Abstract
Melanocortin hormone system plays a key role in maintaining the homeostasis of our body via their neuro-immune-endocrine activities and regulates a diverse array of physiological functions, including melanogenesis, inflammation, immunomodulation, adrenocortical steroidogenesis, hemodynamics, natriuresis, energy homeostasis, sexual function, and exocrine secretion. The pathobiologic actions of all melanocortins are conveyed by melanocortin receptors. As the last melanocortin receptor to be cloned and characterized, melanocortin receptor 5 (MC5R) is widely expressed in both central nervous system and a number of peripheral organ systems in man. However, the exact effect of the MC5R mediated melanocortinergic signaling remains largely uncertain. Owing to the recent advances in developing highly selective peptidomimetic agonists and antagonists of MC5R and also to studies in MC5R knockout animals, our understanding of MC5R pathobiology has been greatly expanded and strengthened. Evidence suggests that MC5R plays a key role in governing immune reaction and inflammatory response, and is pivotal for the regulation of sexual behavior, thermoregulation, and exocrine secretion, like sebogenesis, lacrimal secretion and release of sex pheromones. As such, recent translational efforts have focused on developing novel sebum-suppressive therapies for seborrhoea and acne vulgaris based on antagonizing MC5R. Conversely, selective MC5R agonists have demonstrated promising beneficial effects in immune-mediated diseases, metabolic endocrinopathies and other disease conditions, such as glomerular diseases and dry eyes, skin and mouth. Thus, MC5R-mediated signaling is essential for health. Therapeutic targeting of MC5R represents a promising and pragmatic therapeutic strategy for diverse diseases. This review article delineates the biophysiology of MC5R-mediated biophysiology of the melanocortin hormone system, discusses the existing data on MC5R-targeted therapy in experimental disease models, and envisages the translational potential for treating human diseases.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The melanocortins are a family of neuropeptide hormones. As a key part of the neuroimmunoendocrine axis, the melanocortin system plays a pivotal role in a number of homeostatic processes and physiological functions, including melanogenesis, inflammation, immunomodulation, adrenocortical steroidogenesis, hemodynamics, natriuresis, energy homeostasis, sexual function, and exocrine secretion [1,2,3,4,5,6,7,8]. All melanocortin neuropeptides are derived from the preprohormone proopiomelanocortin (POMC) that is mainly synthesized by the anterior pituitary gland and post-translationally cleaved into different peptides in response to stress [9, 10]. The biological functions of melanocortins are dictated by the melanocortin receptors (MCRs) that are bound. To date, five types of melanocortin receptors (MC1R–MC5R) have been identified with distinct distributions in different tissues [11,12,13]. MCRs belong to the family of guanine nucleotide-protein coupled receptors (GPCRs) and are composed of an intracellular carboxylic domain, seven transmembrane helices connected by intracellular and extracellular loops, and an extracellular amino terminus [14, 15]. The last MCR to be characterized was MC5R, which is widely expressed in a number of organ systems in mammals [16, 17]. MC5R has been implicated in the regulation of exocrine gland secretion [4, 18,19,20], immune regulation [21], muscle fatty acid oxidation [22] and glucose uptake [23], adipocyte lipolysis and re-esterification [24]. Since the discovery of rodent and human MC5R gene in 1994 [25] and 1995 [26], it has been almost 30 years and the number of MC5R-related publications per year has increased exponentially (Fig. 1). However, much remains unknown about its biological function and signaling mechanism. Here, we delineate the current understandings of the biophysiology and molecular biology of MC5R with a focus on its role in health and disease as well as its implication as a novel therapeutic target for treating human diseases.
Number of PubMed citations for the term melanocortin 5 receptor or MC5R from 1994 to 2019. MC5R was described for the first time in 1994 (garnet red bar), when molecular cloning of mouse and rat MC5R genes were completed and reported. Afterwards, molecular cloning of human MC5R gene was published in 1995
Molecular biology of MC5R gene
As one of the smallest known GPCRs, human MC5R consists of 325 amino acids that are encoded by the intronless MC5R gene located on chromosome 18p11.21 in human genome [27]. Being a typical class A GPCR, MC5R is featured by short amino- and carboxyl-terminal ends, seven-helical transmembrane (TM) segments, a very small second extracellular loop (loop 4) and intracellular loops connecting the TM helices (Fig. 2) [28]. The 3-dimensional structure model reveals 7 α-helical TM domains and a ligand-binding pocket of human MC5R (Fig. 3), consisting of an ionic pocket formed by amino acids Asp115 and Asp119 in TM3, as well as an aromatic binding pocket constituted by Phe195 in TM5 and Phe254 in TM6. MC5R protein seems to be highly conserved across various species, including human, rat, mouse, chicken, fish, etc. (Table 1 and Fig. 2). Indeed, the PhyloP basewise conservation analysis based on Multiz alignment of 46 vertebrate species measures evolutionary conservation and demonstrates that the PhyloP score for MC5R gene is 3.51 ± 3.39, signifying that MC5R is highly conserved and its evolution is much slower than neutral drift [29]. Moreover, MC5R gene is rich of CpG islands, where the DNA region has a high frequency of a cytosine nucleotide followed by a guanine nucleotide in the linear sequence of bases along its 5′ to 3′ direction. Importantly, DNA methylation involving covalent modification of cytosine nucleotides at the C5 position in CpG dinucleotides is a key mechanism for epigenetic gene regulation, implying that MC5R gene expression is subjected to considerable influence by epigenetic regulations. As a primitive vertebrate, zebrafish (Danio rerio) carries two copies of MC5R gene that encode the MC5R with 69.8% amino acid homology to the human ortholog [30]. The developed degeneration complementation (DDC) model predicts that the usual mechanism of duplicate gene preservation is the partitioning of ancestral functions rather than the evolution of new functions. The remarkable conservation of amino acid sequence of MC5R throughout more than 400 million years of vertebrate evolution entails a critical role of MC5R required for normal physiological function [27].
