Abstract
Although many studies have described the role of periostin in various diseases, the function of the periostin protein structures derived from alternative splicing and proteinase cleavage at the C-terminal remain unknown. Further experiments revealing the protein structures that are highly related to diseases are essential to understand the function of periostin in depth, which would accelerate its clinical application by establishing new approaches for curing intractable diseases. Furthermore, this understanding would enhance our knowledge of novel functions of periostin related to stemness and response to mechanical stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The number of periostin-related publications in PubMed has recently reached more than 1000, and this number has been increasing every year. The majority of publications correspond to incurable diseases related to the heart, cancer, bone, and immune system. Thus, detailed knowledge about periostin would provide opportunities to cure these diseases. In this review, a new concept of periostin function is proposed, called the “Periostin Switch”.
Periostin gene
The gene structure including the genome structure among vertebrates
Mouse or human periostin gene
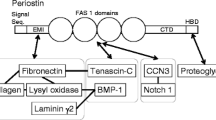
The osteoblast-specific factor 2 gene, which is the original name for periostin, was cloned using a subtraction library from the mouse pre-osteoblastic cell line, MC3T3-E1, minus the fibroblastic cell line, NIH3T3 [1]. The obtained gene encoded 811 amino acids (aa), and this was the exon 17 deleted-type of periostin. Using this mouse-derived probe, the full-length human periostin gene was cloned, which encodes 836 aa (calculated MW 93331), whereas the mouse full-length periostin gene encodes 838 aa (MW 93159) as shown in Fig. 1. The prospective leader sequence encoding a signal peptide is 24 aa for mouse periostin and 22 aa for human periostin, indicating the same size for mature periostin, that is 814 aa with molecular weight around 90 kDa. Comparison of amino acid sequences between the mouse and human periostin shows 90% homology for the mature form. The mouse periostin gene is located on chromosome 3, whereas the human gene exists on chromosome 13, with both mouse and human genes consisting of 23 exons [2]. In the periostin protein, four repeated domains including two short highly conserved amino acid sequences (13 and 14 aa, respectively) for one domain are found to be specific. This protein structure termed as the fas1 domain is conserved in other proteins including fasciclin 1 in drosophila, βigh3 in humans and mice, Algal-CAM in Volvox, and MPB70 in mycobacterium, indicating the presence of the fasciclin 1 family [3]. Drosophila fasciclin 1 functions in growth cone guidance in the nervous system [4].
Alternative splicing of the mouse or human periostin gene
The splice variants of periostin are found at the C-terminal side in mice between exons 17 and 21. Initially, 3 isoforms were found with deletion of one of 3 exons b, d, and f, which correspond to exon 17, 20, and 21, respectively, in addition to full-length periostin. TGF-β1 specifically induces splice variants in periostin, fibronectin, and tenascin C. Upon treatment with TGF-β1 in primary osteoblasts, a new band with a smaller molecule is apparent and its amount increases with increase in TGF-β1 concentration [3]. Moreover, myofibroblasts produce periostin, and also express the splice variants of fibronectin and tenascin-C induced by TGF-β, suggesting the presence of a functional complex involving these three molecules. Han et al. [5] reported that TGF-β1 regulates the expression of fibronectin isoforms and the splicing factor SRp40, suggesting the involvement of a common splicing system in the same cell and developmental stage, organized by the same splicing factor. In a mouse model of myocardial infarction (MI), the specific splice variant formed by the deletion of exons b and e (exons 17 and 21) of the periostin gene is preferentially expressed in the early stage of infarction [6]. The level of full length of periostin is increased later towards scar formation. This splice variant with high potential of secretion induces the phosphorylation of FAK, which is downstream of integrin signaling, suggesting that this variant is fundamentally located in the extracellular matrix to induce cell migration and proliferation, whereas the full-length periostin protein is hardly secreted and probably functions within the cell for fibrillogenesis in scar formation. Similarly, 3 days after mouse coronary ligation in MI, two isoforms of tenascin-C, 220 and 280 kDa, were found [7]; though tenascin-C deficient mice showed no significant increase in rupture, decreased collagen positive area was found after MI. Interestingly, tenascin-C expression during MI is transiently upregulated, which peaks at day 5 and disappears at day 28, indicating that the meshwork structure of type I collagen formed with periostin, tenascin-C, and fibronectin [8], is only temporarily present in the early stage of MI. Morra et al. [9] found more splice variants of periostin in human tissues and tumors such as renal cell carcinoma or non-small cell lung cancer [10]. They found five additional splice patterns of variants in humans including deletion of 2 exons (17 and 18 or 17 and 21), 3 exons (17, 18, and 19 or 17, 18, and 21), and 4 exons (17, 18, 19, and 21). Taken together, in humans, 8 splice variants and full-length periostin have been investigated. Furthermore, a tissue and disease specific periostin variant was described by Litvin et al., which is mouse the periostin-like factor (PLF) with the deletion of exon e (exon 21). In retinal neovascularization, two splice variants (deletion of exon 17 or 21) were reported to be specifically expressed [11]. Exon 17 functions in promoting both pre-retinal pathological neovascularization and physiological revascularization in the retina, whereas exon 21 also promotes pre-retinal pathological neovascularization. In another case, one splice variant termed PDL–POSTN with deletion of 3 exons, 17, 18, and 21, was shown to be predominantly expressed in the periodontal ligament and found to function in cytodifferentiation and mineralization with strong activation of the integrin αvβ3-focal adhesion kinase (FAK) signaling pathway [12]. In patients with idiopathic pulmonary fibrosis (IPF), exon 21 is most likely to be spliced out [13].
