Abstract
There is growing interest in rates of nitrate uptake and denitrification in restored streams to better understand the effects of restoration on nitrogen processing. This study quantified nitrate uptake in two restored and two unrestored streams in Baltimore, Maryland, USA using nitrate additions, denitrification enzyme assays, and a 15N isotope tracer addition in one of the urban restored streams, Minebank Run. Restoration included either incorporation of stormwater ponds below a storm drain and catch basins to attenuate flow or hydrologic “reconnection” of a stream channel to its floodplain. Stream restoration was conducted for restoring aging sanitary and bridge infrastructure and introducing some stormwater management in watersheds developed prior to current regulations. Denitrification potential in sediments was variable across streams, whereas nitrate uptake length appeared to be significantly correlated to surface water velocity, which was low in the restored streams during summer baseflow conditions. Uptake length of NO3 −–N in Minebank Run estimated by 15N tracer addition was 556 m. Whole stream denitrification rates in Minebank Run were 153 mg NO3 −–N m−2 day−1, and approximately 40% of the daily load of nitrate was estimated to be removed via denitrification over a distance of 220.5 m in a stream reach designed to be hydrologically “connected” to its floodplain. Increased hydrologic residence time in Minebank Run during baseflow likely influenced rates of whole stream denitrification, suggesting that hydrologic residence time may be a key factor influencing N uptake and denitrification. Restoration approaches that increase hydrologic “connectivity” with hyporheic sediments and increase hydrologic residence time may be useful for stimulating denitrification. More work is necessary, however, to examine changes in denitrification rates in restored streams across different seasons, variable N loads, and in response to the “flashy” hydrologic flow conditions during storms common in urban streams.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Rapidly urbanizing areas may represent sources of nitrogen to streams, rivers, and estuaries (Howarth et al. 1996; Grimm et al. 2008; Kaushal et al. 2008a). Both nitrogen concentration and export to rivers are strongly correlated to human population density and wastewater inputs (Peierls et al. 1991; Howarth et al. 1996). In the Chesapeake Bay region of the USA, increased nitrogen export associated with agriculture and urbanization in coastal areas has contributed to eutrophication and hypoxia, decreased plant diversity, enhanced formation of harmful algal blooms, and caused fish kills (Boesch et al. 2001; Kemp et al. 2005; Kaushal et al. 2008a).
Although human activities have greatly increased nitrogen inputs to watersheds, retention of nitrogen by headwater streams and riparian wetlands can be an important sink for anthropogenic nitrogen under a certain range of hydrologic conditions and N concentrations. Retention of nitrogen in streams can occur by several mechanisms such as temporary uptake by autotrophic algae and plants, heterotrophic uptake by microbes, storage in sediments, and also permanent removal via denitrification (Böhlke et al. 2004; Grimm et al. 2005; Kaushal et al. 2008b). Nitrate is a major form of nitrogen in many streams and rivers (Howarth et al. 1996; Groffman et al. 2004; Mayer et al. 2007), and there is considerable interest in its removal via denitrification. In situ measurements of denitrification using 15N isotopic techniques in streams have shown that denitrification can account for 15% of nitrate uptake in a forest stream with very low concentrations of nitrate (Mulholland et al. 2004), 50% of nitrate uptake in an agricultural stream with higher concentrations of nitrate (Böhlke et al. 2004), and a median of 16% of total nitrate uptake in a larger survey of 72 streams and 8 regions representing several biomes (Mulholland et al. 2008). Other studies have also recently suggested the importance of denitrification in influencing N flux in running waters (Alexander et al. 2000; Peterson et al. 2001; Seitzinger et al. 2002; Pribyl et al. 2005).
Stream degradation due to land use change may impair denitrification in streams by increasing channel incision, headwater stream burial, amplifying nitrate export during wet years, and decreasing the hydrologic “connectivity” between the stream channel, the hyporheic zone, and riparian areas (Groffman et al. 2002; Böhlke et al. 2007; Elmore and Kaushal 2008; Kaushal et al. 2008b). Furthermore, elevated nitrate loads may “saturate” the ability of headwater streams to attenuate nitrate pollution and contribute to increased nitrate uptake lengths and decreased nitrate retention in streams (Bernot and Dodds 2005; Grimm et al. 2005; Meyer et al. 2005; Mulholland et al. 2008).
There has been a growing interest in the use of stream restoration as a partial means to offset the water quality effects of urbanization and other forms of land use change as indicated by the completion and planning of over 37,000 river restoration projects in the United States (Bernhardt et al. 2005). The goals of these diverse projects are often to restore water quality, restore riparian zones, improve in-stream habitat, reduce channel erosion, promote bank stability, and protect and repair aging infrastructure (Bernhardt et al. 2005; Palmer and Bernhardt 2006; Doyle et al. 2008). Little work has been done to actually evaluate the effectiveness of different stream restoration designs and more research is necessary to understand variability in N cycling (Bernhardt et al. 2005). Decreased flow velocity, increased hydrologic “connectivity” with floodplain wetlands and hyporheic zones, and increased hydrologic residence time may influence N cycling in restored streams during baseflow (Bukaveckas 2007; Roberts et al. 2007; Kaushal et al. 2008b). There is still little data regarding denitrification rates in restored streams, however, and it is not certain how these rates may vary across different restoration designs.
A key question may be related to the efficacy of different stream restoration designs on stimulating ecosystem functions such as nitrate uptake and denitrification. Some experimental designs may choose not to strictly focus on “restoration” and “re-creation” of pre-disturbance conditions, but may attempt to improve strategies for stormwater management practices while repairing aging urban infrastructure along stream valleys (Kaushal et al. 2008b). The objectives of this study were to (1) conduct background measurements of nitrate uptake and sediment denitrification potential in two restored and two unrestored streams in Baltimore, Maryland, USA and (2) to quantify rates of in situ denitrification in a reach of restored urban stream using a 15N stable isotope addition. The study reaches of the restored streams, Minebank Run and Spring Branch, were restored primarily to repair aging infrastructure along the stream corridor (exposed sewer lines, a damaged bridge) and enhance stormwater management and dissipation of erosive force using either hydrologically “connected” floodplains or stormwater ponds and catch basins below storm drains (US EPA River Corridor and Wetland Restoration 2002; Kaushal et al. 2008b). The work described here was part of two long-term research projects: the Baltimore Ecosystem Study (BES, http://beslter.org), an urban Long-term Ecological Research (LTER) project supported by the US National Science Foundation (Groffman et al. 2004; Kaushal et al. 2005, 2008a), and a long-term study of a restored stream, Minebank Run, supported by the US Environmental Protection Agency (Mayer et al. 2003; Groffman et al. 2005; Kaushal et al. 2008b; Gift et al. 2009).
