Abstract
Dysfunction of many ciliary proteins has been linked to a list of diseases, from cystic kidney to obesity and from hypertension to mental retardation. We previously proposed that primary cilia are unique communication organelles that function as microsensory compartments that house mechanosensory molecules. Here we report that primary cilia exhibit membrane swellings or ciliary bulbs, which based on their unique ultrastructure and motility, could be mechanically regulated by fluid-shear stress. Together with the ultrastructure analysis of the swelling, which contains monosialodihexosylganglioside (GM3), our results show that ciliary bulb has a distinctive set of functional proteins, including GM3 synthase (GM3S), bicaudal-c1 (Bicc1), and polycystin-2 (PC2). In fact, results from our cilia isolation demonstrated for the first time that GM3S and Bicc1 are members of the primary cilia proteins. Although these proteins are not required for ciliary membrane swelling formation under static condition, fluid-shear stress induced swelling formation is partially modulated by GM3S. We therefore propose that the ciliary bulb exhibits a sensory function within the mechano-ciliary structure. Overall, our studies provided an important step towards understanding the ciliary bulb function and structure.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Primary cilia, presumed to be a kind of sensory organelles in 1898 [1], have been intensively studied due to their association to a group of diseases, called ciliopathies [2]. Among other ciliopathies, polycystic kidney disease is thought to be pertinent with defective in mechanosensory cilia function [3, 4]. Activation of primary cilia results in an increased calcium level in cilioplasm and cytoplasm of renal epithelial cells [5, 6]. The role of mechanosensory cilia has also been demonstrated in other cell types, including endothelial [7, 8], bone [9], cartilage [10, 11], liver [12], nodal [13, 14], fibroblast [15], kidney [16–20], pancreatic [21, 22], and stem cells [23].
Ultrastructurally, primary cilia have hair-like structures supported by microtubules and enclosed by the ciliary membrane. These microtubules are generally arranged circumferentially without a central pair. The basal body and centriole join together to form a centrosome, which serves as the cell’s main microtubule organization. The ciliary necklace, which is made up by a distinct set of membrane proteins at the transition zone, helps to differentiate the ciliary membrane from the cell’s plasma membrane and cilia membrane [24]. In addition, receptors, ion channels, transporters, and sensory proteins with various functions reside on the ciliary membrane. Furthermore, the cilioplasm of a primary cilium is enriched with many signaling intermediates and is believed to play a role in calcium signaling upon cilia bending or activation [6].
Primary cilia have a bulging or swelling structure, known as bulb. The presence of ciliary swelling was initially observed in 1977 from motile cilia of marine invertebrates, Rhabdopleura zooids [25]. It was proposed that ciliary swelling region is a result of material transport within the cilia [26]. Although motile cilia with paddle-shaped (paddle cilia) or disc-shaped (discocilia) are known to enclose a curved end of the axoneme in a variety of marine invertebrates, it was later argued that the ciliary swelling region observed in motile cilia of marine invertebrates was a fixation artifact, resulted from the changes in osmotic pressure during sample preparations [27]. Interestingly, it was further argued that cilia were structurally not genuine organelles in marine invertebrates [28]. Fixatives that were isosmotic with seawater did not form paddle cilia or discocilia. Hypotonic seawater, however, induced formation of cilia and swelling of the ciliary membrane as observed in the hypertonic microscopy fixative solution.
Throughout this manuscript, we will use the terms “ciliary membrane swelling” or “ciliary bulb” interchangeably. To our knowledge, the term “ciliary bulb” was coined from the presence of a pouch or swelling-like structure seen in the epithelial cilia of the olfactory bulb. Primary cilia of olfactory epithelial cells tend to be wider at the tips. Supporting cells of the olfactory bulb also show swelling or bulb-like structures at the tips of primary cilia [29, 30]. It was thus proposed that the bulbs are responsible for the reception and initial transduction processes of smells [31]. The ciliary bulb was also observed in renal epithelial cells [32]. It was suggested that the ciliary bulb is associated with the ciliary shaft and may represent a circumscribed region of the ciliary membrane. Like the bulbs observed in paddle cilia and discocilia of marine invertebrates, the renal ciliary bulb was hypothesized to be sensitive to environmental stimuli, including osmotic pressure [32].
Unfortunately, the role of the ciliary bulb has never been previously studied. Most of the observational studies of ciliary membrane swelling were performed in the fixed tissues/cells, and live imaging on the ciliary bulb had never been previously done. In this study, we now show for the first time that the membrane swelling of the primary cilium is actually a dynamic structure. We further identified biomechanical property of the ciliary bulb and proteins that are present in the swelling region of a cilium.
Results
Ciliary bulb is a dynamic structure that can be modulated by fluid-shear stress
We previously designed an experimental setup that allowed us to examine primary cilia from the side [6]. We noticed that when a cell population was challenged with fluid flow, the ciliary membrane swellings seemed to appear more often and were preferentially located closer to the tip of cilia. On the other hand, fewer swelling structures were observed at the middle of the cilia under static non-flow condition. Based on this observation, we hypothesized that the ciliary membrane swelling is a dynamic structure and that its movement can be regulated by the surrounding microenvironment.
To test our hypothesis, we used LLCPK cells grown on flexible substratum (formvar). Ciliary membrane swellings were studied during absence (static) and presence of fluid-shear stress. In line with our initial observation under static conditions, ciliary swelling tended to oscillate up and down along the ciliary shaft and was never able to reach the tip of the cilium (Fig. 1a; Supp Movie 1). To examine the sensitivity of a cilium in response to mechanical stimulus, we provided an abrupt pulse of fluid flow, enough to generate small movement of the cilium. To our surprise, this induced the appearance of another swelling (Fig. 1b; Supp Movie 2). However, one of the swellings was reabsorbed into the cilia immediately after the cessation of fluid flow.