Other vertebral periostin genes
First, zebrafish periostin gene was cloned and found to encode 782 aa in total and contain the conserved fourfold-repeated fas 1 domain, but a c-terminal repeat sequence (5 or 6 repeats of 13 aa for one) was newly found as characteristic to fish periostin [13]. Similarly in medaka fish, medaka periostin-a and -b were cloned [15], in which the published medaka periostin-a gene was shorter; after a 3′RACE experiment, additional 24 amino acids at the C-terminal were obtained in medaka periostin-a (Fig. 2), which also includes one amino acid correction (from V to A at the C-terminal in ref. 15). Thus, the full length of medaka periostin-a is 824 aa, whereas that of medaka periostin-b is 719 aa as shown in Fig. 2. Next, the Xenopus periostin gene from Xenopus laevis was reported, encoding 794 aa in total length [16], with the conserved EMI and fas 1 domains. Interestingly, the neighboring genes of the periostin gene in the genome are well conserved among medaka, mice, and humans as shown in Fig. 3, demonstrating the presence of genes like SAMAD9, ALG5, ExoSC8, Fam48a, and TRPC4. In medaka, the periostin-a gene is highly similar to mouse and human periostin.
Transcriptional regulation
Transactivator and cis-element
Initially, before generating a knock-out mouse, periostin was only characterized by its specific expression pattern in bone-related tissues like periodontal ligament and periosteum. To investigate the function of periostin, one method was to investigate its transcriptional regulation, resulting in the bone-specific expression, which led to the discovery of the bHLH transcription factor, twist [17]. Twist binds to the promoter of periostin, consistent with the similar expression pattern of twist and periostin in calvarial bones or periodontal ligaments with downregulation due to occlusal hypofunction [18]. Twist behaves as a negative regulator of osteoblast differentiation in vitro; therefore, cells overexpressing Twist remain in an undifferentiated osteoprogenitor-like state, and cells expressing Twist-antisense progress to more differentiated, mature osteoblasts [19]. In vivo, Twist suppresses the activity of Runx2 to regulate bone formation as identified by generating twist-1 and -2 deficient mice [20]. In embryonic heart development, BMP-2 induced the expression of twist and periostin in valvulogenesis [21], and Twist 1 affects the expression of periostin in endocardial cushion cells [22]. Moreover, in humans, Twist 2 binds the promoter of periostin, and its binding affinity is stronger than that for Twist 1, which was observed in Setleis Syndrome, a rare autosomal recessive disease characterized as abnormal facial development [23].
In the transcriptional regulation of the periostin gene during embryogenesis, an evolutionarily conserved YY1-binding 37-bp region within a 304-bp periostin core enhancer is found between −2509 and −2205 of the promoter, which is capable of regulating the simultaneous novel tissue-specific periostin expression in the cardiac outflow-tract cushion mesenchyme and Schwann cell lineages [24]. Although YY1 is known to be both a transcriptional repressor and/or activator and has been shown to physically interact with more than a dozen proteins, it could be acting as a novel SMAD-interacting protein that represses SMAD transcriptional activities in a gene-specific manner, and therefore, regulates cell differentiation induced by the TGF-β superfamily pathways [25]. A tag-single nucleotide polymorphism (SNP) based on an association method revealed that several SNPs of periostin were associated with bone mineral density or vertebral fractures [26]. The most significant polymorphism site located at −2327 bp upstream of periostin, binds to CDX1 (caudal type homeobox 1), which is a member of the caudal-related homeobox transcription factor family, encoding a DNA-binding protein that regulates intestine-specific gene expression and enterocyte differentiation, and direct interaction between CDX1 and Hoxa-7 affects skeletal formation [27]. Fibrous dysplasia is a benign bone disease characterized by high expression of c-Fos/c-Jun, and transgenic mice overexpressing c-fos, which develop sclerotic lesions, induce periostin expression [28], consistently with the fact that there are two potential binding sites for c-Fos/AP-1 in the periostin promoter [17].