Materials and methods
Study design
Background measurements of denitrification in stream sediments (similar to Groffman et al. 2005) and nitrate uptake using reach scale nutrient additions of nitrate (following the methods described in Stream Solute Workshop 1990) were conducted in two restored streams and two degraded urban streams in the Baltimore metropolitan area from June to August of 2006. These types of background measurements were collected to provide a context for interpretation of in situ denitrification rates at Minebank Run, also conducted during the summer of 2006, using 15N isotopic tracer techniques (following the methods described in Mulholland et al. 2004, 2008). All study sites were located in Baltimore County, Maryland, USA in the Gwynns Falls and Gunpowder Falls watersheds (Fig. 1).
Map of sites located within Baltimore County, Maryland. All sites are pictured within their 12 digit watershed. Minebank Run (MNBK) and Spring Branch (SPBR) are both part of the larger Gunpowder Falls watershed while Glyndon (GLYN) and the tributary of Dead Run (DR5) are both part of the larger Gwynns Falls watershed. Baltimore City is represented by the area highlighted in gray within the Baltimore County map
Site descriptions
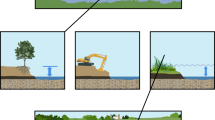
Minebank Run (MNBK) is a second order stream in the Gunpowder Falls watershed, a predominantly suburban watershed within Baltimore County, Maryland (Doheny et al. 2007; Fig. 1). The Lower Gunpowder watershed is approximately 11,828 ha with 30% agricultural, 32% forested, 18% urban, 19% suburban land cover, and 1% other land use. The section of Minebank Run chosen for this study was restored in 1998 and 1999 (Mayer et al. 2003; Kaushal et al. 2008b). Average baseflow discharge in this reach of Minebank Run during the study period was approximately 3.6 L s−1 but could show peaks of 20.4 L s−1 based on precipitation events (Ed Doheny and Jon Dillow, US Geological Survey unpublished results; Doheny et al. 2006). Restoration in the stream included techniques such as installing step-pool structures designed to reduce erosion. The primary restoration manipulation, however, was a reshaping of the stream banks to create hydrologically “connected” floodplains for improved stormwater management, dissipation of erosive force, and re-establishing riparian vegetation in contrast to high armored banks and channelized structures (Mayer et al. 2003; Groffman et al. 2005; Kaushal et al. 2008b) (Fig. 2a).
a Stream reach with hydrologically “connected” floodplain at Minebank Run where experimental nitrate injection and 15N tracer experiment were performed (Photo by P. Mayer). b Series of stormwater ponds below a storm drain in the headwaters of Spring Branch (Photo by T. Newcomer). c Spring Branch downstream of stormwater ponds and catch basins where experimental nitrate injection was performed (Photo by T. Newcomer)
Spring Branch (SPBR), a restored first order stream in Baltimore County, Maryland, drains the suburban Loch Raven watershed, eventually emptying into the Loch Raven Reservoir, a major drinking supply for the Baltimore Metropolitan area (Fig. 1). Land use for the Loch Raven, 9,437 ha, watershed was 12% agriculture, 36% forested, 14% urban, 29% suburban, and 9% other. Routine discharge measurements were not available at Spring Branch during the study period, but baseflow discharge can be 2–3 L s−1 with variations based on precipitation events (T. Newcomer and G. Stanko, unpublished results). The goal of restoration at Spring Branch, completed in 1997, was to manage stream flow to control for erosion and floods (US EPA River Corridor and Wetland Restoration 2002). The primary restoration feature included upstream stormwater ponds below the storm drain outfall, plunge pools below storm pipe outfalls, catch basins to attenuate flow, and hydrologically “connected” floodplain access for bankfull discharges (US EPA River Corridor and Wetland Restoration 2002; Gift et al. 2009). The present study reach of Spring Branch was downstream from a series of shaded headwater stormwater ponds (Fig. 2b, c).
Two degraded suburban/urban streams, which were routinely monitored as part of the National Science Foundation supported Baltimore Long-term Ecological Research (LTER) site were also studied, Glyndon and DR5. Glyndon (GLYN) is the first order headwater sub-watershed of the 19,000 ha Gwynns Falls (Groffman et al. 2004; Kaushal et al. 2005) (Fig. 1). The 4,607 ha Upper Gwynn Falls watershed that Glyndon is within consists of 7% agriculture, 24% forested, 50% urban, 17% suburban, and 1% other land cover. Average baseflow discharge in Glyndon during the study period was approximately 2.6 L s−1, but discharge can show very strong peaks of >100 L s−1 during storms (US Geological Survey, unpublished data). DR5 is a headwater tributary of the larger third order Dead Run stream in the lower Gwynns Falls watershed (Fig. 1). Land use for the 12,233 ha of the Lower Gwynns Falls watershed is 2% agriculture, 14% forested, 75% urban, 8% suburban, and 1% other. Routine discharge was not available at DR5 during the study period, but discharge can be 2–3 L s−1 during baseflow conditions and can show very strong peaks >100 L s−1 during storms (USGS, provisional data http://waterdata.usgs.gov/md/nwis/dv?referred_module=sw&site_no=01589312).
Streamwater chemistry at Minebank Run
Approximately 52 surface water samples from Minebank Run were collected routinely from 1 October 2005 to 1 October 2006. Time-series samples for nitrate concentrations were collected at USGS station 0158397925, Minebank Run at Intervale Court, Towson, Maryland, USA, since June of 2004 (further information on Minebank Run can be found at http://waterdata.usgs.gov/nwis/uv?0158397967). Samples were stored, filtered and analyzed as described below.
Denitrification enzyme assays (DEA)
Sediment samples were collected from each of the four study reaches in June 2006 to measure denitrification potential. The upper 10 cm of sediment was collected using a random sampling design from the measured center of the stream every 25 m (along the 200 m designated reach) and were refrigerated until analysis within a week of collection. Nine sediment samples were collected per each stream reach, and denitrification enzyme activity was measured using a short term denitrification enzyme assay on four replicate subsamples in the laboratory (Groffman et al. 2005). Briefly, we amended sieved sediments with NO3 −, dextrose, chloramphenicol, and acetylene, and incubated them in sealed flasks under anaerobic conditions for 90 min. We took samples from the air space of flasks at 30 and 90 min, stored them in evacuated glass tubes, and then analyzed them for N2O by electron capture gas chromatography (Groffman et al. 2005).