The ciliary bulb had a dynamic movement in both static and shear stress conditions. Representative time-lapse images are shown with time in seconds in each panel. Under static conditions, a swelling structure of a cilium moves along the ciliary shaft but never reaches the tip of cilium (a). A very gentle fluid movement was initiated at 15 s (flow on) and stopped at 19 s (flow off). The new swelling formation was observed immediately (2 s). The cilium eventually retained only one swelling structure when shear stress was ceased (b). Black arrows indicate the positions of ciliary swelling, and white arrows indicate a newly formed ciliary swelling
In some instances, especially when the additional swelling appeared earlier, an extended fluid flow tended to cause the cilium to maintain an additional swelling longer, even after the flow was stopped (Fig. 2a; Supp Movie 2). One of the swellings was always maintained at the tip of the cilium, while the second swelling seemed to move very close to the tip of the cilium during fluid flow. In some cases, swelling appearance induced by fluid flow was observed from the middle of ciliary shaft (Fig. 2b; Supp Movie 3). When a new swelling appears, it always traveled to the tip of cilia.
The ciliary bulb could be induced by shear stress. Representative time-lapse images are shown with time in seconds in each panel. Fluid flow-induced cilia bending promoted the movement of the ciliary swelling to the tip of cilium. Fluid flow was initiated immediately at 76 s (flow on) after the baseline and terminated at 310 s (flow off) (a). Two swelling structures were observed at the tip of the cilium. In a different experiment, a fluid movement was initiated at 2 s (flow on) and stopped after 282 s (flow off) (b). Formation of a new swelling in the middle of the ciliary shaft was seen at 170 s, and the bulb immediately migrated to the tip of the cilium (176 s). This swelling was reabsorbed when shear stress was ceased, and the physical swelling along the cilia is thus no longer observed. Black arrows indicate the positions of ciliary swellings, and white arrows indicate newly formed ciliary swellings
Quantifying these events, we found that formation of ciliary membrane swelling was significantly increased from static conditions (30.1 %) to mechanical inductions (85.2 %; Fig. 3a). The average cilia length in LLCPK cells was about 6 μm (Fig. 3b). The position of the swellings was about 4 and 6 μm away from the apical cell surface under static and mechanically induced conditions, respectively. Regardless of a new or pre-existing swelling, the swelling always moved to the ciliary tip under fluid-shear stress (Fig. 3c). This supported our hypothesis that the ciliary membrane swelling was a dynamic structure and could be modulated by the physical force of fluid movement.
Fluid-shear stress modulated position and formation of ciliary bulb along the cilium. Total number of observations on individual cilium was tabulated, and the number of the ciliary swelling was seen more frequently under fluid-shear stress than static condition (a). Average ciliary length was shown, and ciliary swellings significantly moved away from the apical membrane under fluid-shear stress (b). When individual swelling was normalized to its own ciliary length, ciliary swelling significantly moved toward the ciliary tip (c). Asterisks denote significant difference between groups (p < 0.05; n > 615 for each group)
Ciliary bulb was glycosylated and contained GM3S, PC2, and Bicc-1
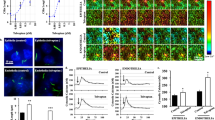
Because cilia have been shown to have specific lipid and carbohydrate moiety [33], we investigated the presence of monosialodihexosylganglioside (GM3) in the ciliary membrane swelling. To our surprise, we found that GM3 specifically localized only on the swelling region (Fig. 4a). Because GM3 is synthesized by GM3 synthase (GM3S), we further investigated the presence of GM3S in ciliary bulb. Like GM3, GM3S was specifically localized in the ciliary membrane swelling (Fig. 4b). Because ciliary swelling could be modulated by fluid-shear stress, we next investigated if polycystin-2 (PC2) known as a mechanosensory protein was localized to the swelling. While PC2 has been shown to localize to the entire cilia, some studies have shown a more distinct pattern [34, 35]. Our results showed that PC2 was localized more specifically to the swelling region rather than the ciliary shaft (Fig. 4c). PC2 has been shown to be modulated by many proteins, but its expression is specifically regulated by Bicc-1 [36]. Indeed, Bicc-1 was also specifically localized to the ciliary membrane swelling (Fig. 4d).
GM3, GM3S, PC2, and BICC-1 were localized to ciliary bulb. Monosialodihexosylganglioside (GM3; a), GM3 synthase (GM3S; b), polycystin-2 (PC2; c) and bicaudal-C (Bicc-1; d) were localized to ciliary bulb (red). Acetylated-α-tubulin (acet-α-tub; green) and DAPI (blue) were used as ciliary and nuclear markers, respectively. High-resolution differential interference contrast (DIC) images are shown to visualize and confirm the presence of ciliary swellings. n > 65 for each study. n is referred to the number of individual cell in each experiment with localization of the protein to the ciliary bulb
To our knowledge, we were the first to show that the ciliary membrane swelling was glycosylated, possibly by GM3S and that Bicc-1 was localized to the cilia. To verify our finding, we next confirmed if GM3S and Bicc-1 could be detected in cilia using cilia isolation and Western blot analyses. Using stably transfected-SST3R in LLCPK cells to help visualize isolated cilia, we were able to isolate cilia by shearing the cilia of the cell surface (Fig. 5a) [37]. This was confirmed using non-transfected cells, where isolated cilia were immunostained with ciliary marker acetylated-α-tubulin. In the Western Blot analyses, we compared lysates from the isolated cilia and the cell body (Fig. 5b). GAPDH as a marker for cell body and acetylated-α-tubulin as a ciliary marker were used to demonstrate the purity of our cilia isolation. Moreover, GM3S was mainly localized in cilia of fully differentiated cells, while Bicc-1 was detected in both cilia and the cell body. This confirmed that GM3S and Bicc1 were localized on the primary cilia.