MC5R, a guanine nucleotide-binding protein coupled receptor, is highly conserved in various organisms. Multiple sequence alignment constructed with COBALT for MC5R in various species. Nonidentical residues to the human MC5R are marked with white boxes. Fully conserved residues are marked with black boxes. Residues with strongly similar properties are marked with pink boxes, and those with weakly similar properties are marked with blue boxes. Diverse domains of MC5R are indicated, including transmembrane (TM) domains, intracellular (IC) domains, extracellular (EC) domains, and N and C-terminal domains
Protein 3-dimensional structure modeling of human MC5R. The full-length 3-dimensional protein structure of human MC5R was generated by I-TASSER server, a hierarchical protocol for automated protein structure modeling. (A-C) Front, side and top views of the 3- dimensional model of MC5R, highlighting the 7 α-helical transmembrane domains and the ligand-binding pocket. (D) Bottom view of a 3-dimensional model of a proposed molecule with high confidence score of prediction docking inside the ligand-binding pocket of MC5R
Distribution of MC5R in mammalian organs
In mammals, MC5R has been demonstrated to express in a multitude of organ systems, including the adrenal glands, fat cells, liver, lung, lymph nodes, bone marrow, thymus, mammary glands, testis, ovary, pituitary glands, uterus, esophagus, stomach, duodenum, skin, and skeletal muscle [2, 7, 13, 18, 21]. In particular, abundant MC5R expression has been detected in exocrine glands like preputial glands, lacrimal glands, pancreas, testis, prostate, and in the terminally differentiated lipid-laden sebocytes in sebaceous glands [22, 31]. In addition, MC5R is also evidently expressed by immune competent cells like B and T lymphocytes, denoting a potential role in immunomodulation [32]. Moreover, there is also evidence suggesting limited distribution of MC5R in the central nervous system, where MC5R expression was probed in the hypothalamus, cortex, cerebellum, hippocampus, substantia nigra aside from the pituitary [33, 34]. More recently, there is a growing body of evidence suggesting the expression of MC5R in the kidney. In renal glomeruli, MC5R has been reported to be expressed in glomerular visceral epithelial cells or podocytes [35], while in renal tubulointerstitium, MC5R expression was detected in interstitial cells [36]. The pattern of MC5R expression enables the MC5R-specific melanocortinergic signaling to be conveyed to selective cell types in target organ systems.
Signaling mechanism of MC5R
The MC5R shares 40 ~ 60% amino acid similarity with other melanocortin receptors [37]. However, it remains to be delineated which domain of the MCRs specifies their unique signaling activities and biological functions. Furthermore, how the MC5R signaling is different from other MCRs has been barely studied, though it is well known that the biological function of MC5R is completely distinct from other MCRs (Fig. 4). Similar to other MCRs, MC5R is a typical GPCR and able to activate an associated guanine nucleotide-binding protein by exchanging its bound GDP for a GTP [38]. The resultant dissociation of guanine nucleotide-binding protein subunits will further activate downstream intracellular signaling proteins, including adenylyl cyclase and phospholipase C, thus triggering the cAMP-PKA signaling pathway [39]. In turn, the cAMP-PKA pathway is able to trigger the downstream signaling cascades, including the lipolysis pathway that involves repression of acetyl-CoA carboxylase, and activation of hormone-sensitive lipase (HSL), perilipin and adipose triglyceride lipase (ATGL) [24], as well as the cAMP response element binding protein (CREB) pathway that plays a pivotal role in balancing the pro- and anti-inflammatory response [40, 41]. In addition, cellular signal transduction pathways other than cAMP-PKA may also be involved in MC5R signaling. Indeed, Rodrigues et al. reported that neither cAMP nor PKA was required for α-melanocyte stimulating hormone (α-MSH)-induced ERK1/2 activation in HEK293 cells overexpressing green fluorescent protein-tagged MC5R [42]. Instead, α-MSH-induced ERK1/2 activation was successfully abolished by wortmannin and LY294002, selective inhibitors of the phosphatidylinositol 3-kinase (PI3K), suggesting that MC5R may activate ERK1/2 through a PI3K-dependent signaling mechanism [42]. Subsequent to ERK1/2 activation, MC5R has been reported to suppress fatty acid re-esterification [24], mediate cellular proliferation/differentiation and induce the expression of immediate early genes implicated in immune responses, like c-fos [42]. Furthermore, it is also tempting to speculate that the same MC5R may trigger different cell signal transduction pathways when bound to different agonistic melanocortins or when activated in different cell types, resulting in different cellular effects and biological functions. In support of this notion, in murine Ba/F3 pro-B lymphocytes and IM-9 human B-lymphoblasts that express MC5R, α-MSH uniquely stimulated Janus kinase 2 (JAK2) and signal transducers and activators of transcription 1 (STAT1) tyrosine phosphorylation and thereby regulated B lymphocyte function [43]. In contrast, in erythroid cells, MC5R signaling promoted actomyosin contractility via the PI3K/Akt/MLC2 pathway and induced cell enucleation [44]. However, in cardiac myocytes, the MC5R activated PI3K reduced the GLUT1/GLUT4 ratios and resulted in an attenuated cellular hypertrophy [45].
