainly found in the dry river beds. Sticks, branches and logs were associated with the invertebrate assemblage patterns in the Moonie River catchment, having the strongest statistical association. Bare ground and ground vegetation cover were weakly associated with invertebrate patterns in the Tagliamento River catchment. Although over 90% of the dry river bed habitats in the Tagliamento were bare, the substrate was coarser than that of the riparian habitats, providing interstitial spaces and complexity that differs from the fine substrates and vegetation cover of the riparian zone. Aspects of the environment that we did not measure could be more strongly associated with the invertebrate patterns than substrate, canopy cover and ground cover. We measured structural attributes of each habitat, whereas temperature, humidity, and soil moisture may also be important to terrestrial invertebrates and should be considered in future studies.
Dry river beds—management and future research
Human activities that change the environmental conditions of dry river beds are likely to influence invertebrate assemblage composition. Cattle trampling, weed invasion, siltation, and altered hydrology can impact rivers and streams, the shoreline, and gravel bars during the wet phase (Balneaves and Hughey 1990; Wood and Armitage 1997; Nilsson et al. 2005; Bates et al. 2007; Sadler and Bates 2008), and are likely stressors on dry river beds during the dry phase. Cattle trampling during the dry phase may compact the river bed sediments, siltation may reduce substrate diversity through in-filling, and weed invasion would increase canopy cover or ground vegetation cover, possibly affecting the quality of dry river beds as habitats.
Under climate change scenarios, global surface temperatures are predicted to increase by 1–4°C during the twenty-first century (Meehl et al. 2007), and these changes may impact invertebrate assemblages of dry river beds. Temperatures recorded in dry river beds can exceed the thermal tolerances of many organisms; therefore future temperature increases may extend the duration of periods when dry river beds are inhospitable to most life. The combined effects of climate change and water management may increase or decrease the duration of the wet and dry phases in rivers (Jackson et al. 2001; Chiew and McMahon 2002; Lehner et al. 2006). Reduced flood frequency has negatively impacted the aquatic biota of temporary rivers and streams (Jenkins and Boulton 2007), and may have negative effects on habitat and diversity of terrestrial invertebrates in dry river beds. Permanent wetting after the construction of instream barriers such as dams or weirs will be detrimental to the terrestrial invertebrates of dry river beds, eliminating dry river bed habitat altogether. Similarly, increased dry periods may impact dry river bed invertebrates by reducing the opportunities for terrestrial predators and scavengers to consume stranded aquatic material, which may be important for their survival or recruitment.
Our study has highlighted the significance of these habitats in supporting unique biota. More studies are needed to better understand how the terrestrial invertebrates of dry river beds are influenced by natural and anthropogenic disturbances. First, biotic responses to alterations of the environmental attributes of dry river beds need to be better described. Second, a more complete understanding of how modifications to wetting and drying regimes of temporary rivers effect successional changes in terrestrial invertebrates needs to be developed. If a link between human impacts and terrestrial invertebrate response is established, then terrestrial invertebrates could be considered as biological indicators of dry river health, in the same way that aquatic invertebrates are often used as indicators of aquatic ecosystem health.
References
Adis J (1986) An “aquatic” millipede from a Central Amazonian inundation forest. Oecologia 68:347–349
Adis J (1992) How to survive six months in a flooded soil: strategies in Chilopoda and Symphyla from Central Amazonian floodplains. Stud Neotrop Fauna Environ 27:117–129
Adis J, Junk WJ (2002) Terrestrial invertebrates inhabiting lowland river floodplains of Central Amazonia and Central Europe: a review. Freshw Biol 47:711–731
Balneaves JM, Hughey K (1990) The need for control of exotic weeds in braided river-beds for conservation of wildlife. In: Proceedings of the 9th Australian Weeds Conference, Adelaide, South Australia, 1990, pp 103–108
Bastow J, Sabo J, Finlay J, Power M (2002) A basal aquatic–terrestrial trophic link in rivers: algal subsidies via shore-dwelling grasshoppers. Oecologia 131:261–268