Factors inducing periostin expression
Originally, periostin was identified as a TGF-β inducible gene, indicating that its expression including the patterns of splice variants was increased in a TGF-β-dose dependent manner, and specifically the expression of the lowest molecule derived from the splice variant was significantly increased in the mouse pre-osteoblast cell line, MC3T3-E1 [3], suggesting that TGF-β itself induces the splice variation. In the human periodontal ligament, treatment with TGF-β1 significantly increases periostin mRNA levels, which are blocked by a focal adhesion kinase (FAK) inhibitor [29]. Concurrently, the Th2 cytokines, IL-4 and IL-13, enhance the expression and secretion of periostin in lung fibroblasts [30], atopic dermatitis (AD), and bronchial asthma [31]. In the human embryonic lung fibroblast cell line, MRC5, 3 periostin bands were detected at 84, 80, and 78 kDa, derived from alternative splicing and generated by the deletion of exons 17, 18, 19, and 21, that of exons 17, 18, and 21, and that of exons 17 and 21, respectively. Interestingly in comparison with other matricellular proteins like fibronectin and tenascin-C, IL-4 and IL-13 can dominantly induce splice variants of periostin but not those of fibronectin and tenascin-C, whereas TGF-β induces splice variants of all 3 genes [30]. IL-4 and IL-13 can enhance periostin expression more effectively compared to TGF-β. In an animal model of asthma, epithelial cell-derived periostin activates TGF-β or sensitizes against TGF-β signaling [32], resulting in collagen gel elasticity in asthma. This new function of periostin for activation of TGF-β signaling is coincident with the action of M2 macrophages. Considering macrophages, M2 macrophages are induced by the T helper 2 (Th2) cytokines, IL-4 and IL-13, distinct from the interferon-γ (IFN-γ)-mediated classical activation required for M1 macrophages [33]. In glioblastoma (GBM), which is the most common malignant brain tumor, glioma stem cells (GSCs) contribute to GBM tumor growth through periostin that is expressed in GSCs enriched in the perivascular niche, and recruit tumor-associated macrophages (TAMs), characterized as M2 macrophages [34, 35]. These TAMs are mainly monocyte-derived macrophages from the peripheral blood, and periostin maintains the M2 subtype of TAMs to accelerate tumor growth by promoting cancer cell survival in GBMs [34]. Similarly, hypoxia enhances the recruitment of TAMs, and polarizes these macrophages toward the M2 subtype by increasing the expression of M-CSFR in macrophages and that of TGF-β in glioma cells [36]. In injury of the central nervous system, the same action of periostin is observed; periostin expression is predominantly induced at the scar-forming pericytes in the spinal cord injury to promote the migration of macrophages for scar formation [37]. Taken together, these results indicate that the Th2 cytokines, IL-4 and IL-13, upregulate the periostin expression as well as activation of M2 macrophages, and periostin then recruits these macrophages for tissue repair or tumor growth.
Periostin protein
Domains in association with other extracellular molecules
Periostin has a protein structure composed of an amino-terminal EMI domain, a tandem repeat of 4 fas 1 domains, and a carboxyl-terminal domain including a heparin-binding site at its C-terminal end (Fig. 1); therefore, we characterized it as a member of the fasciclin 1 family based on these typical fas 1 domains. Fasciclin 1 is a GPI-anchored Drosophila protein functioning in axon growth guidance, containing four tandem fas 1 domains composed of about 150 aa residues each, which are not related to any other protein domain of the known structures [38]. In humans, fas 1 domains are found in βigh3 [39] and stabilins [40] as well as in periostin. Periostin and βigh3 are most similar and share uninterrupted tandem repeats of 4 fas 1 domains. Interestingly, mutations in fas 1 domains of human βigh3 result in corneal dystrophy due to deposition of insoluble protein aggregates in the cornea [41]. The EMI domain, which is a small module rich in cysteine residues that is found in the EMILIN family, is a site for protein–protein interaction [42] and can bind to fibronectin [43], whereas tenascin-C binds to the fas 1 domain [8]. For collagen cross-linking in fibrillogenesis, upon the interaction of periostin with fibronectin and tenascin-C, the association of periostin with BMP-1, a metalloproteinase for digestion, followed by activation of lysyl oxidase (LOX) for the formation of covalent cross-links in collagen and elastic fibers, is essential [44]. In addition to activation of LOX, BMP-1 functions in processing the C-propeptides of procollagens types I–III to yield the major fibrous components of vertebrate ECM, and that of NH2-terimal globular domains and C-propeptides of types of V and XI procollagen chains, to yield monomers that control the diameters of collage type I and II fibrils by its incorporation [45]. Furthermore, BMP-1 processes the precursors of laminin 5 (γ2) and collagen type VII, both of which are involved in securing the epidermis to the underlying dermis. Consistently, in wound healing models, at the basement membrane, periostin is associated with laminin γ2, and this association enhances BMP-1-mediated proteolytic cleavage of the laminin γ2 long form to produce its short from [46]. Regarding the importance of BMP-1 in broader fields, BMP-1 mutation on human chromosome 8p21 or BMP-1 deficient mice show some dentin defects and alveolar bone loss, which have shown to be causal in the development of osteogenesis imperfecta (OI) type XIII [47, 48], and a similarly abnormal alveolar bone loss is observed in periostin deficient mice [49]. Recently, it was indicated that periodontal ligament cells contribute to alveolar bone formation, and it is appealing that periostin is a key factor expressed in the stem-like cells of both periodontal ligaments and alveolar bones. As for LOX, in bone metastasis, increased stiffness of ECM on bones by the action of LOX is sensed by cancer cells, which in turn focus their activities towards invasion, and drive them to migrate to distant metastatic sites [50, 51]. In lung cancer, lysyl hydroxylase 2 that catalyzes the hydroxylation of lysine residues in the telopeptides of fibrillar collagens leading to collagen cross-links enhances metastatic propensity by generating stiffer tumor stroma by collagen cross-linking [52]. Thus, periostin is possibly involved in bone metastasis through the activation of BMP-1 and LOX actions.