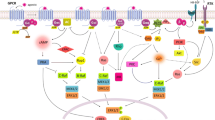
Schematic of MC5R signaling pathways. As a typical G-protein coupled receptor with the seven membrane-spanning domain structure, MC5R is activated after binding to synthetic peptidomimetic agonists or to naturally occurring ligands like ACTH and α,β,γ-MSH with the order of potency in activating MC5R being α-MSH > ACTH = β-MSH > > γ-MSH. Following activation, MC5R couples to diverse G proteins and thereby triggers a variety of signaling pathways, including JAK2/STAT1 pathway, cAMP/PKA/CREB pathway and PI3K/ERK1/2/MSK1/c-fos pathway, which are likely implicated in the immunoregulatory function of MC5R. The MC5R signaling may activate the ERK1/2 pathway via either the transient PI3K/c-Raf/MEK1/2 cascade or the sustained βarr1/2/c-Raf/MEK1/2 cascade, possibly resulting in cellular proliferation and differentiation. In addition, MC5R signaling may induce lipolysis via the cAMP/PKA/ACC-HSL-PLIN1-ATGL pathway and suppress fatty acid re-esterification by the ERK1/2/PEPCK pathway, leading to a possible beneficial effect on metabolic endocrinopathies. Furthermore, the MC5R activated PI3K may trigger the Akt/MLC2 signaling and promote actomyosin contractility, which is likely involved in erythroid enucleation or improvement of cytoskeletal integrity in glomerular podocytes. Alternatively, MC5R may reduce the GLUT1/GLUT4 ratios via PI3K pathway and thereby attenuate cellular hypertrophy in cells like cardiac myocytes. ACC acetyl-CoA carboxylase; ATGL adipose triglyceride lipase; βarr β-arrestin; CREB cAMP response element binding protein; ERK extracellular signal-regulated kinase; GLUT glucose transporter; HSL hormone-sensitive lipase; JAK Janus kinase; MEK MAPK/ERK kinase; MLC myosin light chain; MSK mitogen- and stress-activated protein kinase; PEPCK phosphoenolpyruvate carboxykinase; PI3K phosphatidylinositol 3-kinase; PKA protein kinase A; PLIN perilipin; RSK ribosomal S6 kinase; STAT signal transducers and activators of transcription
MC5R agonists and antagonists
A number of natural and synthetic ligands of MC5R have been characterized or developed with varying agonizing or antagonizing activities. The naturally occurring endogenous MC5R agonists, termed melanocortins, are a group of structurally related peptides and derive from the same precursor peptide, POMC, which is biosynthesized in the anterior pituitary gland in response to stress signals transmitted from the hypothalamus [2, 10, 14]. Post-translational modification of POMC by proteolytic cleavage generates many bioactive peptides, including adrenocorticotropic hormone (ACTH), α-MSH, β-MSH, γ-MSH, β-lipotropic hormone (β-LPH), and β-endorphin. Among these neuropeptides, ACTH, α-MSH, β-MSH, and γ-MSH are categorized into the family of melanocortins due to their melanotropic activities [10]. All of the melanocortins share a conserved common tetrapeptide sequence, namely His-Phe-Arg-Trp, which is the minimal sequence required for selectivity and stimulation of the cognate MCRs. In addition, α-MSH, β-MSH and ACTH share a heptapeptide sequence, i.e., Met-Glu-His-Phe-Arg-Trp-Gly, which is presumably crucial for the melanogenic effects [6]. MC5R has the most sequence homology to MC4R and the least homology to MC2R. Besides, MC5R is similar to MC1R and MC4R in its ability to respond to almost all melanocortins, except γ-MSH [30, 37]. The order of potency of the natural melanocortins in activating MC5R is α-MSH > ACTH = β-MSH > > γ-MSH [14, 46]. Because the half-life time of natural melanocortin peptides is very short in vivo, it is impossible to utilize these peptides as investigational new drugs to conduct pre-clinical/clinical studies and examine the biological effects of MCRs. Moreover, it is technically challenging to prepare large amounts of highly purified natural melanocortins. To overcome these drawbacks of natural MC5R agonists, more and more synthetic MC5R agonists with varying MC5R specificity are being developed based on molecular and structural modification of α-MSH [47, 48]. One of the most useful compounds that emerged from the early research was NDP-α-MSH (Melanotan-I). Base on the amino acid sequence of α-MSH, substituting Phe-7 with D-Phe and Met-4 with Nle, respectively, resulted in NDP-MSH, which was found to have high affinity for all non-steroidogenic MCRs, including MC5R. Indeed, NDP-MSH is a potent agonist of MC5R and other MCRs including MC1R, MC3R and MC4R. Although the sequence of NDP-MSH peptide is not particularly selective for MC5R, there is evidence that its selectivity for MC5R may still be higher than that for MC1R, MC3R and MC4R [49]. Similar to NDP-MSH, Melanotan II is another derivative of α-MSH and is also a pan-agonist of MCRs. It appears to be metabolically stable and possesses considerable agonistic activity on MC5R [50]. SHU9119 and HS014 are partial agonists of both MC1R and MC5R, while they antagonize MC3R and MC4R [48]. RO27-4680, a novel selective MC4R agonist, was later found to also have an agonistic activity on MC5R [12]. The first highly selective agonist of MC5R, Ac-Nle-c[Asp-Pro-DNal(2)-Arg-Trp]-Lys-NH2 or PG-901 was invented in 2002 and exhibited almost 40 times higher activity on MC5R than the super-agonistic compound MT-II [11]. PG-911, another selective agonist of MC5R developed at the same time, is less potent than PG-901 in activating MC5R [51]. More recently, another oligopeptide [H-Tyr-Val-Nle-Gly-His-DNal(2′)-Arg-DPhe-Asp-Arg-Phe-Gly-NH2] was described as a selective agonist of MC5R [52]. Besides, N-terminal modification of the tetrapeptide His-D-Phe-Arg-Trp-NH2 with an aromatic group resulted in 3,3,3-triphenylpropionyl-His-D-Phe-Arg-Trp-NH2 with a 100-fold selective agonistic activity on MC5R [7, 53]. So far, most of these agonists have been safely applied in in vitro and in vivo research studies [14], paving the way for translating these novel MC5R agonists into future clinical trials.