Bates A, Sadler J, Fowles A (2007) Livestock trampling reduces the conservation value of beetle communities on high quality exposed riverine sediments. Biodivers Conserv 16:1491–1509
Batzer DP (2004) Movements of upland invertebrates into drying seasonal woodland ponds in northern Minnesota, USA. Wetlands 24:904–907
Beattie AJ, Oliver I (1994) Taxonomic minimalism. Trends Ecol Evol 9:488–490
Boulton AJ, Lake PS (1992) The ecology of two intermittent streams in Victoria, Australia. II. Comparisons of faunal composition between habitats, rivers and years. Freshw Biol 27:99–121
Brooks AP, Shellberg JG, Knight J, Spencer J (2009) Alluvial gully erosion: an example from the Mitchell fluvial megafan, Queensland, Australia. Earth Surf Proc Land 34:1951–1969
Cardoso P, Silva I, de Oliveira NG, Serrano ARM (2004) Higher taxa surrogates of spider (Araneae) diversity and their efficiency in conservation. Biol Conserv 117:453–459
Chevaldonné P, Fisher CR, Childress JJ, Desbruyeres D, Jollivet D, Zal F, Toulmond A (2000) Thermotolerance and the ‘Pompeii worms’. Mar Ecol Prog Ser 208:293–295
Chiew FHS, McMahon TA (2002) Modelling the impacts of climate change on Australian streamflow. Hydrol Process 16:1235–1245
Clarke K, Gorley R (2006) PRIMER v6: User Manual/Tutorial. PRIMER-E, Plymouth
Clarke K, Gorley R (2007) PRIMER-6. 6.1.10 edn. PRIMER-E Ltd, Plymouth
Clarke KR, Warwick RM (1994) Change in marine communities: an approach to statistical analysis and interpretation. Plymouth Marine Laboratory, Plymouth
Colwell RK (2006) EstimateS: statistical estimation of species richness and shared species from samples (software and user’s guide), 8th edn. http://purl.oclc.org/estimates
Cummins KW (1962) An evaluation of some techniques for the collection and analysis of benthic samples with special emphasis on lotic waters. Am Midl Nat 67:477–504
Döring M, Uehlinger U, Rotach A, Schläpfer DR, Tockner K (2007) Ecosystem expansion and contraction dynamics along a large Alpine alluvial corridor (Tagliamento River, Northeast Italy). Earth Surf Proc Land 32:1693–1704
European Commission (2000) Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy. Off J Eur Commun 327:1–7
Gregory SV, Swanson FJ, McKee WA, Cummins KW (1991) An ecosystem perspective of riparian zones. Bioscience 41:540–551
Hering D, Plachter H (1997) Riparian ground beetles (Coleoptera, Carabidae) preying on aquatic invertebrates: a feeding strategy in alpine floodplains. Oecologia 111:261–270
Hickey DA, Singer GAC (2004) Genomic and proteomic adaptations to growth at high temperature. Genome Biol 5:117
Jackson RB, Carpenter SR, Dahm CN, McKnight DM, Naiman RJ, Postel SL, Running SW (2001) Water in a changing world. Ecol Appl 11:1027–1045
Jenkins KM, Boulton AJ (2007) Detecting impacts and setting restoration targets in arid-zone rivers: aquatic micro-invertebrate responses to reduced floodplain inundation. J Appl Ecol 44:823–832
Kennard MJ, Pusey BJ, Olden JD, Mackay SJ, Stein JL, Marsh N (2010) Classification of natural flow regimes in Australia to support environmental flow management. Freshw Biol 55:171–193
Lalley JS, Viles HA, Henschel JR, Lalley V (2006) Lichen-dominated soil crusts as arthropod habitat in warm deserts. J Arid Environ 67:579–593
Larned S, Datry T, Robinson C (2007) Invertebrate and microbial responses to inundation in an ephemeral river reach in New Zealand: effects of preceding dry periods. Aquat Sci 69:554–567
Larned ST, Datry T, Arscott DB, Tockner K (2010) Emerging concepts in temporary-river ecology. Freshw Biol 55:717–738
Lehner B, Doell P, Alcamo J, Henrichs T, Kaspar F (2006) Estimating the impact of global change on flood and drought risks in Europe: a continental, integrated analysis. Clim Change 75:273–299
Marshall JC, Steward AL, Harch BD (2006) Taxonomic resolution and quantification of freshwater macroinvertebrate samples from an Australian dryland river: the benefits and costs of using species abundance data. Hydrobiologia 572:171–194
Meehl GA, Stocker TF, Collins WD, Friedlingstein P, Gaye AT, Gregory JM, Kitoh A, Knutti R, Murphy JM, Noda A, Raper SCB, Watterson IG, Weaver AJ, Zhao ZC (2007) Global climate projections. In: Solomon S, Qin D, Manning M et al (eds) Climate change 2007: the physical science basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change (IPCC). Cambridge University Press, Cambridge, pp 747–845
Moring JB, Stewart KW (1994) Habitat partitioning by the wolf spider (Araneae, Lycosidae) guild in streamside and riparian vegetation zones of the Conejos River, Colorado. J Arachnol 22:205–217
Naiman RJ, Decamps H (1997) The ecology of interfaces: riparian zones. Annu Rev Ecol Syst 28:621–658