Stream nitrate injections
Nitrate injections were conducted once at all four sites during July and August of 2006. A solution of KNO3 − and NaBr was dripped at the upstream location of all four study reaches for 6–10 h, allowing enough time for concentrations at all stations to plateau. Concentrations of NO3 −N are typically between 1 and 2 mg L−1 in the urban streams of Baltimore (Groffman et al. 2004; Kaushal et al. 2008a), therefore the goal of the injection was to raise background stream concentrations of nitrate by at least 500 μg N L−1.
Stations were located in areas of the stream where the channel was constricted to ensure well-mixed samples at each station (LINX II 2004; Mulholland et al. 2008). Stream study reaches ranged from 74 to 212 m in length with 5 sampling stations located throughout the reach. Samples collected before nutrient additions were analyzed to determine background concentrations of nitrate and bromide in the stream at each station. Samples taken throughout the addition at each site (approximately every 30 min) were used to estimate the time of arrival of the nitrate and bromide at each station as well as to determine when concentration plateau or equilibrium had been reached at each site (Stream Solute Workshop 1990; Webster and Ehrman 1996; LINX II 2004; Mulholland et al. 2008). Samples of water were collected and transported to the University of Maryland Center for Environmental Science Appalachian Laboratory, Frostburg, Maryland for filtration and storage. Bromide analyses were performed using a Dionex 500 ion chromatograph. Analyses of nitrate, nitrite, ammonium and total nitrogen were performed on all pre and plateau samples using a Lachat Quick Chem 8000 autoanalyzer (Lachat Instruments, Milwaukee, WI, USA).
Because of low flow and difficulty in making accurate measurements with a flow meter, reach travel time, stream discharge, and average stream surface water velocity were estimated using channel measurements and analysis of the conservative tracer data. Channel measurements of wetted width and reach length were collected within the study reaches using a meter tape (Webster and Ehrman 1996). Reach travel time was calculated as the difference between the times at which the bromide breakthrough curves for the upstream and downstream stations reached the maximum rate of increase (Houser et al. 2005; Roberts et al. 2007). Average stream surface water velocity (u) was then calculated as reach length divided by reach travel time. Discharge (Q) was calculated as:
where Q pump = the injection rate of the pump, Brinj = the concentration of bromide in the injection solution, Brp = the concentration of bromide at the station during plateau, Brb = the background concentration of bromide at the station (Webster and Ehrman 1996; Houser et al. 2005; Roberts et al. 2007).
Nitrate uptake length in the four stream reaches was estimated using the nutrient spiraling metrics equations (Newbold et al. 1981; Stream Solute Workshop 1990; Webster and Ehrman 1996). Plateau concentrations of both nitrate and bromide were corrected for background concentrations. Nitrate was also corrected for dilution using the ratio of nitrate to bromide (Stream Solute Workshop 1990; Webster and Ehrman 1996). Uptake length (S w) was calculated as the negative inverse slope of the regression line of the natural log of the dilution-corrected concentration of nitrate versus distance downstream (Stream Solute Workshop 1990; Grimm et al. 2005; Gücker and Pusch 2006). The slope of this line is the fractional rate of decline of the nitrate, k. Uptake rate and uptake velocity were calculated using the spiraling metric equations:
where S w = uptake length (m), U = uptake rate (μg m−2 s−1), V f = uptake velocity (mm h−1), Q = discharge (L s−1), C = concentration of the nutrient (mg L−1), and w = stream wetted width (m).
Isotope addition and denitrification in Minebank Run
15N–NO3− addition and in situ denitrification
A 15N–NO3 − isotope addition was conducted at Minebank Run from 16 to 17 August 2006, to provide a more intensive measurement of the three nutrient spiraling metrics and to quantify actual denitrification rates at the reach scale without raising the ambient concentration of nitrate in the stream. The length of the study reach was 220.5 m. A solution of 99% 15N labeled KNO3 − along with the conservative tracer Br− was dripped in the stream for 27 h to increase the δ15N of nitrate in the stream by approximately 20,000‰ (LINX 2004).
Methods were similar to LINX II (2004) and water samples were collected at each station before and at plateau for 15NO3 − analysis. Nitrate was reduced to ammonium using Devarda’s alloy and an ammonium alkaline headspace diffusion procedure was used to collect 15N from the sample for analysis (Sigman et al. 1997; LINX II 2004). Water samples were collected in plastic syringes for extraction of 15N labeled gases (Mulholland et al. 2004, 2008). Helium headspace was added to each syringe while under stream water to further guard against contamination of N2 from the atmosphere (Mulholland et al. 2004; LINX II 2004; Hamilton and Ostrom 2007; Mulholland et al. 2008). Equilibrated headspace was injected into evacuated exetainers (Labco, Buckinghamshire, England). Exetainers were stored in centrifuge tubes filled with water to prevent diffusion of N2 gas from the atmosphere (Mulholland et al. 2004; LINX II 2004; Hamilton and Ostrom 2007; Mulholland et al. 2008). Filters infused with 15N and gas samples were sent to the University of California-Davis Stable Isotope Laboratory to be analyzed for 15N/14N isotopic ratios using isotope ratio mass spectrometry, and precision was 0.3‰ for replicate analyses of reference standards (Cloern et al. 2002).
Reaeration rates and whole stream metabolism
Reaeration rate of O2 and whole stream metabolism were determined following methods described by Marzolf et al. (1994) and adapted by Young and Huryn (1998). The gas exchange rate of propane was determined by the slope of a regression of the natural log of the dilution-corrected concentration of propane versus distance downstream, and a reaeration rate (k 2) for dissolved O2, N2 and N2O was determined (Marzolf et al. 1994; Young and Huryn 1998; Mulholland et al. 2004).
15N mass flux calculations
Tracer 15N flux was calculated from measured δ15N using a series of equations developed by Mulholland et al. (2004). Briefly, δ15N values were converted to mole fraction ratios using the equation:
The 15NO3 − mass flux (15N flux i ) was then calculated as
where MF i = the plateau mole fraction at the station, MFbi = is the background (or Pre) mole fraction at the station, [NO3 −-N] is the concentration of nitrate at the station and Q i = stream discharge at each station. Q i was calculated from the same equation used for the nutrient additions (Eq. 1). The ln 15N flux was plotted against distance downstream to calculate fractional rate of decline of the nitrate (k total) and uptake length (S w). Uptake rate (U) and uptake velocity (V f) were then calculated similarly to the nitrate additions using Eqs. 3 and 4.