Distinct from other endocrine hormone systems, the melanocortin hormone system is equipped with a naturally occurring antagonizing mechanism, represented by the agouti signaling protein (ASIP) in man and the agouti-related peptide (AgRP) in rodents. ASIP is the only naturally occurring antagonistic peptide in human body that binds to MC5R and mitigates the agonistic activity of all known melanocortins with varying efficacy [11]. It is also a potent antagonist of human MC1R, MC4R and a relatively weak antagonist of MC2R and MC3R. In mice, AgRP antagonizes MC1R and MC4R and to a lesser extent MC3R, but has no effect on MC5R. Other synthetic selective antagonists of MC5R include PG14N, PG17N, PG20N and JNJ-10229570 [54]. To better define the role of MC5R in pathophysiology and also to develop novel therapeutic modalities targeting MC5R, it is imperative to develop synthetic agonists and antagonists of MC5R with improved specificity.
MC5R in health
Although MC5R is expressed extensively in peripheral organs and tissues [19, 55]. The MC5R-mediated physiological functions are not fully understood. Gene expression profiling indicates that MC5R mRNA is expressed at high levels in exocrine glands, such as lacrimal and harderian glands [1]. In addition, MC5R has also been reported to express abundantly in skeletal muscles and in skin tissues, particularly in sebaceous glands [56]. The high expression levels of MC5R in exocrine glands suggest that MC5R may play an important role in the secretion of exocrine glands. Deletion of the MC5R gene in mice results in nearly total loss of NDP-MSH binding sites in the skeletal muscles and in the harderian, lachrymal and preputial glands, entailing that MC5R represents the predominant MCR in these tissues [7, 22].
To date, the most robust evidence in support of the key role of MC5R signaling in physiology and health was derived from genetic ablation of MC5R. MC5R knockout mice exhibit a severe dysfunction of exocrine secretion, affecting hair follicle-associated sebaceous, harderian, lachrymal and preputial glands and resulting in reduced hair lipid content, defects in water repulsion, and reduced coat insulation against cold environments that leads to an impaired thermoregulatory function [20, 57]. These phenotypes suggest that MC5R plays a key role in the regulation of sebaceous lipid production, water repulsion, and thermal regulation, and that MC5R is centrally involved in sebogenesis [58]. Sebogenesis is a process of producing sebum-specific lipids within the sebaceous gland, which produces sebum, a lipid mixture of squalene, wax esters, triglycerides, cholesterol esters, and free fatty acids [59, 60]. After its production, sebum is released from sebocytes by a holocrine secretion into the infundibulum and hair canal. Then, it is delivered to the hair and skin surface for protective coating and moisturization. Excessive sebum production is one of the major factors in the pathogenesis of acne [61, 62]. MC5R has been associated with sebocyte differentiation and sebum production [63]. In support of this contention, production of sebaceous lipids is down-regulated in MC5R knockout mice. In contrast, α-MSH acts as a sebotropic hormone in rodents. In addition, MC5R is physiologically expressed in the acinar cells of the lacrimal gland [4] and this expression is repressed in experimental dry eye in rats induced by preganglionic parasympathetic denervation of the lacrimal gland [64]. Furthermore, MC5R may have a direct, trophic role in maintaining lacrimal function and secretion as the MC5R-deficient rat develops alacrimia [64].
Farnesenes are male pheromones that induce estrus and regulate sexual behavior in female mice, but are aversive olfactory signals that discourage territorial urine marking in male mice. The preputial gland is the only known source of farnesenes in the urine of male mice. MC5R-deficiency appears to produce a physiological defect similar to preputialectomy [20]. Mice lacking MC5R exhibit a decreased sensitivity to the stimulatory effects of systemic melanocortin injections on aggressive behavior [57]. The levels of sex pheromones, α- and β-farnesene as well as sesquiterpenes and ethyl nonanoate secreted from preputial glands and urine by MC5R-deficient mice were lower than those by wide-type littermates [20].