Proteinase digestion
Initially, 4 days after induction of myocardial infarction (MI), proteolytic cleavage was found at the C-terminal site, indicating one major lower band, using an antibody recognizing the first fas 1 domain at the N-terminal site but not by an antibody recognizing the C-terminal end of periostin, indicating that at least one site in the C-terminal domain was digested by a proteinase; after 28 days of MI, multiple bands were observed. Similarly, in a model of lung injury by bleomycin administration, several bands were found because of the proteolytic cleavage of periostin [53]. The possible proteinase cleavage sites are shown in Fig. 4. What is the function of this shorter periostin protein? The first candidate to determine the action of the shorter periostin is the commercial recombinant periostin provided by Bio Vender, because this recombinant molecule has deleted 167 aa at the C-terminal site, which mainly consists of the EMI domain and the four fas 1 domains but does not have almost the entire C-terminal region. In an interesting experiment for collagen I gene expression induced by periostin in airway fibroblasts, this recombinant periostin could not induce collagen I gene expression even though periostin itself can induce it, suggesting that the C-terminal region functions to enhance collagen I gene expression with the activation of TGF-β by periostin [32]. Recombinant periostin markedly induces the elastic modules of gels formed by type I collagen because addition of recombinant periostin generates a more densely cross-linked gel. In another model of fibrosis in bronchial asthma, the C-terminal region-deleted periostin shows much stronger binding to fibronectin and tenascin-C [30]. Originally, Kii et al. [8] reported that the cleavage of the C-terminal end of periostin is essential for binding to tenascin-C. These 3 reports suggest that the truncated form of periostin with the deleted C-terminal region, tightly binds to fibronectin and tenascin-C for enhanced cross-linking of type I collagen. Furthermore, the EMI domain of periostin is essential for its multimerization, which facilitates the collagen cross-link through formation of a meshwork structure with fibronectin and tenascin-C [8]. Accordingly, recombinant periostin easily enters the dimeric form [30]. In the analysis of the medaka periostin gene with long or short periostin molecules, the short periostin, periostin-b with 719 aa, shows negative regulation in osteoblast differentiation, whereas the long periostin, periostin-a, behaves in a positive manner for osteoblast differentiation (data not shown). Taken together, a new action of periostin termed as a “Periostin Switch” is proposed as shown in Fig. 5, indicating that in the early stage of periostin expression, it behaves as a positive regulator of cell proliferation, movement, and collagen production, and after cleavage of the C-terminal site, the action of periostin changes to collagen cross-linking.
Heparin binding site
What constitutes the C-terminal end of periostin? We found an arginine-rich heparin-binding site at this end, and observed that periostin protein could be purified using a heparin column [54]. Fibronectin contains two heparin-binding sites, the high-affinity heparin II binding domain located at the C-terminal site is thought to interact with cell surface glycosaminoglycans to facilitate cell adhesion and spreading, and also plays an important role in matrix assembly, whereas the heparin I domain is also involved in matrix assembly, and in particular, fibronectin self-assembly [55]. Assembly of dimeric fibronectin into the extracellular matrix involves multiple consecutive binding interactions with integrin receptors, with itself, and with matrix components such as type I collagen [56].
Elastin fiber formation
In fibrillogenesis, elastin fiber formation, in which extracellular short fibulins, fibulin-3, -4, and -5 are components of the elastic fiber/microfibril system and are implicated in the formation and homeostasis of elastic tissues, has properties similar to collagen fiber formation; (a) lox interaction, (b) integrin signaling, (c) heparin binding, (d) proteinase digestion, and (e) multimerization [57]. Thus, the periostin-like action is possibly utilized in this system. Among fibulins, fibulin-4 can work on proper elastogenesis in interaction with LOX that is an elastin-cross-linking enzyme [58], and these fibulins form multimers, indicating that fibulin-4 behaves like periostin in elastin fiber formation, whereas fibulin-5 binds to human umbilical vein endothelial cells in an RGD-dependent manner via integrins, indicating that fibulin-5 behaves like fibronectin. Moreover, both fibulins may bind cell surface-located heparin sulfate [57]. Taken together, these observations indicate that fibulin-4 and -5 are utilized in the formation of elastin fibers instead of periostin and fibronectin. Thus, knowledge of elastin fiber formation is very useful to understand the mechanism of collagen fiber formation.
γ-glutamyl-carboxylase
Modification of glutamic acid residues to γ-carboxyglutamic acid (Gla) is a post-translational modification catalyzed by the vitamin K-dependent enzyme, γ-glutamylcarboxylase. The most abundant Gla-containing protein secreted by bone marrow-derived mesenchymal stromal cells is periostin, in which the fas 1 domain is carboxylated [59]. Since only 12 vitamin K-dependent Gla-containing proteins such as osteocalcin and matrix Gla protein, which play a pivotal role in bone development and repair, have been identified in humans, periostin and βigh3 are the 13th and 14th of these proteins, respectively. Periostin was found to be abundantly deposited in bone nodules, in areas where osteoblastic cells are tightly embedded in the mineralized extracellular matrix, suggesting that the Gla residues on periostin might provide hydroxyapatite binding properties that have an important structural role.
Periostin paralogue, TGFB1 (βigh3)
Generally, the expression pattern between periostin and the periostin paralogue, TGFB1 (βigh3) is different. However, in bone cells, periostin and βigh3 are expressed in both osteoblasts and osteoclasts in vitro [60], suggesting that both genes possibly act co-operatively in bone formation.