In order to determine the concentrations of 15N2 and 15N2O, the measured headspace δ15N values were first corrected for isotopic fractionation (Mulholland et al. 2004, 2008). N mass values were corrected for incomplete gas transfer into the headspace using the volumes of headspace and water for each sample along with the Bunsen coefficients for N2 and N2O at the same pressure and temperature the headspace equilibration was performed (Mulholland et al. 2004). Mole fraction values were calculated from the fractionation corrected δ15N using Eq. 5. Fluxes of 15N2 and 15N2O were then calculated using Eq. 6.
Production rates of N2 and N2O from denitrification were estimated by fitting a denitrification model, created by Mulholland et al. (2004), to the average tracer 15N flux data for N2 and N2O at each station. A least squares fitting technique was used with the model to estimate values for fractional rate of decline of the nitrate due to denitrification (k den) from the 15N mass flux data for N2 and N2O separately. Denitrification rates were also calculated as a nitrate mass removal rate per unit area for N2 and N2O using the uptake rate equation (Eq. 3) and the model predicted k den values for each (Mulholland et al. 2004). To quantify uncertainty, model simulations were run while varying the gas exchange rate of N2 and N2O (k 2) by 0.5 k 2 and 2 k 2 and by varying k den.
Statistical analysis
All statistical analyses were performed using SAS Analyst (version 9.1, SAS Institute, Cary, North Carolina, USA). Significance for all of the reported data was determined at α = 0.05. Regression analyses were used to examine the longitudinal pattern of N–NO3 − uptake within each stream and the produced slope (k) was used to calculate the uptake length (S w) for each stream. To evaluate the differences in uptake length, and the related uptake rate and uptake velocity, an analysis of covariance (ANCOVA) was used on the pooled regression data. Analysis of variance (ANOVA) followed by the Tukey–Kramer adjusted least square means test, was used to examine differences in denitrification potential and background [N–NO3 −] between sites. For comparisons of denitrification potential and uptake length, pairwise Pearson correlations were used on pooled data across all streams.
Results
Nitrate concentrations in the stream water at Minebank Run ranged between 0.76 and 1.36 mg L−1 for the months of June–August, with a mean concentration of 1.03 mg L−1 (0.12 SE) for the month of June, 1.04 mg L−1 (0.08 SE) for July, and 0.87 mg L−1 (0.04 SE) for August (Fig. 3). Nitrate concentrations varied significantly (p < 0.05) in the four streams; SPBR had the highest nitrate–N concentrations, DR5 had the lowest nitrate–N concentrations, and MNBK and GLYN were intermediate (Table 1).
Denitrification rates in surface sediments of the stream bed were variable and showed no predictable pattern across sites or with stream water nitrate concentrations. MNBK, SPBR, and DR5 had significantly higher mean denitrification potential than GLYN (Table 2). DR5 had the highest mean denitrification potential in streambed sediments of the four streams and was significantly higher than GLYN and MNBK, but not SPBR. DR5 and SPBR had similar denitrification potentials in streambed sediments.
Surface water velocity measurements were low in all four streams, particularly the two restored sites MNBK and SPBR (2.1 and 2.0 cm s−1, Table 1). Travel times for the four reaches ranged from 1 to 3 h over distances of 74–212 m, with plateau at the final stations not occurring until several hours after additions began (Fig. 4a). Travel time for MNBK was especially slow, as it took 5.5 h to reach plateau at the furthest station, only 74 m from the injection site (Fig. 4a).
a An example of bromide concentrations and travel times for a nitrate addition at Minebank Run. b Relationship between stream surface water velocity, u, and nitrate uptake length, Sw, for the four urban streams; (R2 = 0.92, p < 0.05, n = 4). Restored streams (MNBK and SPBR) had the slowest stream water velocities
MNBK had the shortest uptake length, 357 m, while DR5 had the longest, 1,341 m (Table 2), but there were no significant differences in uptake slopes (k, the fractional rate of decline of nitrate) among the four sites. There was a significant decline in the ln corrected concentration of N–NO3 − with distance downstream in DR5 and GLYN (R 2 = 0.84 and 0.98, respectively), but not in MNBK and SPBR. There was a significant relationship between uptake length (S w) and mean surface velocity (u) in the four streams (R 2 = 0.92, p < 0.05; Fig. 4b). There was no correlation between S w and Q or between S w and either background [N–NO3 −] or experimental [N–NO3 −]. There were significant correlations between background [N–NO3 −] and both U and V f.
Isotope addition and in situ measurements of denitrification
Background physical and chemical properties of Minebank Run during the isotope addition experiment are presented in Table 3. A significant longitudinal linear decrease was observed for the tracer 15NO3 − flux versus distance downstream, R 2 = 0.89, p < 0.05 (Fig. 5a). An uptake length (S w) of 556 m was calculated from the slope of this line (k tot). Uptake rate (U) was calculated to be 1.75 μg m−2 s−1 and uptake velocity (V f) was 1.80 × 10−3 mm s−1 (Table 4). When examining patterns at the first three stations, the portion of the reach also used for the nitrate additions, uptake length (S w) was shorter at 204 m (R 2 = 0.99 and k tot = 0.0049). The corresponding uptake rate (U) and uptake velocity (V f) were 5.0 μg m−2 s−1 and 4.7 mm s−1, respectively.
The air water exchange rate of propane for the entire stream reach was 0.0223 m−1. This rate was then used to calculate an exchange rate (k 2) for N2, 0.0292 m−1, and N2O, 0.0286 m−1. A hump shaped curve, similar to those reported by other researchers (Mulholland et al. 2004), was found for the values of 15N mole fraction for N2 versus distance downstream (Fig. 5b). In addition, the propane addition and dissolved oxygen measurements were used to estimate a gross primary production (GPP) of 1.12 g O2 m−2 day−1 during the time of the isotope tracer addition and community respiration (CR24) of 9.26 g O2 m−2 day−1 (calculated similarly to Marzolf et al. 1994; Young and Huryn 1998). Net ecosystem metabolism (NEM), calculated as community respiration (CR24) subtracted from GPP, NEM = GPP − CR24 (Marzolf et al. 1994; Young and Huryn 1998; Meyer et al. 2005) was −8.14 g O2 m−2 day−1, indicating that metabolism during the isotope addition was dominated by heterotrophic activity (which was conducive to denitrification) in the subsurface hyporheic zone versus autotrophic activity in the stream channel.