More recent data revealed that MC5R is basally expressed in kidney glomeruli and interstitium under homeostatic conditions. Ablation of MC5R exacerbated proteinuria and glomerular injury in murine models of focal and segmental glomerulosclerosis [65]. In addition, up-regulation of MC5R in the rat adrenal cortex was observed as a consequence of chronic stress [66]. There is a possibility that MC5R function seems to be associated with stress response. Other plausible functions of MC5R are associated with the anti-inflammatory effect, regulation of aldosterone secretion and involvement in adipose metabolism [67].
The wide expression of MC5R in many tissues throughout the body suggests that the role of MC5R in physiology is by no means only limited to the above. Unfortunately, this field has been less studied. More in-depth studies are warranted to harness the MC5R knockout mice to elucidate the role of MC5R in the homeostasis of diverse organ systems, like the kidney, heart, liver, etc. [2].
Therapeutic targeting of MC5R in disease
A growing body of evidence indicates that MC5R contributes to the differentiation and regulation of sebaceous lipid regulation. In consistency, ablation of the neurointermediate lobe of the pituitary (the major source of circulating α-MSH), caused a decrease in sebaceous lipid production [68]. In addition, POMC-deficient mice were found to have a similar lipid-related phenotype as observed in MC5R-deficient mice. It has been reported that expression of MC5R was seen only in lipid-laden differentiated mature sebocytes, but not in the basal cells of the sebaceous gland, entailing the association of this receptor with sebaceous differentiation [63]. Moreover, sebocyte differentiation could be enhanced by melanocortins or by cholera toxin, a selective activator of the adenylate cyclase-cAMP pathway that is downstream of MC5R signaling [57, 69]. Sebaceous disorders, such as acne vulgaris and seborrheic dermatitis, are characterized by increased sebum production. The current treatments for acne, including isotretinoin and androgen modulators, directly affect sebaceous gland differentiation, but have serious undesired side effects. Therefore, there is biological and clinical plausibility to target MC5R for the treatment of diverse sebaceous disorders. In fact, it has been postulated that MC5R antagonists may be utilized as potential sebum-suppressive agents and contribute to the treatment of seborrhoea and acne vulgaris, which involve dysfunction of sebaceous glands. Conversely, MC5R agonists may alleviate conditions of dry eyes, skin and mouth [2]. Further investigations on the physiological function of MC5R and selective antagonists/agonists of MC5R would pave the way for developing novel therapeutic modalities.
In addition, multiple lines of evidence suggest that melanocortins contribute to immune modulation. The expression of MC5R is evident in a number of immune cells, including T and B lymphocytes, mast cells, antigen presenting cells and others. It is postulated that MC5R may play a key role in the regulation of immune response. In support of this view, pollen allergy is associated with an increased expression of MC5R but not MC1R in the trachea in a murine model of pollen allergy, concomitant with an increased level of α-MSH and ACTH in plasma [12]. The same study also demonstrated that sneezing and IgA production were suppressed by α-MSH antibody treatment in murine models of pollen allergy, but they remained unchanged after MC1R antagonist (agouti) treatment. These findings indicated that sneezing due to pollen allergy is likely associated with an increased MC5R activity in the trachea.
More recent research suggested a beneficial role of MC5R activation in experimental autoimmune uveoretinitis (EAU), a model of human autoimmune uveitis induced in mice by immunization with the retinal antigen interphotoreceptor retinoid-binding protein (IRBP) [21]. Taylor et al. demonstrated that MC5R is involved in the protection of retina against the inflammatory damage of autoimmune uveoretinitis and in the induction of ocular autoantigen-responsive CD4 + Treg cells in the post EAU spleen [21]. In consistency, MC5R knockout mice with EAU sustained greater damage to their retinal structures, and did not generate regulatory immunity to ocular autoantigen in their spleens [70]. These findings suggest that there is not only a localized role of MC5R in the protection of retina against inflammatory damage, but also a systemic role of MC5R in the establishment of peripheral tolerance to ocular autoantigens following ocular autoimmune disease. Similarly, Lee et al. also noted that antigen presenting cells promoted or selectively activated IRBP-specific FoxP3 + TGF-β + CD25 + CD4 + Treg cells in the spleen of mice recovered from EAU in an MC5R dependent mode [70, 71].
In addition to immune regulation, additional evidence suggests that MC5R is also involved in the protection against metabolic organ injuries, like high-glucose induced cardiomyocyte hypertrophy and diabetic retinopathy [45, 51]. In cultured cardiac H9c2 cells exposed to high glucose, both the highly selective MC5R peptidomimetic agonist PG-901 and α-MSH abrogated the impaired cell survival and reduced the total protein per cell, denoting a pro-survival and anti-hypertrophic effect. Moreover, in a model of streptozotocin (STZ)-induced diabetic retinopathy in mice, intravitreal injections of PG-901 conferred significant protection of retina, evidenced by regular course and caliber of retinal vessels without microvascular changes or vessel leakage. In contrast, intravitreal injections of PG20N, an antagonist of MC5R, augmented the lesions of diabetic retinopathy in STZ-injured mice [51]. The glycemic levels were not altered, suggesting that a direct protective effect of MC5R on retinal parenchymal cells may contribute. More recent data also revealed a direct protective effect of other MC5R peptidomimetic agonists on kidney podocytes and thereby prominently ameliorated glomerular damage in experimental glomerulopathy [65].