GM3S and BICC-1 were detected in isolated primary cilia. Stably transfected LLCPK cells with SST3R-GFP were used as confirmation for the primary cilia isolation (a, upper panels). Isolated cilia from LLCK cells were immunostained with ciliary marker, acetylated-α-tubulin (acet-α-tub; lower panels). The DIC and green fluorescence images demonstrate the purity of primary cilia isolation. The isolated primary cilia were immunoblotted to further confirm the presence of GM3S and BICC-1 in the primary cilia (b). GAPDH was used as a negative control while acetylated-α-tubulin was used as a positive control for ciliary marker
Shear-induced bulb formation was partly modulated by GM3S, but not Bicc-1 or PC2
To identify if formation of ciliary membrane swelling was regulated by GM3S, Bicc-1 or PC2, we generated knockdown cell lines for the respective genes (St3gal5, Bicc-1 or Pkd2). We tested knockdown efficiencies of these genes using four different knockdown sequences (Fig. 6a). In the case of Bicc-1, we used both chemical siRNA transfection and shRNA viral infection approaches. All stably knockdown cell lines were verified for their corresponding protein expressions, and Bicc-1 #B, St3gal5 #D, and Pkd2 #D shRNA knockdown cell lines were selected for further studies. Because the shRNA expression was tagged with fluorescence GFP, we confirmed the stability of the shRNA integration in these knockdown cell lines after several passages by immunefluorescence microscopy (Fig. 6b) and flow-cytometry (Supplemental Fig. 1).
Stable Bicc-1, St3gal5, and Pkd2 knockdown cell lines were generated. Knockdown efficiency was first examined with silencing different regions of each gene, and siRNA was also tested as an earlier approach (a). Total cell lysate from scramble (control) and various siRNAs or shRNA-GFP lentivirus sequences (A, B, C, D) of each gene were analyzed. Once the shRNA-GFP knockdown efficiency was selected, infection efficiency among cell population was visualized with DIC and fluorescence microscopy (b)
To test the hypothesis that GM3S, Bicc-1, and/or PC2 were involved in ciliary membrane swelling formation, we studied the swelling formation in St3gal5, Bicc-1, and Pkd2 knockdown cells. The presence of membrane swelling was apparent in St3gal5 knockdown cells (Fig. 7a). As expected, GM3S was not observed in the ciliary swelling in St3gal5 knockdown cells. Surprisingly, Bicc-1 was also not detected in ciliary swelling, whereas PC2 was still present. Swelling formation was detected in Bicc-1 knockdown cells (Fig. 7b). As expected, Bicc-1 was not observed in the ciliary swelling in Bicc-1 knockdown cells. Interestingly, GM3S was also not detected in ciliary swelling, whereas PC2 was still present. As in St3gal5 and Bicc-1 knockdown cells, swelling formation was detected in Pkd2 cells (Fig. 7c). As expected, PC2 was not observed in the ciliary swelling in Pkd2 knockdown cells. Surprisingly, GM3S was also not detected in ciliary swelling, whereas Bicc-1 was still present. Our studies indicated that swelling localization or expression of GM3S, in part, could potentially be regulated by Bicc-1 and PC2. More importantly, our results demonstrated that GM3S, Bicc-1, and/or PC2 were not involved in swelling formation. To further investigate if flow-induced swelling formation was altered in these cells, we challenged the cells with fluid-shear stress and quantified formation of the swelling in each of these cells (Fig. 7d). Our results showed that unlike for PC2 and Bicc-1, GM3S was in part required for flow-induced swelling formation.
Flow-induced ciliary membrane swelling formation was modulated by GM3S. GM3S, Bicc-1 or PC2 expression (red) in ciliary swelling was examined in St3gal5 (a), Bicc-1 (b), and Pkd2 (c) knockdown cell lines. Acetylated-α-tubulin (acet-α-tub; green) and DAPI (blue) were used as ciliary and nuclear markers, respectively. High-resolution differential interference contrast (DIC) images are shown to visualize and confirm the presence of ciliary swellings. All knockdown cells had primary cilia and swelling formation. However, only St3gal5 knockdown cells lost swelling formation in response to fluid-shear stress (d). Asterisks denote significant difference between groups (p < 0.05; n = 65–242 for each group). “n” denotes the number of individual cell in each experiment
Global expressions of miRNA17, Bicc-1, GM3S, and PC2 were interrelated at the cell level
We next examined if Bicc-1 and PC2 regulated swelling localization or overall expression of GM3S. To understand the global effect of the knockdown genes at the cell level, we performed immunoblot studies to understand the interrelationship between the ciliary and cytoplasmic proteins. In particular, Bicc-1 [36], GM3S [38] and PC2 [39] have been detected at the cell body. miRNA-17 (Mir-17) was included in this experiment, because Mir-17 has been known to modulate PC2 and Bicc-1 [36]. Our studies indicated while knockdown of Mir-17 induced Bicc-1 expression level, knockdown of Bicc-1, St3gal5, or Pkd2 could have an effect on the global expression of each other (Fig. 8).