Periostin action
Periostin action in stemness
Periostin is recognized as an important molecule in severe diseases such as scar formation in myocardial infarction, fibrosis in asthma, cancer cell migration [61], and others are shown in Fig. 6. Recently, interesting reports have appeared regarding periostin action in stem cell biology. In hematopoietic stem cells (HSC), Khurana et al. [62] reported that periostin via interaction with integrin-αv, regulates HSC maintenance in the bone marrow niche, and Tanaka et al. [63] reported that periostin from stromal cells supports both normal hematopoietic progenitor cells and leukemia-initiating cells. It is also reported that periostin is associated with a matricellular protein, CCN3 (NOV), and that a functional relationship is found in the periodontal ligament [64]. In this regard, CCN3 functions as a regulator of human HSC or hematopoietic progenitor cells as demonstrated by the loss of CCN3, which diminishes the functional capacity of the primitive hematopoietic compartment [65], and in mice, CCN3 functions in the maintenance of HSCs [66], suggesting an intrinsic activity of periostin together with CCN3 for maintaining stemness. Moreover, Notch signaling is involved in this stem system, and periostin–Notch1 and periostin–CCN3 interactions suggest the involvement of periostin–CCN3–Notch in stemness. In another type of stem cells, periostin is secreted from mesenchymal stem cells to support tendon formation [67], characterized by the overexpression of periostin.
Periostin in mechanical stress
From its initial naming as periostin derived from periosteum and periodontal ligament, this protein has been expected to be mechanical stress-sensitive [3], because the periosteum on bone and periodontal ligament in teeth, or in the lately found cardiac valves [68] are very sensitive to mechanical stress for tissue regeneration and development; however, no direct evidence of stress sensitivity has been reported. It is presumed that periostin is more functional in humans but not in other animal models because humans walks upright, which induces severe mechanical stress. Interestingly, a report has described a signal-linking mechanical stress with the upregulation of periostin expression; Rosselli-Murai et al. [69] found that both periostin and mammalian target of rapamycin (mTOR) are coordinately upregulated with tension forces during wound healing to induce cell proliferation and migration, indicating that the same signal derived from tendon forces activates the expression of both periostin and mTOR, after which periostin enhances the mTOR signals. In addition, the mTOR signal activates osteoclast differentiation for the initiation of bone remodeling [70], which reasonably suggests that periostin functions in the maintenance of bone mass during bone remodeling [8]. A potential candidate signal for mTOR and periostin activation is neuronal nitric oxide synthase (nNOS). In overload-induced skeletal muscle hypertrophy in mice, nNOS is transiently activated within 3 min of overload, which then activates the transient receptor potential cation channel, subfamily V member 1 (TRPV1), resulting increased intracellular Ca2+ concentration that subsequently triggers mTOR activation [71].
References
Takeshita S, Kikuno R, Tezuka K, Amann E (1993) Osteoblast-specific factor 2: cloning of a putative bone adhesion protein with homology with the insect protein fasciclin I. Biochem J 294:271–274
Hoersch S, Andrade-Navarro MA (2010) Periostin shows increased evolutionary plasticity in its alternatively spliced region. BMC Evo Biol 10:30
Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katsuura M, Ozawa H, Toyama Y, Bonewald LF, Kudo A (1999) Identification and characterization of a novel protein, periostin with restricted expression to periosteum and periodontal ligament and increased expression by transforming growth factor β. J Bone Miner Res 14:1239–1249
Zinn K, McAllister L, Goodman CS (1988) Sequence analysis and neuronal expression of fasciclin I in grasshopper and Drosophila. Cell 53:577–587
Han F, Gilbert JR, Harrison G, Adams CS, Freeman T, Tao Z, Zaka R, Liang H, Williams C, Tuan RS, Norton PA, Hickok NJ (2007) Transforming growth factor-β1 regulates fibronectin isoform expression and splicing factor SRp40 expression during ATDC5 chondrogenic maturation. Exp Cell Res 313:1518–1532
Shimazaki M, Nakamura K, Kii I, Kashima T, Amizuka N, Li M, Saito M, Fukuda K, Nishiyama T, Kitajima S, Saga Y, Fukayama M, Sata M, Kudo A (2008) Periostin is essential for cardiac healing after acute myocardial infarction. J Exp Med 205:295–303