Future perspectives
MC5R is widely expressed in a number of organs and tissues, but the exact pathobiology of MC5R remains elusive. The highly conserved amino acid sequence of MC5R among various species implies the critical role of MC5R in biophysiology over evolution in the past 400 million years. Analysis of the MC5R knockout mouse is the first step to elucidate the function of MC5R. Converging evidence suggests that MC5R is involved in inflammatory response, sebogenesis, farnesene signaling, exocrine secretion, regulation of sexual behavior, immunoregulation, and other physiological functions. With the development of highly selective peptidomimetic agonists and antagonists of MC5R, it is feasible that MC5R may serve as an actionable therapeutic target for treating acne, autoimmune uveitis, diabetic retinopathy, allergy, autoimmune diseases and kidney diseases. Future work will continue to focus on developing potent agonists and antagonists of MC5R with minimal off-target activities and negligible adverse effects, which will not only improve our understanding of the pathophysiology of MC5R but also advance the clinical translatability of MC5R-targeted therapy. Hopefully these efforts will help shed further light on the nature of MC5R-mediated melanocortin hormone system.
References
Hadley ME, Haskell-Luevano C (1999) The proopiomelanocortin system. Ann N Y Acad Sci 885:1–21
Wikberg JE, Muceniece R, Mandrika I, Prusis P, Lindblom J, Post C, Skottner A (2000) New aspects on the melanocortins and their receptors. Pharmacol Res 42:393–420
Norman RA, Permana P, Tanizawa Y, Ravussin E (1999) Absence of genetic variation in some obesity candidate genes (GLP1R, ASIP, MC4R, MC5R) among Pima indians. Int J Obes Relat Metab Disord 23:163–165
van der Kraan M, Adan RA, Entwistle ML, Gispen WH, Burbach JP, Tatro JB (1998) Expression of melanocortin-5 receptor in secretory epithelia supports a functional role in exocrine and endocrine glands. Endocrinology 139:2348–2355
Chhajlani V, Muceniece R, Wikberg JE (1993) Molecular cloning of a novel human melanocortin receptor. Biochem Biophys Res Commun 195:866–873
Datta PC, King MG (1982) Alpha-melanocyte-stimulating hormone and behavior. Neurosci Biobehav Rev 6:297–310
Gantz I, Fong TM (2003) The melanocortin system. Am J Physiol Endocrinol Metab 284:E468–E474
Catania A, Gatti S, Colombo G, Lipton JM (2004) Targeting melanocortin receptors as a novel strategy to control inflammation. Pharmacol Rev 56:1–29
Zhang L, Anthonavage M, Huang Q, Li WH, Eisinger M (2003) Proopiomelanocortin peptides and sebogenesis. Ann N Y Acad Sci 994:154–161
Millington GW (2006) Proopiomelanocortin (POMC): the cutaneous roles of its melanocortin products and receptors. Clin Exp Dermatol 31:407–412
Abdel-Malek ZA (2001) Melanocortin receptors: their functions and regulation by physiological agonists and antagonists. Cell Mol Life Sci 58:434–441
Getting SJ (2002) Melanocortin peptides and their receptors: new targets for anti-inflammatory therapy. Trends Pharmacol Sci 23:447–449
Camadro JM, Thome F, Brouillet N, Labbe P (1994) Purification and properties of protoporphyrinogen oxidase from the yeast Saccharomyces cerevisiae. Mitochondrial location and evidence for a precursor form of the protein. J Biol Chem 269:32085–32091
Voisey J, Carroll L, van Daal A (2003) Melanocortins and their receptors and antagonists. Curr Drug Targets 4:586–597
Andersen GN, Hagglund M, Nagaeva O, Frangsmyr L, Petrovska R, Mincheva-Nilsson L, Wikberg JE (2005) Quantitative measurement of the levels of melanocortin receptor subtype 1, 2, 3 and 5 and pro-opio-melanocortin peptide gene expression in subsets of human peripheral blood leucocytes. Scand J Immunol 61:279–284
Gantz I, Shimoto Y, Konda Y, Miwa H, Dickinson CJ, Yamada T (1994) Molecular cloning, expression, and characterization of a fifth melanocortin receptor. Biochem Biophys Res Commun 200:1214–1220
Griffon N, Mignon V, Facchinetti P, Diaz J, Schwartz JC, Sokoloff P (1994) Molecular cloning and characterization of the rat fifth melanocortin receptor. Biochem Biophys Res Commun 200:1007–1014
Thiboutot D, Sivarajah A, Gilliland K, Cong Z, Clawson G (2000) The melanocortin 5 receptor is expressed in human sebaceous glands and rat preputial cells. J Invest Dermatol 115:614–619
Chen W, Kelly MA, Opitz-Araya X, Thomas RE, Low MJ, Cone RD (1997) Exocrine gland dysfunction in MC5-R-deficient mice: evidence for coordinated regulation of exocrine gland function by melanocortin peptides. Cell 91:789–798
Morgan C, Thomas RE, Ma W, Novotny MV, Cone RD (2004) Melanocortin-5 receptor deficiency reduces a pheromonal signal for aggression in male mice. Chem Senses 29:111–115
Taylor AW, Kitaichi N, Biros D (2006) Melanocortin 5 receptor and ocular immunity. Cell Mol Biol 52:53–59