Relative gene expression was analyzed in the whole cell lysate. To examine if there is any interrelationship between the proteins located in the ciliary swelling at the cell level, protein expressions from the total cell lysate were analyzed in wild-type (control), scramble, miRNA17, st3gal5, Bicc-1, and Pkd2 knockdown cells. A flow diagram shows the proposed mechanistic pathway for the identified ciliary proteins. Asterisks denote significant difference between groups (p < 0.05; n = 3)
Discussion
Previous studies have shown the presence of the ciliary membrane swelling in both primary and secondary cilia of vertebrates [15, 32, 40–42], as well as invertebrates [25, 26]. The presence of the ciliary swelling in these studies was observed in the tip and at the middle of the ciliary shaft. Some have suggested that these ciliary swellings are a result of chemical fixation artifacts. Along this assumption, another study suggested that the ciliary swelling represents circumscribed regions of the ciliary membrane that are sensitive to osmotic pressure. In addition, ciliary swelling has also been observed in vivo, which argued that the bulb is not an artifact [32].
Observing the entire cilium from the side view allowed us to visualize and study the dynamics of ciliary swelling for the very first time in a single living cell. The presence of the swelling was observed in the pig kidney LLCPK and mouse renal epithelial cilia. We also showed that ciliary bulb was observed in vascular endothelial cilia (Supplemental Fig. 2). Proximal tubular epithelial LLCPK cells were used throughout our studies, because of their long cilia, which enabled us to image and differentiate ciliary membrane swelling from the cell body. It is important to note that the ciliary swelling was neither a result of chemical fixation artifacts nor changes in osmotic pressure. Our time-lapse imaging studies confirmed that ciliary swelling was observed in the absence of chemical reagents and differential osmotic pressure (Supp Movie 1). Furthermore, ciliary swelling was also observed to move dynamically along the ciliary shaft without the presence of fluid-shear stress. This indicated that ciliary bulb movement along the ciliary shaft could occur (1) without the bending of the cilia and (2) without external stimuli, such as osmotic pressure.
It has also been suggested that the formation of ciliary swelling is only observed in the ciliary tip of growing cilia due to differences in the growth rates between the ciliary membrane and the axonemal microtubules [43]. Another suggestion is that intraflagellar transport (IFT) molecules within the cilia are the main modulator of the ciliary swelling formation, and IFT forms the swelling-like structure in the absence of elongating axonemal microtubules [26, 44]. Our current results further indicated that ciliary swellings were dynamically observed in the ciliary tip only during fluid-shear stress, but during static condition ciliary bulbs were mostly observed along the ciliary shaft. Noteworthy, our analyses were performed in fully differentiated LLCKP cells with optimal length of cilia.
Based on our studies, a cilium can have multiple swelling regions along its ciliary shaft (Supp Movie 2). Interestingly, the multiple ciliary swellings could come very close to each other. In some cases, the swellings may have arrived from a split of an existing swelling. Consistent with this idea, while most swellings are round in shape, some swellings observed in our immunofluorescence were not. We believe that a swelling with an irregular shape may actually contain two or three swelling structures, either about to fuse together or undergoing separation. Our studies also indicate that the appearance of swellings could be induced with fluid-shear stress. One of the most obvious examples was seen from a cilium that did not initially have a swelling structure (Supp Movie 3). In this case, swelling appearance was induced by fluid flow and was observed from the middle of ciliary shaft. Interestingly, when formation of a new swelling structure was induced by fluid flow, it always traveled to the tip of cilia. This phenomenon was also confirmed in our immunofluorescence studies, where most of the swellings were observed on the tips of the cilia. Of note is that immunostaining method required frequent washing steps, before and after fixation, which would generate fluid flow on the top of the cells. This fluid flow helped the ciliary swelling to move to the tip of the cilia, which further supported our live-imaging studies and our hypothesis that ciliary membrane swelling was a sensitive structure responsive to the surrounding microenvironment. It is important to note that under static condition without flow, the swelling still could move momentarily to the ciliary tip and move back along the ciliary shaft (Supplemental Fig. 3). Furthermore, in a very rare occasion the swelling could maintain at the bottom of a cilium under fluid flow. Thus, the swelling could actively move to the tip of a cilium, and it was not simply moved to the tip passively by the flow.
To further examine if the magnitude of shear stress would induce swelling formation differentially, we challenged the cells at low (10.3 ± 2.1 μL/min), medium (21.6 ± 2.4 μL/min) or high (35.3 ± 1.1 μL/min) flow rate (Supplemental Fig. 4). All in all, flow-induced swelling formation was observed in about 80–90 % of the cells. For those cells that responded to fluid-shear stress, there was no indication that magnitude of fluid-shear stress would differentially affect swelling formation (magnitude-independent). Our present studies unfortunately did not produce any meaningful data to help explain the physiological roles of the flow-induced swelling formation or movement to the tips of cilia. We, however, speculate that the swelling may be shed as an exosome or exosome-like vesicle (ELVs), similar to the scenario in chlamydomonas where vesicles are shed from flagella and contain enzymes which assist in the digestion of the mother cell wall, a process critical in hatching of the exospore after dormancy [45]. It has also been reported that renal primary cilia can interact with ELVs which are PC2-positive, and it has been postulated that these can transduce a `urocrine’ signaling event [46]. Recent work in the nematode shows that extracellular vesicles (ECVs) are also released from the plasma membrane at the base of the primary cilium and maintain a close association with the shaft until they are shed [47]. The ECVs are positive for Lov-2, a homolog of PC2. These observations in the worm suggest a unifying hypothesis where ECVs can be released, perhaps after a signaling event. Certainly, the ECV from the male worm have potent effect on other males suggesting that the ELVs might represent an ancient signaling mechanism. Consistent with this idea, it has been hypothesized that ciliary proteins can be released from the primary cilium, a mechanism by which is regulated by fluid-shear stress [48].