Denitrification rates were determined using the Mulholland et al. (2004) denitrification model, which was separately fitted to the 15N flux data for both N2 and N2O. Due to detection problems with N2O, the model was fit to data from only the first 50 m of the reach for N2O. The best fit k den for N2 production was 0.0016, 89% of the k total (0.0018). N2O production was considerably less and the best fit k den was 1.96 × 10−4, approximately 11% of k den. The mass flux rates of N2 and N2O production per unit area (U den-N2 and U den-N2O) were 136.6 and 16.5 mg N m−2 day−1, respectively, totaling 153 mg N m−2 day−1 removed by denitrification (Table 4). When adjusting values of the gas exchange rates (k 2) and k den for uncertainty analysis, k den for N2 production ranged from 0.0010 to 0.0025 m−1, and ranged from 1.10 × 10−4 to 2.95 × 10−4 m−1 for N2O.
Discussion
Our results suggest that nitrate uptake and denitrification processes in sediments and at the stream reach scale may be important in influencing nitrate dynamics in some unrestored and restored streams in urban Maryland, USA, and that hydrologic residence time during baseflow may be an important variable. Previous work at some of the present study sites has shown that low-density suburban catchments export total nitrogen and nitrate loads mostly at relatively low flows, whereas more urbanized sites export total nitrogen and nitrate at higher and less frequent flows (Shields et al. 2008). Therefore, variations in export distribution should be considered when targeting stream channel restoration efforts at the watershed scale (Shields et al. 2008) and when considering variability in the efficacy of different stream restoration designs.
We investigated the importance of nitrogen transformations in several different ways including denitrification enzyme assays, nitrate solute injections, and use of a 15NO3 − tracer injection at one of the sites. Denitrification enzyme activity from surface sediments in the stream bottom showed no obvious patterns among streams and was highly variable. Nonetheless, results showed that substantial denitrification in surface sediments could be occurring in Minebank Run, Spring Branch, and the tributary of Dead Run at rates similar to ranges reported for urban streams in Baltimore (Groffman et al. 2005; Gift et al. 2009). Urban streams may have sediment microzones with low oxygen and high organic matter content that function as “hot spots” of denitrification (McClain et al. 2003). Denitrification studies in stream sediments have found rates to be highly variable and “patchy” both within and among surface sediments in streams contributing to the variability observed in the present study (Groffman et al. 2005). For example, previous work in streams of the present study have shown significant variations in denitrification rates in debris dams, pools, riffles, vegetated gravel bars, and mucky gravel bars (Groffman et al. 2005). More research is needed regarding the effects of restoration and watershed land use on the spatial distribution of stream benthic habitats and implications for N processing.
Nitrate uptake in Minebank Run relative to the other streams suggested that travel times can be long in both the restored and degraded urban streams during summer baseflow conditions as indicated by the long time for conservative tracers to reach a stable plateau after beginning continuous additions. We did not observe a strong relationship between uptake length, S w, and discharge, Q, as is often found in stream nutrient addition studies (Peterson et al. 2001; Grimm et al. 2005). We did observe an increase in uptake length (S w) with increasing surface water velocity (u), however. Interpretation of these results should be considered with caution given the small number of measurements of uptake length. Given the restoration strategies of hydrologically “connected” floodplains at Minebank Run and upstream flow through a series of stormwater ponds at Spring Branch, it is not surprising that restoration could alter flow conditions. Minebank Run and Spring Branch, the two restored streams, had the lowest surface water velocities as well as the shortest uptake lengths of the four sites, but there was no significant difference in other uptake metrics between restored and degraded streams. Increasing hydrologic contact with sediments may influence N cycling under certain stream restoration conditions (Kasahara and Hill 2006; Bukaveckas 2007; Roberts et al. 2007; Kaushal et al. 2008b; Craig et al. 2008). For example, previous work at Minebank Run has shown a strong relationship between mass removal of nitrate and hydrologic residence time (Kaushal et al. 2008b), and integrating restoration approaches with stormwater management in uplands, watersheds, and stream valleys to slow the flow of water may be critical to maximizing N removal (Kaushal et al. 2008a).
Although many studies have focused on reporting uptake length, S w, there can be variability in S w among streams due to both biogeochemical and hydrogeomorphic effects (Doyle et al. 2003). Therefore, there is also an interest in uptake velocity, V f, the rate at which a nutrient travels vertically before being taken up by the stream bed, because it normalizes for the effects of stream hydrogeomorphology and is more strongly related to biogeochemical changes (Davis and Minshall 1999; Doyle et al. 2003). Our uptake velocity values were lower than those typically reported (Davis and Minshall 1999; Grimm et al. 2005; Gücker and Pusch 2006) and were lower than the average V f value for first order streams (168 mm h−1) determined in a comprehensive review of 52 reported literature values by Ensign and Doyle (2006). Uptake rate and length, when compared to other streams, would indicate that Minebank Run is highly retentive, yet uptake velocity indicated otherwise. We speculate that low discharges in Minebank Run and the three other study sites during baseflow conditions may have contributed to the estimates of low uptake velocity when compared to other studies. V f represents the vertical velocity of the movement of nutrient molecules through the water column towards the benthos (Ensign and Doyle 2006). Another way of expressing this metric may be u × h ÷ S w, where u = stream velocity, h = depth, and S w = uptake length (Ensign and Doyle 2006). Therefore, very low values of stream velocity in the present study could have contributed to low estimations of V f.
Nitrate uptake length measured from the 15N isotope tracer addition was 556 m in Minebank Run. When comparing our isotope spiraling metric results to other studies that used the 15N isotope tracer technique, uptake length for Minebank Run was longer than the forested Walker Branch (35.7 m) studied by Mulholland et al. (2004) and the arid desert Agua Fria River (36 m) studied by Grimm et al. (2005) but shorter than the agricultural streams (800–9,600 m) studied by Bernot et al. (2006). Minebank Run was similar to and within the mid-range for the three urban arid streams also studied by Grimm et al. (2005). Surprisingly, we found no relationship between nitrate uptake and background nitrate concentrations at Minebank Run and the other three stream sites from unenriched additions. This may have been due to small sample size, small range in background concentrations, or the importance of hydrologic factors during baseflow conditions.
According to the Mulholland et al. (2004) model, the best fit fractional NO3 − removal rate due to denitrification (k den) was 0.0018 m−1, with an uncertainty of approximately ±0.001 m−1. Thus, the model suggests 100% of uptake was explained by denitrification, with the uncertainty being ±40%. Production of N2 made up approximately 89% of denitrification while N2O production made up the remaining 11%. The uncertainty was relatively high and may have been due to analytical factors such as incomplete gas transfer (although these samples were adjusted based on equations by Mulholland et al. 2004).