Nishioka T, Onishi K, Shimojo Y, Matsusaka H, Ikeuchi M, Ide T, Tsutsui H, Hiroe M, Yoshida T, Imanaka-Yoshida K (2010) Tenascin-C may aggregate left ventricular remodeling and function after myocardial infarction. Am J Physiol Heart Circ Physiol 298:H1072–H1078
Kii I, Nishiyama T, Li M, Matsumoto K, Saito M, Amizuka N, Kudo A (2010) Incorporation of tenascin-C into the extracellular matrix by periostin underlies an extracellular meshwork architecture. J Biol Chem 285:2028–2039
Morra L, Rechsteiner M, Casagrande S, Duc Luu V, Santimaria R, Diener PA, Sulser T, Kristiansen G, Schraml P, Moch H, Soltermann A (2011) Relevance of periostin splice variants in renal cell carcinoma. Am J Pathol 179:1513–1521
Morra L, Rechsteiner M, Casagrande S, von Teichman A, Schraml P, Moch H, Soltermann A (2012) Characterization of periostin isoform pattern in non-small cell lung cancer. Lung Cancer 76:183–190
Nakama T, Yoshida S, Ishikawa K, Kobayashi Y, Abe T, Kiyonari H, Shioi G, Katsuragi N, Ishibashi T, Morishita R, Taniyama Y (2016) Different roles played by periostin splice variants in retinal neovascularization. Exp Eye Res 153:133–140
Yamada S, Tauchi T, Awata T, Maeda K, Kajikawa T, Yanagita M, Murakami S (2014) Characterization of a novel periodontal ligament-specific periostin isoform. J Dent Res 93:891–897
Nance T, Smith KS, Anaya V, Richardson R, Lawrence H, Pala M, Mostafavi S, Battle A, Feghali-Bostwick C, Rosen G, Montgomery SB (2014) Transcriptome analysis reveals differential splicing events in IPF lung tissue. PLoS One 9:e92111
Kudo H, Amizuka N, Araki K, Inohaya K, Kudo A (2004) Zebrafish periostin is required for the adhesion of muscle fiber bundles to the myoseptum and for the differentiation of muscle fibers. Dev Biol 267:473–487
Ito K, Morioka M, Kimura S, Tasaki M, Inohaya K, Kudo A (2014) Differential reparative phenotypes between zebrafish and medaka after cardiac injury. Dev Dyn 243:1106–1115
Tao S, Kuhl M, Kuhl SJ (2011) Expression of periostin during Xenopus laevis embryogenesis. Dev Genes Evol 221:247–254
Oshima A, Tanabe H, Yan T, Lowe GN, Glackin CA, Kudo A (2002) A novel mechanism for the regulation of osteoblast differentiation: transcription of periostin, a member of the fasciclin I family, is regulated by the bHLH transcription factor, Twist. J Cell Biochem 86:792–804
Afanador E, Yokozeki M, Oba Y, Kitase Y, Takahashi T, Kudo A, Moriyama K (2005) Messenger RNA expression of periostin and twist transiently decrease by occlusal hypofunction in mouse periodontal ligament. Arc Oral Biol 50:1023–1031
Lee MS, Lowe G, Strong DD, Wergedal J, Glackin CA (1999) TWIST, a basic-loop-helix transcription factor, can regulate the human osteogenetic lineage. J Cell Biochem 75:566–567
Bialek P, Kern B, Yang X, Schrock M, Sosic D, Hong N, Wu H, Yu K, Ornitz DM, Olson EN, Justice MJ, Karsenty G (2004) A twist code determines the onset of osteoblast differentiation. Develop Cell 6:423–435
Inai K, Norris RA, Hoffman S, Markwald RR, Sugi Y (2008) BMP-2 induces cell migration and periostin expression during atrioventricular valvulogenesis. Dev Biol 315:383–396
Shelton EL, Yutzey KE (2008) Twist 1 function in endocardial cushion cell proliferation, migration, and differentiation during heart valve development. Dev Biol 317:282–295
Franco HL, Casasnovas JJ, Lenon RG, Friesel R, Ge Y, Desnick RJ (2011) Nonsense mutations of the bHLH transcription factor TWIST2 found in Setleis Syndrome patients cause dysregulation of periostin. Int J Biochem Cell Biol 43:1523–1531
Lindsley A, Snider P, Zhou H, Rogers R, Wang J, Olaopa M, Kruzynska-Frejtag A, Koushik SV, Lilly B, Burch JBE, Firulli AB, Conway SJ (2007) Identification and characterization of a novel Schwann and outflow tract endocardial cushion lineage-restricted periostin enhancer. Dev Biol 307:340–355
Kurisaki K, Kurisaki A, Valcourt U, Terentiev AA, Pardali K, ten Dijke P, Heldin C-H, Ericsson J, Moustakas A (2003) Nuclear factor YY1 inhibits transforming growth factor {beta}- and bone morphogenetic protein-induced cell differentiation. Mol Cell Biol 23:4494–4510
Xiao S-M, Gao Y, Cheng C-L, Bow CH, Lau K-S, Sham PC, Tan KCB, Kung AWC (2012) Association of CDX1 binding site of periostin gene with bone mineral density and vertebral fracture risk. Osteoporos Int 23:1877–1887
Subramanian V, Meyer BI, Gruss P (1995) Disruption of the murine homeobox gene Cdx1 affects axial skeletal identities by altering the mesodermal expression domains of Hox genes. Cell 83:641–653
Kashima TG, Nishiyama T, Shimazu K, Shimazaki M, Kii I, Grigoriadis AE, Fukayama F, Kudo A (2009) Periostin, a novel marker of intramembranous ossification, is expressed in fibrous dysplasia and in c-Fos-overexpressing bone lesions. Hum Pathol 40:226–237