Although the presence of ciliary swelling has been reported, the ultrastructure of a swelling had not been previously identified. Our studies indicated that ciliary swelling shared an intact structure along with the ciliary shaft, and there is no indication that ciliary swelling was attached to the outer ciliary membrane (Supplemental Fig. 5). This was observed not only in scanning electron microscopy (SEM) but also in freeze fracture transmission electron microscopy (FFTM), which gave us the ability to visualize the ciliary bulb without the use of any chemical fixation. Furthermore, the FFTM images showed particle indentions along the ciliary membrane, including the ciliary swelling. This finding suggested that ciliary swelling and the cilium itself contained integral and/or matrix proteins and that the swelling bilayer was part of the cilia bilayer.
The GM3 ganglioside is one of the members of the glycosphingolipid family. Previous study has shown that GM3 is co-localized with acetylated-α-tubulin to the cilium in MDCK cells [33]. Together with our FFTM study, GM3 was a good candidate to investigate in the ciliary swelling. To our surprise, we found that GM3 specifically localized on the swelling structure of a cilium. To further investigate about the GM3 ganglioside, we looked at the GM3S, which synthesizes GM3 in the Golgi [38]. Like GM3, GM3S was also localized to the ciliary swelling. To our knowledge, this was the first time that GM3S had been shown to localize to the primary cilia, specifically on the ciliary bulb. These findings further confirmed that ciliary swelling had a distinct protein moiety on its surface, consistent with our FFTM result.
PC2 has also been shown to contain N-glycosylation sites where it can be N-glycosylated [49, 50]. In addition, N-liked sugar moiety can be removed by endoglycosidase from PC2. Thus, PC2 might potentially co-localize with GM3 and/or GM3S. Because PC2 is also known as a mechanosensory protein and because shear stress could induce swelling formation, we asked if PC2 could be localized to the ciliary swelling. In agreement with our assumption, we found that PC2 localized to ciliary bulb. Overall, this suggests that (1) GM3S might assist PC2 localization to the bulb; (2) GM3S might be required for PC2 localization during flow-induced bulb formation; and/or (3) GM3S might play an important role in PC2 and Bicc-1 signaling pathway. Worth mentioning, PC2 has been known to localize to the primary cilia. However, we have found that PC2 was expressed and localized more specifically in the ciliary swelling region than in the ciliary shaft, at least in LLCPK cells.
Our immunofluorescence studies and western blot analyses suggested that Bicc-1 was co-localized with PC2 to the swelling structure. Although many other proteins can modulate PC2, the RNA-binding protein Bicc-1 specifically regulates PC2 expression through antagonizing the repressive activity of the microRNA-17 (miR-17) on the 3′UTR of Pkd2 mRNA [36]. Furthermore, both bicc-1 and pkd2 mouse mutants died prenatally and developed severe polycystic kidney disease. This may further implicate the association of Bicc-1 and PC2 in the swelling to ciliopathy.
Our data unfortunately did not indicate any of these proteins were directly involved in the ciliary membrane swelling formation. Knockdown of any of these proteins showed that ciliary swelling formation remained unaffected. When stably knockdown cells for each gene were challenged with fluid-shear stress, only stable cell line with st3gal5 knockdown showed a decrease in swelling formation in response to fluid-shear. This indicated that mutation in st3gal5 gene could be responsible for the modulating ciliary swelling formation during fluid-shear stress.
Interestingly, only PC2 expression was observed on the ciliary swelling in either st3gal5 or bicc-1 cells. This might also indicate that there was a cross talk between GM3S and Bicc-1. Noteworthy, Bicc-1 has been shown to regulate mRNA stability and translation via modulation of mRNA polyadenylation in Drosophila and C. elegans [51–53]. In addition, Mir-17 modulation via Bicc1 has been shown to regulate PC2 expression [36]. However, due to the cross talk between GM3S and Bicc1, it was not known whether Mir-17 was involved in the expression of GM3S. As a result, we included siRNA Mir-17 transfected cells to see its effects on GM3S, Bicc-1, and PC2. Our proposed mechanism indicated that the knockdown of Mir-17 induced an increase in Bicc-1 expression level. This is consistent with previous study [36], which indicates that Mir-17 acts upstream of Bicc-1. In addition, the knockdown of Bicc-1 has an effect on St3gal5 and Pkd2 expression. However, based on our study we propose that St3gal5 might have a negative feedback effect on Bicc-1. On the other hand, GM3S and PC2 expressions seemed to depend on each other, which further confirm previous study that PC2 are N-glycosylated [50].
Overall, our studies demonstrated for the first time that ciliary membrane swelling is a dynamic structure and that its dynamic movement can be regulated by fluid-shear stress. Furthermore, the ciliary swelling was glycosylated, possibly by GM3S, and we showed that Bicc-1, GM3S, and PC2 were localized to the swelling region. Together, our findings provide an opportunity for future studies on the structural complexity of primary cilia that can potentially lead us to a better understanding of cilia-related diseases.
Methods and materials
Cell culture
The porcine kidney epithelial cells (LLC-PK1) were used in this study and cultured in Dulbecco’s modified eagle medium (DMEM) (Corning Cellgro), 10 % fetal bovine serum (FBS) (HyClone, Inc.), and 1 % penicillin/streptomycin (Corning Cellgro) at 37 °C in 5 % CO2 incubator. Prior to experiments, cells were serum starved 24 h for differentiation. In some cases, we also used previously characterized vascular endothelial cells [4].