An JJ et al (2007) Peripheral effect of alpha-melanocyte-stimulating hormone on fatty acid oxidation in skeletal muscle. J Biol Chem 282:2862–2870
Enriori PJ et al (2016) α-Melanocyte stimulating hormone promotes muscle glucose uptake via melanocortin 5 receptors. Mol Metab 5:807–822
Rodrigues AR, Almeida H, Gouveia AM (2013) Alpha-MSH signalling via melanocortin 5 receptor promotes lipolysis and impairs re-esterification in adipocytes. Biochem Biophys Acta 1831:1267–1275
Labbé O, Desarnaud F, Eggerickx D, Vassart G, Parmentier M (1994) Molecular cloning of a mouse melanocortin 5 receptor gene widely expressed in peripheral tissues. Biochemistry 33:4543–4549
Chowdhary BP, Gustavsson I, Wikberg JE, Chhajlani V (1995) Localization of the human melanocortin-5 receptor gene (MC5R) to chromosome band 18p11.2 by fluorescence in situ hybridization. Cytogenet Cell Genet 68:79–81
Logan DW, Bryson-Richardson RJ, Pagan KE, Taylor MS, Currie PD, Jackson IJ (2003) The structure and evolution of the melanocortin and MCH receptors in fish and mammals. Genomics 81:184–191
Grieco P, Han G, Weinberg D, Van der Ploeg LH, Hruby VJ (2002) Design and synthesis of highly potent and selective melanotropin analogues of SHU9119 modified at position 6. Biochem Biophys Res Commun 292:1075–1080
Takahashi A, Kawauchi H (2006) Evolution of melanocortin systems in fish. Gen Comp Endocrinol 148:85–94
Ringholm A, Fredriksson R, Poliakova N, Yan YL, Postlethwait JH, Larhammar D, Schioth HB (2002) One melanocortin 4 and two melanocortin 5 receptors from zebrafish show remarkable conservation in structure and pharmacology. J Neurochem 82:6–18
Cone RD (2006) Studies on the physiological functions of the melanocortin system. Endocr Rev 27:736–749
Taylor A, Namba K (2001) In vitro induction of CD25+ CD4+ regulatory T cells by the neuropeptide alpha-melanocyte stimulating hormone (alpha-MSH). Immunol Cell Biol 79:358–367
Catania A (2008) Neuroprotective actions of melanocortins: a therapeutic opportunity. Trends Neurosci 31:353–360
Shukla C, Britton SL, Koch LG, Novak CM (2012) Region-specific differences in brain melanocortin receptors in rats of the lean phenotype. NeuroReport 23:596–600
Gong R (2011) The renaissance of corticotropin therapy in proteinuric nephropathies. Nat Rev Nephrol 8:122–128
Si J, Ge Y, Zhuang S, Wang LJ, Chen S, Gong R (2013) Adrenocorticotropic hormone ameliorates acute kidney injury by steroidogenic-dependent and -independent mechanisms. Kidney Int 83:635–646
Switonski M, Mankowska M, Salamon S (2013) Family of melanocortin receptor (MCR) genes in mammals-mutations, polymorphisms and phenotypic effects. J Appl Genet 54:461–472
New DC, Wong YH (2007) Molecular mechanisms mediating the G protein-coupled receptor regulation of cell cycle progression. J Mol Signal 2:2
Ichiyama T, Zhao H, Catania A, Furukawa S, Lipton JM (1999) alpha-melanocyte-stimulating hormone inhibits NF-kappaB activation and IkappaBalpha degradation in human glioma cells and in experimental brain inflammation. Exp Neurol 157:359–365
Rodrigues AR, Almeida H, Gouveia AM (2012) Melanocortin 5 receptor signaling and internalization: role of MAPK/ERK pathway and beta-arrestins 1/2. Mol Cell Endocrinol 361:69–79
House JS, Zhu S, Ranjan R, Linder K, Smart RC (2010) C/EBPalpha and C/EBPbeta are required for Sebocyte differentiation and stratified squamous differentiation in adult mouse skin. PLoS One 5:e9837
Rodrigues AR, Pignatelli D, Almeida H, Gouveia AM (2009) Melanocortin 5 receptor activates ERK1/2 through a PI3K-regulated signaling mechanism. Mol Cell Endocrinol 303:74–81
Buggy JJ (1998) Binding of a-melanocyte-stimulating hormone to its G-protein-coupled receptor on B-lymphocytes activates the Jak/STAT pathway. Biochem J 331:211–216
Simamura E et al (2015) Melanocortins contribute to sequential differentiation and enucleation of human erythroblasts via melanocortin receptors 1, 2 and 5. PLoS One 10:e0123232–e0123232
Trotta MC, Maisto R, Alessio N, Hermenean A, D'Amico M, Di Filippo C (2018) The melanocortin MC5R as a new target for treatment of high glucose-induced hypertrophy of the cardiac H9c2 cells. Front Physiol 9:1475–1475
Abdel-Malek ZA (2001) Melanocortin receptors: Their functions and regulation by physiological agonists and antagonists. Cellul Mol Life Sci 58:434–441
Diano S (2011) New aspects of melanocortin signaling: a role for PRCP in alpha-MSH degradation. Front Neuroendocrinol 32:70–83
Grieco P, Cai M, Han G, Trivedi D, Campiglia P, Novellino E, Hruby VJ (2007) Further structure-activity studies of lactam derivatives of MT-II and SHU-9119: their activity and selectivity at human melanocortin receptors 3, 4, and 5. Peptides 28:1191–1196