Despite the large uncertainty, denitrification still appeared to comprise a majority of NO3 − uptake within Minebank Run during the isotope addition. The denitrification rate for Minebank Run from the isotope addition, expressed as removal per unit of area, was 153 mg N m−2 day−1. Even at our highest uncertainty estimate, denitrification in Minebank Run comprised up to 60% of the NO3 − uptake within the reach and could potentially account for removal of 40% of the daily nitrate load during summer over the 220.5 m study reach. The importance of denitrification at Minebank Run is supported by results from the propane addition and dissolved oxygen measurements which allowed us to estimate a gross primary production. Results from gross primary production estimates indicated that the metabolism during the isotope addition was dominated by heterotrophic activity (e.g., denitrification) in the subsurface hyporheic zone versus autotrophic activity in the stream channel. This reach of Minebank Run has a densely shaded riparian canopy that covers the stream and reduces light penetration. Therefore, denitrification may have been the predominant process controlling N uptake due to riparian canopy shading (Mulholland et al. 2008).
Denitrification enzyme activity (DEA) in the surface sediments and in situ denitrification rates using the 15N tracer approach both showed that denitrification was occurring at Minebank Run. The two measurements use different methodologies, and their measurements target different locations (i.e., DEA measured potential denitrification activity in surface sediments as opposed to in situ denitrification at the stream reach scale using the 15N addition). Although denitrification rates in surface sediments could have been important, results from the 15N tracer addition also suggest that subsurface hydrologic flowpaths may have also been important in influencing denitrification at Minebank Run (Kaushal et al. 2008b). In situ denitrification at Minebank Run was within the range reported elsewhere using similar methodology (Mulholland et al. 2004, 2008; Böhlke et al. 2004). Recently, Mulholland et al. (2008) reported that denitrification accounted for a median of 16% of nitrate uptake in a large study of 72 streams across biomes. Other studies have shown that denitrification accounted for 16% of uptake within Walker Branch, a forested stream in Tennessee (Mulholland et al. 2004), and 52% of uptake within Sugar Creek, an agricultural stream in Indiana (Böhlke et al. 2004).
Despite the relatively high denitrification rates during baseflow conditions at Minebank Run, the rate of N loading in urban streams can be larger than biotic demand, thus exceeding the potential for removal via denitrification (Mulholland et al. 2008). Furthermore, high flow conditions during “flashy” storm events may greatly compromise urban stream and watershed retention processes (Booth 2005; Walsh et al. 2005). Stormwater management strategies that increase hydrologic residence time in watersheds (uplands, wetlands, and streams) may be extremely critical for managing N during high flow conditions (Booth 2005; Walsh et al. 2005; Kaushal et al. 2008a). Our isotopic study was conducted in the summer under baseflow conditions and high temperature, which are optimal conditions for maximum denitrification. Denitrification performance across differing restoration designs and hydrologic conditions is likely to be highly variable and contain large uncertainties. Because there can be large variability in the effectiveness of different designs (Kaushal et al. 2008b), stream restoration by itself is currently not adequate to mitigate for excess N inputs or to compensate for stream destruction and degradation. Certain forms of stream restoration aimed at increasing hydrologic residence time and hydrologic “connectivity” with denitrification “hot spots” may have the potential to complement comprehensive watershed management strategies when also focusing on reducing effective impervious surface coverage in uplands (Walsh et al. 2005) and repairing aging infrastructure (Doyle et al. 2008). This is particularly important in Baltimore, Maryland, where many restorations have been conducted while repairing urban infrastructure such as sewer lines, and watersheds were urbanized before current stormwater practices could be implemented. Future studies should investigate the effects of varying nitrate loads, hydrologic residence times, hydrological “connectivity,” and seasonality on denitrification rates in restored streams. Future studies should also include the use of models, such as the OTIS and OTIS-P models (Gooseff et al. 2003; Runkel 2007), which are transport-based, time-series approaches that provide estimates of U and V f independent of hydrologic effects, allowing for separation of hydrologic and non-hydrologic processes. Finally, more studies are necessary to evaluate the efficacy of various stream restoration designs on denitrification rates under different land uses and stream flow conditions (Tague et al. 2008).
In conclusion, stream restoration approaches that increase hydrologic “connectivity” with hyporheic sediments and increase hydrologic residence times may influence denitrification at the stream reach scale. Although stream restorations are likely to continue or grow in the future (Bernhardt et al. 2005; Palmer and Bernhardt 2006), very little work has been done on measuring nitrogen uptake and denitrification in restored streams (Bukaveckas 2007; Roberts et al. 2007; Kaushal et al. 2008b). Stream restoration is still a highly experimental field and designs that improve biogeochemical function may not be necessarily related to recreating pre-disturbance conditions (Palmer 2009). More empirical research combining water quality monitoring and measurements of ecosystem functions is necessary across different sites and under varying hydrologic conditions to evaluate the effectiveness of different stream restoration designs.
References
Alexander RB, Smith RA, Schwartz GE (2000) Effect of stream channel size on the delivery of nitrogen to the Gulf of Mexico. Nature 403:758–761
Bernhardt ES, Palmer MA, Allan JD, Alexander G, Barnas K, Brooks S, Carr J, Clayton S, Dahm C, Follstad-Shah J, Galat D, Gloss S, Goodwin P, Hart D, Hassatt B, Jenkinson R, Katz S, Kondolf GM, Lake PS, Lave R, Meyer JL, O’Donnell TK, Pagano L, Powell B, Sudduth E (2005) Synthesizing US river restoration efforts. Science 308:636–637