Formvar technique
We have adopted a new technique to visualize cilium from the side [6]. Briefly, a special flexible substratum (Formvar; Electron Microscopy Science) was dissolved in ethylene dichloride to make a 2–4 % Formvar solution. A 18 × 18 glass slide was then coated with the fomvar solution, and the glass slide was placed in a six-well plate until dry, followed with 50 μg/ml collagen coating on the top of the Formvar layer. The six-well plate was then placed under ultraviolet light for 30 min for sterilization. After sterilization, the fomvar cover slide was briefly washed with phosphate buffer saline (PBS, pH 7.4). Cells were then grown on this collagen-coated formvar flexible substratum (FFS). A pair of sharp forceps was used to peel the FFS and gently folded to form a triangle shape where cilia were oriented upward for visualization. After folding, the FFS was placed on a special made glass-bottom plate and held with a cover glass slide.
Live imaging
The FFS was put on a Nikon TiU stage. A special thin pipette tip (Fisher scientific, Inc.) was connected with inlet and outlet clear plastic PVC tubes with a 0.031 inch inside diameter (Nalgene, Inc.). The tubes were inserted into the in-flow and out-flow pumps (InsTech P720) and the pipette tips were inserted between the bottom glass plate and the glass cover slide as described previously [6].
Immunostaining
LLCPK cells were immunostained using a standard protocol. Briefly, cells were preferred to be at 80–90 % confluence before immunostaining. Cells were rinsed with sodium cacodylate buffer, fixed with 3 % glutaraldehyde in 0.2 M sodium cacodylate buffer for 10 min, and permeabilized with 1 % Triton-X in sodium cacodylate for 5 min. All primary antibodies were diluted in 10 % FBS solution; acetylated-α-tubulin (1:10,000; Sigma, Inc.), BICC-1 (1:500; ABGENT, Inc.), GM3 (1:200; Seikagaku Biobusiness, Inc) GM3S (1:800; LSCBIO, Inc.), and PC2 (1:50; Santa Cruz, Inc.). All secondary antibodies were also diluted in 10 % FBS to decrease background florescence; FITC fluorescence secondary antibody (1:500; Pierce, Inc.) and TexasRed fluorescence secondary antibody (1:500; Pierce, Inc.). Cells were then washed twice with cacodylate buffer and mounted with DAPI (Vector laboratories, Inc.). Images were taken with Nikon TE2000 using Metamorph software.
RNAi knockdown
To generate stable knockdown cell lines, HEK-293T cells were transfected with shRNA lentiviral vectors specific to St3gal5, Bicc1 or Pkd2 (Origene; pGFP-C-shLenti). Viral particles were collected after 48 h, centrifuged at 4,000 rpm, and passed through a 0.45 μm filter. Cells were then centrifuged at 2,500 rpm for 30 min at 30 °C with viral particles containing 8 μg/ml polybrene and then cultured for up to 72 h. Gene silencing was further confirmed by Western blot, and only the shRNA lentivirus with higher knockdown efficiency was selected. Stable knockdown cell lines were obtained through cell sorting (BD Bioscience, Inc.) and puromycin dihydrochloride (Santa Cruz, Inc.). The shRNA sequences used in this study are listed below.
Descriptions | Sequences |
|---|---|
Scrambled | 5′-TGA CCA CCC TGA CCT ACG GCG TGC AGT GC-3′ |
St3gal5 | |
A | 5′-CAA GAT GGT CCT GAG TGT CCT CAC TTC GT-3′ |
B | 5′-CAT AAT GCA CTG CTC GCG CTG TTG TAT CC-3′ |
C | 5′-GAG CTGT AGG CGT TGT GTG GTC ATC GGA A-3′ |
D | 5′-TCT TGC GGT GAT CGG AAC AGA CAA GTT CT-3′ |
Bicc1 | |
A | 5′-CGA ATG TAT GGT GCT ACT GTGA TAG TTC G-3′ |
B | 5′-ATG GAA CAG CTC GAT GTC TTC ATC AGT AT -3′ |
C | 5′-GAA CTC GCT CCT GAA TGC TCT CAA TAG CT-3′ |
D | 5′-AGA TCG ACC TTC AGA CAT TCC TCA CTC TC-3′ |
Pkd2 | |
A | 5′-TTG TGC ATC TTG ACC TAC GGC ATG ATG AG-3′ |
B | 5′-TAC GGC ATG ATG AGC TCC AAT GTG TAC TA-3′ |
C | 5′-TTT GAT TTC TTC CTG GCA GCC TGT GAG AT-3′ |
D | 5′-GTC TGG ATT AAG CTC TTC AAA TTC ATC AA-3′ |
siRNA transfection
Cells were plated so that they reached 60–80 % confluence upon the time of transfection as previously described [7]. Diluted lipofectamine reagent and siRNA were used according to the manufacturer guideline (Invitrogen, Inc.). Cells were transfected for 48–72 h, and the siRNA sequences used in this study are listed below.
Descriptions | Sequences |
|---|---|
Scrambled | 5′-ACG UGA CAC GUU CGG AGA ATT-3′ |
Bicc1(A) | 5′-UUA AAG GAC ACA GAA ACG C-3′ |
Bicc1(B) | 5′-CCA ACC ACG UAU CCU AUA A-3′ |
Bicc1(C) | 5′- GGA AGC AAU GGC UGU AAC CUG AAC A-3′ |
Mir-17 | 5′-CUC AAA GUG CUU ACA GUG CAG GUA GUU-3′ |
Immunoblotting
Cell lysate was prepared using a standard technique as previously described [7]. Briefly, cells were rinsed with PBS, added fresh radioimmunoprecipitation assay (Ripa) buffer containing protease inhibitor, placed on ice, gently shaken for 30 min, scraped from the culture dish, transferred to microcentrifuge tube, and spun for 15 min at 10,000 g (accuSpin Micro 17, Fisher scientific, Inc.). Total cell lysate was transferred into another microcentrifuge tube and stored in −20 °C and analyzed using a standard 6–10 % gradient sodium dodecyl sulfate—polyacrylamide gel electrophoresis. Antibody against GM3S (LSCBIO, Inc.), bicaudal C (ABGENT, Inc.), polycystin-2 (Santa Cruz, Inc.), GAPDH (cell signaling, Inc.), and acetylated-α-tubulin (Sigma, Inc.) were used at dilutions of 1:800, 1:1,000, 1:200, 1:1,000 and 1:1,000, respectively.