Qiao Y et al (2016) MC1R is dispensable for the proteinuria reducing and glomerular protective effect of melanocortin therapy. Sci Rep 6:27589
Gong R (2014) Leveraging melanocortin pathways to treat glomerular diseases. Adv Chronic Kidney Dis 21:134–151
Rossi S et al (2016) Activation of melanocortin receptors MC 1 and MC 5 attenuates retinal damage in experimental diabetic retinopathy. Mediators Inflamm 2016:7368389
Barkey NM et al (2011) Development of melanoma-targeted polymer micelles by conjugation of a melanocortin 1 receptor (MC1R) specific ligand. J Med Chem 54:8078–8084
Holder JR, Marques FF, Xiang Z, Bauzo RM, Haskell-Luevano C (2003) Characterization of aliphatic, cyclic, and aromatic N-terminally “capped” His-d-Phe-Arg-Trp-NH2 tetrapeptides at the melanocortin receptors. Eur J Pharmacol 462:41–52
Getting SJ (2006) Targeting melanocortin receptors as potential novel therapeutics. Pharmacol Ther 111:1–15
Nimura M, Udagawa J, Hatta T, Hashimoto R, Otani H (2006) Spatial and temporal patterns of expression of melanocortin type 2 and 5 receptors in the fetal mouse tissues and organs. Anat Embryol 211:109–117
Enriori PJ et al (2016) alpha-Melanocyte stimulating hormone promotes muscle glucose uptake via melanocortin 5 receptors. Mol Metab 5:807–822
Morgan C, Cone RD (2006) Melanocortin-5 receptor deficiency in mice blocks a novel pathway influencing pheromone-induced aggression. Behav Genet 36:291–300
Eisinger M, Li WH, Anthonavage M, Pappas A, Zhang L, Rossetti D, Huang Q, Seiberg M (2011) A melanocortin receptor 1 and 5 antagonist inhibits sebaceous gland differentiation and the production of sebum-specific lipids. J Dermatol Sci 63:23–32
Moller CL, Pedersen SB, Richelsen B, Conde-Frieboes KW, Raun K, Grove KL, Wulff BS (2015) Melanocortin agonists stimulate lipolysis in human adipose tissue explants but not in adipocytes. BMC Res Notes 8:559
Berg AL, Rafnsson AT, Johannsson M, Dallongeville J, Arnadottir M (2006) The effects of adrenocorticotrophic hormone and an equivalent dose of cortisol on the serum concentrations of lipids, lipoproteins, and apolipoproteins. Metabolism 55:1083–1087
Sanchez E, Rubio VC, Cerda-Reverter JM (2009) Characterization of the sea bass melanocortin 5 receptor: a putative role in hepatic lipid metabolism. J Exp Biol 212:3901–3910
Moller CL, Raun K, Jacobsen ML, Pedersen TA, Holst B, Conde-Frieboes KW, Wulff BS (2011) Characterization of murine melanocortin receptors mediating adipocyte lipolysis and examination of signalling pathways involved. Mol Cell Endocrinol 341:9–17
Zhang L, Li WH, Anthonavage M, Eisinger M (2006) Melanocortin-5 receptor: a marker of human sebocyte differentiation. Peptides 27:413–420
Nguyen DH, Toshida H, Schurr J, Beuerman RW (2004) Microarray analysis of the rat lacrimal gland following the loss of parasympathetic control of secretion. Physiol Genomics 18:108–118
Chang M, Dworkin LD, Gong R (2019) Melanocortin 5 receptor (MC5R) signaling protects against podocyte injury and proteinuria. J Am Soc Nephrol 30:677
Pignatelli D, Maia M, Castro AR, da Conceicao Magalhaes M, Vivier J, Defaye G (2000) Chronic stress effects on the rat adrenal cortex. Endocr Res 26:537–544
Jackson DS, Ramachandrappa S, Clark AJ, Chan LF (2015) Melanocortin receptor accessory proteins in adrenal disease and obesity. Front Neurosci 9:213
Zhang L, Li WH, Anthonavage M, Pappas A, Rossetti D, Cavender D, Seiberg M, Eisinger M (2011) Melanocortin-5 receptor and sebogenesis. Eur J Pharmacol 660:202–206
Nimura M, Udagawa J, Hatta T, Hashimoto R, Otani H (2006) Spatial and temporal patterns of expression of melanocortin type 2 and 5 receptors in the fetal mouse tissues and organs. Anat Embryol (Berl) 211:109–117
Lee DJ, Taylor AW (2013) Both MC5r and A2Ar are required for protective regulatory immunity in the spleen of post-experimental autoimmune uveitis in mice. J Immunol 191:4103–4111
Lee DJ, Taylor AW (2011) Following EAU recovery there is an associated MC5r-dependent APC induction of regulatory immunity in the spleen. Invest Ophthalmol Vis Sci 52:8862–8867
Acknowledgements
This work was supported in part by the U.S. National Institutes of Health grant DK114006.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dr. Gong reports research funding from the Mallincrodt Pharmaceuticals, which is not related to this study. Dr. Gong served as a consultant to the Questcor Pharmaceuticals and the Mallincrodt Pharmaceuticals. All the other authors declared no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Xu, Y., Guan, X., Zhou, R. et al. Melanocortin 5 receptor signaling pathway in health and disease. Cell. Mol. Life Sci. 77, 3831–3840 (2020). https://doi.org/10.1007/s00018-020-03511-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-020-03511-0