Sidhu SS, Yuan S, Innes AL, Kerr S, Woodruff PG, Hou L, Muller SJ, Fahy JV (2010) Roles of epithelial cell-derived periostin in TGF-b activation, collagen production, and collagen gel elasticity in asthma. Proc Natl Acad Sci USA 17:14170–14175
Takayama G, Arima K, Kanaji T, Toda H, Shoji S, McKenzie AN, Nagai H, Hotokebuchi T, Izuhara K (2006) Periostin: a novel component of subepithelial fibrosis of bronchial asthma downstream of IL-4 and IL-13 signals. J Allergy Clin Immunol 118:98–104
Masuoka M, Shiraishi H, Ohta S, Suzuki S, Arima K, Aoki S, Toda S, Inagaki N, Kurihara Y, Hayashida S, Takeuchi S, Koike K, Ono J, Noshiro H, Furue M, Conway SJ, Narisawa Y, Izihara K (2012) Periostin promotes chronic allergic inflammation in response to Th2 cytokines. J Clin Invest 122:2590–2600
Wen W, Chau E, Jackson L, Elliott C, Daley TD, Hamilton DW (2010) TGF-β1 and FAK regulate periostin expression in PDL fibroblasts. J Dent Res 89:1439–1443
Gordon S, Martinez FO (2010) Alternative activation of macrophages: mechanism and functions. Immunity 32:593–604
Zho W, Ke SQ, Huang Z, Flavahan W, Fang X, Paul J, Wu L, Sloan AE, McLendon RE, Li X, Rich JN, Bao S (2014) Periostin secreted by glioblastoma stem cells recruits M2 tumor-associated macrophages and promotes malignant growth. Nat Cell Biol 17:170–182
Wu T, Ouyang G (2015) Periostin: a potent chemotactic factor for recruiting tumor-associated macrophage. Protein Cell 6:235–237
Guo X, Xue H, Shao Q, Wang J, Guo X, Chen X, Zhang J, Xu S, Li T, Zhang P, Gao X, Qiu W, Liu Q, Li G (2016) Hypoxia promotes glioma-associated macrophage infiltration via periostin and subsequent M2 polarization by upregulating TGF-beta and M-CSFR. Oncotarget 7:80521–80542
Yokota K, Kobayakawa K, Saito T, Hara M, Kijima K, Ohkawa Y, Harada A, Okazaki K, Ishihara K, Yoshida S, Kudo A, Iwamoto Y, Okada S (2017) Periostin promotes scar formation through the interaction between pericytes and infiltrating monocytes/macrophages after spinal cord injury. Am J Pathol 187:639–653
Hortsh M, Goodman CS (1991) Cell and substrate adhesion molecules in Drosophila. Annu Rev Cell Biol 7:505–557
Kim JE, Kim SJ, Lee BH, Park RW, Kim KS, Kim IS (2000) Identification of motifs for cell adhesion within the repeated domains of transforming growth factor-beta-induced gene, beta ig-h3. J Biol Chem 275:30907–30915
Politz O, Gratchev A, McCourt PA, Schledzewski K, Guillot P, Johansson S, Svineng G, Franke P, Kannicht C, Kzhyshkowska J, Longati P, Velten FW, Johansson S, Goerdt S (2002) Stabilin-1 and -2 constitute a novel family of fasciclin-like hyaluronan receptor homologues. Biochem J 362:155–164
Munier FL, Korvatska E, Djemai A, Le Paslier D, Zografos L, Pescia G, Schorderet DF (1997) Kerato-epithelin mutations in four 5q31-linked corneal dystrophies. Nat Genet 15:247–251
Doliana R, Bot S, Bonaldo P, Colombatti A (2000) EMI, a novel cysteine-rich domain of EMILINs and other extracellular proteins, interacts with the gC1q domains and participates in multimerization. FEBS Lett 484:164–168
Kii I, Nishiyama T, Kudo A (2016) Periostin promotes secretion of fibronectin from the endoplasmic reticulum. Biochem Biophy Res Commun 470:888–893
Maruhashi T, Kii I, Saito M, Kudo A (2010) Interaction between periostin and BMP-1 promotes proteolytic activation of lysyl oxidase. J Biol Chem 285:13294–13303
Ge G, Greenspan DG (2006) Developmental roles of the BMP1/TLD metalloproteinases. Birth Defects Res (Part C) 78:47–68
Nishiyama T, Kii I, Kashima TG, Kikuchi Y, Ohazama A, Shimazaki M, Fukayama M, Kudo A (2011) Delayed re-epithelialization in periostin-deficient mice during cutaneous wound healing. PLoS One 4:e18410
Syx D, Guillemyn B, Symoens S, Sousa AB, Medeira A, Whiteford M, Hermanns-Le T, Coucke PJ, de Paepe A, Malfait F (2015) Defective proteolytic processing of fibrillar procollagens and prodecorin due to biallelic BMP1 mutations results in a severe, progressive form of Osteogenesis Imperfecta. J Bone Miner Res 30:1445–1456
Wang J, Massoudi D, Ren Y, Muir AM, Harris SE, Greenspan DS, Feng JQ (2017) BMP1 and TLL1 are required for maintaining periodontal homeostasis. J Dent Res 96:578–585
Takayama I, Kudo A (2012) Periostin in dental science. Jpn Dent Sci Rev 48:92–98
Gartland A, Erler JT, Cox TR (2016) The role of lysyl oxidase, the extracellular matrix and the pre-metastatic niche in bone metastasis. J Bone Oncol 5:100–103
Cox TR, Rumney RMH, Schoof EM, Perryman L, Hoye AM, Agrawal A, Bird D, Latif NA, Foreest H, Evans HR, Huggins ID, Lang G, Linding R, Gartland A, Erler JT (2015) The hypoxic cancer secretome induces pre-metastatic bone lesions through lysyl oxidase. Nature 522:106–110