Isolation of primary cilia
The LLCPK primary cilia were isolated by shear force [37]. First, LLCPK cells were grown in 150 mm culture dish in 10 % DMEM and 1 % penicillin/streptomycin in a humidified incubator at 37 °C in 5 % CO2. Cells were grown for maximum 8 days and were starved for differentiation before the shaking. Cells were rinsed with PBS briefly and gently, added 10 ml of PBS. The dish was then placed on a rotary shaker and shook for 4 min at 350 rpm (Orbital shaker, Cole-Parmer, Inc.). Carefully, the 10 ml media was transferred to a 50 ml centrifuge tube and spun for 30 min at 3,000g at 4 °C (Eppendrof 5810R). The supernatant was then transferred to a polyallomer tube and spun for 1 h at 70,000g at 4 °C in an ultracentrifuge (Beckman optima L-60). Primary cilia (pellet) were then resuspended in either Ripa buffer or resuspension buffer.
Visualization of isolated primary cilia
A nitrogen smearing technique was used to visualize and verify the efficiency of isolated primary cilia. Right after the isolation process, isolated primary cilia were fixed in 10 μL of 2 % paraformaldehyde, transferred, and spread equally on a glass slide. The coverslip was then placed on top of the glass slide. The whole glass slide was then placed in liquid nitrogen for about 1–2 min. The coverslip was then peeled off with a razor blade from the glass slide, and washed with PBS three times for 5 min each. The glass slide was then let to air dry and stored in −80 °C. Images were taken using Nikon TE2000.
Statistics
All quantifiable experimental values are expressed as mean ± SEM, and values of p < 0.05 are considered significant. All comparisons between two groups were performed with Student’s t test. Comparisons of three or more groups were done using ANOVA, followed with Tukey’s post test. Data analysis was performed using Sigma Plot software version 11.
References
Zimmermann K (1898) Beiträge zur Kenntnis einiger Drüsen und Epithelien. Arch Mikroskop Anat 52:552–706
Tobin JL, Beales PL (2009) The nonmotile ciliopathies. Genetics Med 11(6):386–402. doi:10.1097/GIM.0b013e3181a02882
Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ, Ingber DE, Zhou J (2003) Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet 33(2):129–137. doi:10.1038/ng1076
Nauli SM, Kawanabe Y, Kaminski JJ, Pearce WJ, Ingber DE, Zhou J (2008) Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1. Circulation 117(9):1161–1171. doi:10.1161/CIRCULATIONAHA.107.710111
Praetorius HA, Spring KR (2001) Bending the MDCK cell primary cilium increases intracellular calcium. J Membr Biol 184(1):71–79
Jin X, Mohieldin AM, Muntean BS, Green JA, Shah JV, Mykytyn K, Nauli SM (2014) Cilioplasm is a cellular compartment for calcium signaling in response to mechanical and chemical stimuli. Cell Mol Life Sci 71(11):2165–2178. doi:10.1007/s00018-013-1483-1
Aboualaiwi WA, Muntean BS, Ratnam S, Joe B, Liu L, Booth RL, Rodriguez I, Herbert BS, Bacallao RL, Fruttiger M, Mak TW, Zhou J, Nauli SM (2014) Survivin-induced abnormal ploidy contributes to cystic kidney and aneurysm formation. Circulation 129(6):660–672. doi:10.1161/CIRCULATIONAHA.113.005746
AbouAlaiwi WA, Takahashi M, Mell BR, Jones TJ, Ratnam S, Kolb RJ, Nauli SM (2009) Ciliary polycystin-2 is a mechanosensitive calcium channel involved in nitric oxide signaling cascades. Circ Res 104(7):860–869. doi:10.1161/CIRCRESAHA.108.192765
Takaoki M, Murakami N, Gyotoku J (2004) C-fos expression of osteoblast-like MC3T3-E1 cells induced either by cooling or by fluid flow. Uchu Seibutsu Kagaku 18(3):181–182
McGlashan SR, Haycraft CJ, Jensen CG, Yoder BK, Poole CA (2007) Articular cartilage and growth plate defects are associated with chondrocyte cytoskeletal abnormalities in Tg737orpk mice lacking the primary cilia protein polaris. Matrix Biol 26(4):234–246. doi:10.1016/j.matbio.2006.12.003
Jensen CG, Poole CA, McGlashan SR, Marko M, Issa ZI, Vujcich KV, Bowser SS (2004) Ultrastructural, tomographic and confocal imaging of the chondrocyte primary cilium in situ. Cell Biol Int 28(2):101–110. doi:10.1016/j.cellbi.2003.11.007
Masyuk AI, Masyuk TV, Splinter PL, Huang BQ, Stroope AJ, LaRusso NF (2006) Cholangiocyte cilia detect changes in luminal fluid flow and transmit them into intracellular Ca2+ and cAMP signaling. Gastroenterology 131(3):911–920. doi:10.1053/j.gastro.2006.07.003
Yoshiba S, Shiratori H, Kuo IY, Kawasumi A, Shinohara K, Nonaka S, Asai Y, Sasaki G, Belo JA, Sasaki H, Nakai J, Dworniczak B, Ehrlich BE, Pennekamp P, Hamada H (2012) Cilia at the node of mouse embryos sense fluid flow for left-right determination via Pkd2. Science 338(6104):226–231. doi:10.1126/science.1222538