Bernot MJ, Dodds WK (2005) Nitrogen retention, removal, and saturation in lotic ecosystems. Ecosystems 8:442–453
Bernot MJ, Tank JL, Royer TV, David MB (2006) Nutrient uptake in streams draining agricultural catchments of the midwestern United States. Freshw Biol 51:499–509
Boesch DF, Brinsfield RB, Magnien RE (2001) Chesapeake Bay eutrophication: Scientific understanding, ecosystem restoration, and challenges for agriculture. J Environ Qual 30:303–320
Böhlke JK, Harvey JW, Voytek MA (2004) Reach-scale isotope tracer experiment to quantify denitrification and related processes in a nitrate-rich stream, mid-continent United States. Limnol Oceanogr 49:821–838
Böhlke JK, O’Connell ME, Prestegaard KL (2007) Ground water stratification and delivery of nitrate to an incised stream under varying flow conditions. J Environ Qual 36:664–680
Booth DB (2005) Challenges and prospects for restoring urban streams: a perspective from the Pacific Northwest of North America. J N Am Benthol Soc 24:724–737
Bukaveckas PA (2007) Effects of channel restoration on water velocity, transient storage, and nutrient uptake in a channelized stream. Environ Sci Technol 41:1570–1576
Cloern JE, Canuel EA, Harris D (2002) Stable carbon and nitrogen isotope composition of aquatic and terrestrial plants of the San Francisco estuarine system. Limnol Oceanogr 47:713–729
Craig LS, Palmer MA, Richardson DC, Filoso S, Bernhardt ES, Bledsoe BP, Doyle MW, Groffman PM, Hassett B, Kaushal SS, Mayer PM, Smith SM, Wilcock PR (2008) Stream restoration strategies for reducing river nitrogen loads. Front Ecol Environ 6(10):529–538. doi:10.1890/070080
Davis JC, Minshall GW (1999) Nitrogen and phosphorus uptake in two Idaho (USA) headwater wilderness streams. Oecologia 119:247–255
Doheny EJ, Starsoneck RJ, Striz EA, Mayer PM (2006) Watershed characteristics and pre-restoration surface-water hydrology of Minebank Run, Baltimore County, Maryland, water years 2002–2004: US Geological Survey Scientific Investigations Report 2006–5179, 42 p
Doheny EJ, Starsoneck RJ, Mayer PM, Striz EA (2007) Pre-restoration geomorphic characteristics of Minebank Run, Baltimore County, Maryland, 2002–2004. USGS Scientific Investigations Report 2007–5127
Doyle MW, Stanley EH, Harbor JM (2003) Hydrogeomorphic controls on phosphorus retention in streams. Water Resour Res 39:1147. doi:10.1029/2003WR002038
Doyle MW, Stanley EH, Havlick DG, Kaiser MJ, Steinbach G, Graf WL, Galloway GE, Riggsbee JA (2008) Aging infrastructure and ecosystem restoration. Science 319:286–287
Elmore AJ, Kaushal SS (2008) Disappearing headwaters: Patterns of stream burial due to urbanization. Front Ecol Environ 6:308–312. doi:10.1890/070101
Ensign SH, Doyle MW (2006) Nutrient spiraling in streams and river networks. J Geophysical Res Biogeosciences 111(G4):G04009
Gift D, Groffman PM, Kaushal SS, Mayer PM, Striz EA (2009) Root biomass, organic matter and denitrification potential in degraded and restored urban riparian zones. Restor Ecol. doi:10.1111/j.1526-100X.2008.00438.x
Gooseff MN, Wondzell SM, Haggerty R, Anderson J (2003) Comparing transient storage modeling and residence time distribution (RTD) analysis in geomorphically varied reaches in the Lookout Creek basin, Oregon, USA. Adv Water Resour 26:925–937
Grimm NB, Sheibley RW, Crenshaw CL, Dahm CN, Roach WJ (2005) N retention and transformation in urban streams. J N Am Benthol Soc 24:626–642
Grimm NB, Faeth SH, Golubiewski NE, Redman CL, Wu J, Bai X, Briggs JM (2008) Global change and the ecology of cities. Science 319:756–760
Groffman PM, Boulware NJ, Zipperer WC, Pouyat RV, Band LE, Colosimo MF (2002) Soil nitrogen cycle processes in urban riparian zones. Environ Sci Technol 36:4547–4552
Groffman PM, Law NL, Belt KT, Band LE, Fisher GT (2004) Nitrogen fluxes and retention in urban watershed ecosystems. Ecosystems 7:393–403
Groffman PM, Dorsey AM, Mayer PM (2005) N processing within geomorphic structures in urban streams. J N Am Benthol Soc 24:706–723
Gücker B, Pusch MT (2006) Regulation of nutrient uptake in eutrophic lowland streams. Limnol Oceanogr 51:1443–1453
Gücker B, Brauns M, Pusch MT (2006) Effects of wastewater treatment plant discharge on ecosystem structure and function of lowland streams. J N Am Benthol Soc 25:313–329
Hamilton SK, Ostrom NE (2007) Measurement of the stable isotope ratio of dissolved N2 in 15N tracer experiments. Limnol Oceanogr Methods 5:233–240
Houser JN, Mulholland PJ, Maloney KO (2005) Catchment disturbance and stream metabolism: patterns in ecosystem respiration and gross primary production along a gradient of upland soil and vegetation disturbance. J N Am Benthol Soc 24:538–552
Howarth RW, Billen G, Swaney D, Townsend A, Jaworski N, Lajtha K, Downing JA, Elmgren R, Caraco N, Jordan T, Berendse F, Freney J, Kudeyarov V, Murdoch P, Zhu ZL (1996) Regional nitrogen budgets and riverine N & P fluxes for the drainages to the North Atlantic Ocean: Natural and human influences. Biogeochemistry 35:75–139
Kasahara T, Hill AR (2006) Effects of riffle-step restoration on hyporheic zone chemistry in N-rich lowland streams. Can J Fish Aquatic Sci 63:120–133
Kaushal SS, Groffman PM, Likens GE, Belt KT, Stack WP, Kelly VR, Band LE, Fisher GT (2005) Increased salinization of freshwater in the northeastern United States. Proc Natl Acad Sci USA 102:13517–13520
Kaushal SS, Groffman PM, Band LE, Shields CA, Morgan RP, Palmer MA, Belt KT, Fisher GT, Swan CM, Findlay SEG (2008a) Interaction between urbanization and climate variability amplifies watershed nitrate export in Maryland. Environ Sci Technol 42:5872–5878
Kaushal SS, Groffman PM, Mayer PM, Striz E, Doheny EJ, Gold AJ (2008b) Effects of stream restoration on denitrification at the riparian-stream interface of an urbanizing watershed. Ecol Appl 18:789–804
Kemp WM, Boynton WR, Adolf JE, Boesch DF, Boicourt WC, Brush G, Cornwell JC, Fisher TR, Glibert PM, Hagy JD, Harding LW, Houde ED, Kimmel DG, Miller WD, Newell RIE, Roman MR, Smith EM, Stevenson JC (2005) Eutrophication of Chesapeake Bay: historical trends and ecological interactions. Mar Ecol Prog Ser 303:1–29
LINX II (2004) Stream 15N experiment protocols. http://www.biol.vt.edu/faculty/webster/linx/linx2proto-rev5.pdf