Chen Y, Guo H, Terajima M, Banerjee P, Liu X, Yu J, Momin AA, Karayama H, Hanash SM, Burns AR, Fields GB, Yamauchi M, Kurie JM (2016) Lysyl hydroxylase 2 is secreted by tumor cells and can modify collagen in the extracellular space. J Biol Chem 291:25799–25808
Kondoh H, Nishiyama T, Kikuchi Y, Fukayama M, Saito M, Kii I, Kudo A (2016) Periostin deficiency causes severe and lethal lung injury in mice with bleomycin administration. J Histochem Cytochem 64:441–453
Sugiura T, Takamatu S, Kudo A, Amann E (1995) Expression and characterization of murine osteoblast-specific factor 2 (OSF-2) in a baculovirus expression system. Protein Expr Purif 6:305–311
van Vliet AI, van Alderwegen IE, Baelde HJ, Heer ED, Bruijn JA (2002) Fibronectin accumulation in glomerulosclerotic lesions: self-assembly sites and the heparin II binding domain. Kidney Int 61:481–489
Bultmann H, Santas AJ, Pesciotta Peters DM (1998) Fibronectin fibrillogenesis involves the heparin II binding domain of fibronectin. J Biol Chem 273:2601–2609
Takayama I, Tanabe H, Nishiyama T, Ito H, Amizuka N, Li M, Watanabe Y, Katsube K, Kii I, Kudo A (2017) Periostin is required for matricellular localization of CCN3 in periodontal ligament of mice. J Cell Commun Signal 11:5–13
Gupta R, Hong D, Iborra F, Sarno S, Enver T (2007) NOV(CCN3) functions as a regulator of human hematopoietic stem of progenitor cells. Science 316:590–593
Coutu DL, Hui WuJ, Monette A, Rivard G-E, Blostein MD, Galipeau J (2008) Periostin, a member of a novel family of vitamin K-dependent proteins, is expressed by mesenchymal stromal cells. J Biol Chem 283:17991–18001
Merie B, Bouet G, Rousseau J-C, Betholon C, Garnero P (2014) Periostin and transforming growth factor β-induced protein (TGFβIp) are both expressed by osteoblasts and osteoclasts. Cell Biol Int 38:398–404
Conway SJ, Izuhara K, Kudo Y, Litvin J, Markwald R, Ouyang G, Arron JR, Holweg CTJ, Kudo A (2014) The role of periostin in tissue remodeling across health and disease. Cell Mol Life Sci 71:1279–1288
Khurana S, Schouteden S, Manesia JK, Sanamaria-Martinez A, Huelsken J, Lacy-Hulbert A, Verfaillie CM (2016) Outside-in integrin signaling regulates haematopoietic stem cell function via Periostin-Itgav axis. Nature Commun 7:13500
Tanaka S, Maekawa A, Matsubara L, Imanishi A, Yano M, Roeder RG, Hasegawa N, Asano S, Ito M (2016) Periostin supports hematopoietic progenitor cells and niche-dependent myeloblastoma cells in vitro. Biochem Biophys Res Commun 478:1706–1712
Djokic J, Fagotto-Kaufmann C, Bartels R, Nelea V, Reinhardt DP (2013) Fiblin-3, -4, and -5 are highly susceptible to proteolysis, interact with cells and heparin, and form multimers. J Biol Chem 288:22821–22835
Horiguchi M, Inoue T, Ohbayashi T, Hirai M, Noda K, Marmorstein LY, Yabe D, Takagi K, Akama TO, Kita T, Kimura T, Nakamura T (2009) Fibulin-4 conducts proper elastogenesis via interaction with cross-linking enzyme lysyl oxidase. Proc Natl Acad Sci USA 45:19029–19034
Ishihara J, Umemoto T, Yamato M, Shiratsuchi Y, Takaki S, Petrich BG, Nakauchi H, Eto K, Kitamura T, Okano T (2014) Nov/CCN3 regulates long-term repopulating activity of murine hematopoietic stem cells via integrin avb3. Int J Hematol 99:393–406
Noack S, Seiffart V, Willbold E, Laggies S, Winkel A, Shahab-Osterloh S, Florkemeier T, Hertwig F, Steinhoff C, Nuber UA, Gross G, Hoffmann A (2014) Periostin secreted by mesenchymal stem cells supports tendon formation in an ectopic mouse model. Stem Cell Develop 23:1844–1857
Kruzynska-Freitag A, Machnicki M, Rogers R, Markwald RR, Conway SJ (2001) Periostin (an osteoblast-specific factor) is expressed within the embryonic mouse heart during calve formation. Mech Dev 103:183–188
Rosselli-Murai LK, Almeida LO, Zagni C, Galindo-Moreno P, Padial-Molina M, Volk SL, Murai MJ, Rios HF, Squarize CH, Castilho RM (2013) Periostin responds to mechanical stress and tension by activating the MTOR signaling pathway. PLoS One 8:e83580
Dai Q, Xie F, Han Y, Ma X, Zhou S, Jiang L, Zou W, Wang J (2017) Inactivation of regulatory-associated protein of mTOR (Raptor)/mammalian Target of Rapamycin Complex 1 (mTORC1) signaling in osteoclasts increases bone mass by inhibiting osteoclast differentiation in mice. J Biol Chem 292:196–204
Ito N, Ruegg UT, Kudo A, Miyagoe-Suzuki Y, Takeda S (2013) Activation of calcium signaling through Trpv1 by nNOS and peroxynitrite as a key trigger of skeletal muscle hypertrophy. Nat Med 19:101–106
Acknowledgements
I thank my collaborators involved in the periostin project for providing figures.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kudo, A. Introductory review: periostin—gene and protein structure. Cell. Mol. Life Sci. 74, 4259–4268 (2017). https://doi.org/10.1007/s00018-017-2643-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-017-2643-5