Nilsson C, Reidy CA, Dynesius M, Revenga C (2005) Fragmentation and flow regulation of the world’s large river systems. Science 308:405
Oliver I, Beattie AJ (1996) Invertebrate morphospecies as surrogates for species: a case study. Conserv Biol 10:99–109
Paetzold A, Schubert CJ, Tockner K (2005) Aquatic terrestrial linkages along a braided-river: riparian arthropods feeding on aquatic insects. Ecosystems 8:748–759
Pik AJ, Oliver I, Beattie AJ (1999) Taxonomic sufficiency in ecological studies of terrestrial invertebrates. Aust J Ecol 24:555–562
Queensland Department of Environment and Resource Management (2010) http://www.derm.qld.gov.au/water/monitoring/index.html (Accessed 11 October, 2010)
Sadler JP, Bates AJ (2008) The ecohydrology of invertebrates associated with exposed riverine sediments. In: Wood PJ, Hannah DM, Sadler JP (eds) Hydroecology and ecohydrology. Wiley, pp 37–56
Stanley EH, Buschman DL, Boulton AJ, Grimm NB, Fisher SG (1994) Invertebrate resistance and resilience to intermittency in a desert stream. Am Midl Nat 131:288–300
Stanley EH, Fisher SG, Grimm NB (1997) Ecosystem expansion and contraction in streams. Bioscience 47:427–435
Stark LR, McLetchie DN, Roberts SP (2009) Gender differences and a new adult eukaryotic record for upper thermal tolerance in the desert moss Syntrichia caninervis. J Therm Biol 34:131–137
Tansey MR, Brock TD (1972) The upper temperature limit for eukaryotic organisms. Proc Natl Acad Sci USA 69:2426
Tockner K, Ward JV, Arscott DB, Edwards PJ, Kollmann J, Gurnell AM, Petts GE, Maiolini B (2003) The Tagliamento River: a model ecosystem of European importance. Aquat Sci 65:239–253
Tonolla D, Acuña V, Uehlinger U, Frank T, Tockner K (2010) Thermal heterogeneity in river floodplains. Ecosystems 13:727–740
Tooth S (2000) Process, form and change in dryland rivers: a review of recent research. Earth Sci Rev 51:67–107
Ward JV, Tockner K, Edwards PJ, Kollmann J, Bretschko G, Gurnell AM, Petts GE, Rossaro B (1999) A reference river system for the Alps: the ‘Fiume Tagliamento’. Regul Rivers Res Manage 15:63–75
Williams G (1959) The seasonal and diurnal activity of the fauna sampled by pitfall traps in different habitats. J Anim Ecol 28:1–13
Wishart MJ (2000) The terrestrial invertebrate fauna of a temporary stream in southern Africa. Afr Zool 35:193–200
Wood PJ, Armitage PD (1997) Biological effects of fine sediment in the lotic environment. Environ Manage 21:203–217
Acknowledgments
AS was funded by the Queensland Department of Environment and Resource Management (DERM) and TRaCK (Tropical Rivers and Coastal Knowledge, http://www.track.gov.au). TRaCK received major funding for its research through the Australian Government’s Commonwealth Environment Research Facilities initiative; the Australian Government’s Raising National Water Standards Programme; Land and Water Australia and the Queensland Government’s Smart State Innovation Fund. Additional support was provided from the European Union through the Mediterranean Intermittent River ManAGEment (MIRAGE) project (ref: FP7 ENV 2007 1, http://www.mirage-project.eu). We acknowledge DERM for supplying the flow data for the Australian rivers, and IGB for supplying the flow data for the Tagliamento River. Diego Tonolla and Daniel von Schiller assisted with providing Tagliamento River GIS data. Many thanks to the field volunteers: in Australia—Jon and Jo Blessing, Sara Clifford, Ceaira Cottle, Werner Ehrsam, Jimmy Fawcett, Laurisse Frampton, Jess Haxen, Dean Holloway, Barry Kenway, Jaye Lobegeiger, Kate Masci, Morag McKinnon, Courtenay Mills, Annette Ritchie, Rob Rolls, Michael Rooke, Emily Saeck, Bill Senior, Suzanne Sippel, Ilva Sporne, Hamish Sutherland, Kenn Tews, Dominic Valdez, Ben Woodward, Belinda Young and Farah Zavahir; and in Italy—Claudio Cruciat and Brigitte Zoller. Thanks to Rob Rolls and Ben Stewart-Koster for providing statistical advice. We also wish to thank the landowners, particularly Neil and Helen Peddle. We thank the Australian Rivers Institute’s Aquatic Ecology Discussion Group (‘Dregs’) for providing comments on the manuscript. We thank Thibault Datry and the Guest Editor of this issue Dave Arscott. Finally we thank two anonymous referees for providing valuable criticism and excellent suggestions that greatly improved this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article belongs to the Special Issue “Recent Perspectives on Temporary River Ecology”.
Rights and permissions
About this article
Cite this article
Steward, A.L., Marshall, J.C., Sheldon, F. et al. Terrestrial invertebrates of dry river beds are not simply subsets of riparian assemblages. Aquat Sci 73, 551–566 (2011). https://doi.org/10.1007/s00027-011-0217-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00027-011-0217-4