Marzolf ER, Mulholland PJ, Steinman AD (1994) Improvements to the diurnal upstream–downstream dissolved-oxygen change technique for determining whole-stream metabolism in small streams. Can J Fish Aquatic Sci 51:1591–1599
Mayer PE, Striz E, Shedlock R, Doheny E, Groffman PM (2003) The effects of ecosystem restoration on nitrogen processing in an urban mid-Atlantic Piedmont stream. In: Renard KG et al. (eds) First Interagency Confer. Res. in the Watersheds, Benson, Arizona, 27–30 October 2003. Agric. Res. Serv., US Department of Agric., Washington, DC, pp 536–542 (http://www.tuscon.ars.ag.gov/icrw/Proceedings/mayer.pdf)
Mayer PM, Reynolds SK, McMutchen MD, Canfield TJ (2007) Meta-analysis of nitrogen removal in riparian buffers. J Environ Qual 36:1172–1180
McClain ME, Boyer EW, Dent CL, Gergel SE, Grimm NB, Groffman PM, Hart SC, Harvey JW, Johnston CA, Mayorga E, McDowell WH, Pinay G (2003) Biogeochemical hot spots and hot moments at the interface of terrestrial and aquatic ecosystems. Ecosystems 6:301–312
Meyer JL, Paul MJ, Taulbee WK (2005) Stream ecosystem function in urbanizing landscapes. J N Am Benthol Soc 24:602–612
Mulholland PJ, Valett HM, Webster JR, Thomas SA, Cooper LW, Hamilton SK, Peterson BJ (2004) Stream denitrification and total nitrate uptake lengths measured using 15N tracer addition approach. Limnol Oceanogr 49:809–820
Mulholland PJ, Helton AM, Poole GC, Hall RO, Hamilton SK, Peterson BJ, Tank JL, Ashkenas LR, Cooper LW, Dahm CN, Dodds WK, Findlay SEG, Gregory SV, Grimm NB, Johnson SL, McDowell WH, Meyer JL, Valett HM, Webster JR, Arango CP, Beaulieu JL, Bernot MJ, Burgin AJ, Crenshaw CL, Johnson LT, Niederlehner BR, O’Brien JM, Potter JD, Sheibley RW, Sobota DJ, Thomas SM (2008) Stream denitrification across biomes and its response to anthropogenic nitrate loading. Nature 13:452(7184):202-5 18337819
Newbold JD, Elwood JW, O’Neill RV, VanWinkle W (1981) Measuring nutrient spiraling in streams. Can J Fish Aquatic Sci 38:860–863
Palmer MA (2009) Reforming watershed restoration: science in need of application and applications in need of science. Estuar Coasts 32:1–71. doi:10.1007/s12237-008-9129-5
Palmer MA, Bernhardt ES (2006) Hydroecology and river restoration: ripe for research and synthesis. Water Resour Res 42: Art. No. W03S07
Peierls BL, Caraco NF, Pace ML, Cole JJ (1991) Human influence on river nitrogen. Nature 350:386–387
Peterson BJ, Wollheim WM, Mulholland PJ, Webster JR, Meyer JL, Tank JL, Marti E, Bowden WB, Valett HM, Hershey AE, McDowell WH, Dodds WK, Hamilton SK, Gregory S, Morrall DD (2001) Control of nitrogen export from watersheds by headwater streams. Science 292:86–90
Pribyl AL, McCutchan JH, Lewis WM Jr, Saunders JF III (2005) Whole-system estimation of denitrification in a plains river: a comparison of two methods. Biogeochemistry 73:439–455
Roberts BJ, Mulholland PJ, Houser JN (2007) Effects of upland disturbance on instream restoration on hydrodynamics and ammonium uptake in headwater streams. J N Am Benthol Soc 26:38–53
Runkel RL (2007) Toward a transport-based analysis of nutrient spiraling and uptake in streams. Limnol Oceanogr Methods 5:50–62
Seitzinger SP, Styles RV, Boyer EW, Alexander RB, Billen G, Howarth RW, Mayer B, van Breemen N (2002) Nitrogen retention in rivers: model development and application to watersheds in the northeastern USA. Biogeochemistry 57–58:199–237
Shields CA, Band LE, Law NL, Groffman PM, Kaushal SS, Savvas K, Fisher GT (2008) Streamflow distribution of nitrogen export from urban–rural catchments in the Chesapeake Bay watershed. Water Resour Res 44:W09416. doi:10.1029/2007WR006360
Sigman DM, Altabet MA, Michener R, McCorkle DC, Fry B, Holmes RM (1997) Natural abundance-level measurement of the nitrogen isotopic composition of oceanic nitrate: an adaptation of the ammonia diffusion method. Mar Chem 57:227–242
StreamSolute Workshop (1990) Concepts and methods for accessing solute dynamics in stream ecosystems. J N Am Benthol Soc 9:95–119
Tague C, Valentine S, Kotchen M (2008) Effect of geomorphic channel restoration on streamflow and groundwater in a snowmelt-dominated watershed. Water Resources Research 44: Article Number: W10415
US Environmental Protection Agency River Corridor and Wetland Restoration (2002) Spring Branch Stream Restoration
Walsh CJ, Roy AH, Feminella JW, Cottingham PD, Groffman PM, Morgan RP (2005) The urban stream syndrome: current knowledge and the search for a cure. J N Am Benthol Soci 24:706–723
Webster JR, Ehrman TP (1996) Solute Dynamics. In: Hauer FR, Lamberti GA (eds) Methods in stream ecology. Academic Press, San Diego, pp 145–160
Young RG, Huryn AD (1998) Comment: Improvement to the diurnal upstream–downstream dissolved oxygen change technique for determining whole-stream metabolism in small streams. Can J Fish Aquatic Sci 55:1784–1785
Acknowledgments
Carolyn Klocker received graduate student support from the University of Maryland, College Park and Appalachian Laboratory. Pete Bogush, Tamara Newcomer, and Lauren McChesney provided assistance with field work. Ellen Moon, David Lewis, and Katie Kline provided assistance in the laboratory. Steve Stewart of Baltimore County DEPRM helped with selecting study sites and Bill Stack of Baltimore City DPW provided helpful discussions. Ed Doheny and Jon Dillow of the USGS provided hydrologic information. We thank Dr. Steve Thomas, Dr. Steve Hamilton, and Dr. Pat Mulholland for providing advice regarding the stable isotope tracer addition. This research was supported by Maryland Sea Grant award SA7528085-U, the U.S. EPA through its Office of Research and Development under cooperative agreement CR829676, a grant from the Maryland Water Resources Research Center, and the NSF Baltimore Ecosystem Study LTER program (DEB-9714835). The research has not been subjected to US Environmental Protection Agency review and therefore does not necessarily reflect the views of any of the funding agencies, and no official endorsement should be inferred. This is contribution number 4344 of the University of Maryland Center for Environmental Science.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Klocker, C.A., Kaushal, S.S., Groffman, P.M. et al. Nitrogen uptake and denitrification in restored and unrestored streams in urban Maryland, USA. Aquat. Sci. 71, 411–424 (2009). https://doi.org/10.1007/s00027-009-0118-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00027-009-0118-y