McGrath J, Somlo S, Makova S, Tian X, Brueckner M (2003) Two populations of node monocilia initiate left-right asymmetry in the mouse. Cell 114(1):61–73
Su S, Phua SC, DeRose R, Chiba S, Narita K, Kalugin PN, Katada T, Kontani K, Takeda S, Inoue T (2013) Genetically encoded calcium indicator illuminates calcium dynamics in primary cilia. Nat Methods 10(11):1105–1107. doi:10.1038/nmeth.2647
Rydholm S, Zwartz G, Kowalewski JM, Kamali-Zare P, Frisk T, Brismar H (2010) Mechanical properties of primary cilia regulate the response to fluid flow. Am J Physiol Renal Physiol 298(5):F1096–F1102. doi:10.1152/ajprenal.00657.2009
Xu C, Rossetti S, Jiang L, Harris PC, Brown-Glaberman U, Wandinger-Ness A, Bacallao R, Alper SL (2007) Human ADPKD primary cyst epithelial cells with a novel, single codon deletion in the PKD1 gene exhibit defective ciliary polycystin localization and loss of flow-induced Ca2+signaling. Am J Physiol Renal Physiol 292(3):F930–F945. doi:10.1152/ajprenal.00285.2006
Nauli SM, Rossetti S, Kolb RJ, Alenghat FJ, Consugar MB, Harris PC, Ingber DE, Loghman-Adham M, Zhou J (2006) Loss of polycystin-1 in human cyst-lining epithelia leads to ciliary dysfunction. J Am Soc Nephrol 17(4):1015–1025. doi:10.1681/ASN.2005080830
Siroky BJ, Ferguson WB, Fuson AL, Xie Y, Fintha A, Komlosi P, Yoder BK, Schwiebert EM, Guay-Woodford LM, Bell PD (2006) Loss of primary cilia results in deregulated and unabated apical calcium entry in ARPKD collecting duct cells. Am J Physiol Renal Physiol 290(6):F1320–F1328. doi:10.1152/ajprenal.00463.2005
Liu W, Murcia NS, Duan Y, Weinbaum S, Yoder BK, Schwiebert E, Satlin LM (2005) Mechanoregulation of intracellular Ca2+ concentration is attenuated in collecting duct of monocilium-impaired orpk mice. Am J Physiol Renal Physiol 289(5):F978–F988. doi:10.1152/ajprenal.00260.2004
Cano DA, Sekine S, Hebrok M (2006) Primary cilia deletion in pancreatic epithelial cells results in cyst formation and pancreatitis. Gastroenterology 131(6):1856–1869. doi:10.1053/j.gastro.2006.10.050
Cano DA, Murcia NS, Pazour GJ, Hebrok M (2004) Orpk mouse model of polycystic kidney disease reveals essential role of primary cilia in pancreatic tissue organization. Development 131(14):3457–3467. doi:10.1242/dev.01189
Hoey DA, Tormey S, Ramcharan S, O’Brien FJ, Jacobs CR (2012) Primary cilia-mediated mechanotransduction in human mesenchymal stem cells. Stem Cells 30(11):2561–2570. doi:10.1002/stem.1235
Gilula NB, Satir P (1972) The ciliary necklace. A ciliary membrane specialization. J Cell Biol 53(2):494–509
Dilly PN (1977) Further observations of transport within paddle cilia. Cell Tissue Res 185(1):105–113
Dilly PN (1977) Material transport within specialised ciliary shafts on Rhabdopleura zooids. Cell Tissue Res 180(3):367–381
Beninger PG, Potter TM, Stjean SD (1995) Paddle cilia fixation artifacts in pallial organs of adult Mytilus edulis and Placopecten magennanicus. Canadian J Zool 73:610–614
Short G, Tamm SL (1991) On the nature of paddle cilia and discocilia. Biol Bull 180:466–474
Menco BP, Farbman AI (1985) Genesis of cilia and microvilli of rat nasal epithelia during pre-natal development. II. Olfactory epithelium, a morphometric analysis. J Cell Sci 78:311–336
Menco BP, Farbman AI (1985) Genesis of cilia and microvilli of rat nasal epithelia during pre-natal development. I. Olfactory epithelium, qualitative studies. J Cell Sci 78:283–310
Holley A, Mac Leod P (1977) Transduction and coding of olfactory information. Journal de physiologie 73(6):725–848
Roth KE, Rieder CL, Bowser SS (1988) Flexible-substratum technique for viewing cells from the side: some in vivo properties of primary (9 + 0) cilia in cultured kidney epithelia. J Cell Sci 89(Pt 4):457–466
Janich P, Corbeil D (2007) GM1 and GM3 gangliosides highlight distinct lipid microdomains within the apical domain of epithelial cells. FEBS Lett 581(9):1783–1787. doi:10.1016/j.febslet.2007.03.065
Yoder BK, Hou X, Guay-Woodford LM (2002) The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol 13(10):2508–2516
Pazour GJ, San Agustin JT, Follit JA, Rosenbaum JL, Witman GB (2002) Polycystin-2 localizes to kidney cilia and the ciliary level is elevated in orpk mice with polycystic kidney disease. Curr Biol 12(11):R378–R380
Tran U, Zakin L, Schweickert A, Agrawal R, Doger R, Blum M, De Robertis EM, Wessely O (2010) The RNA-binding protein bicaudal C regulates polycystin 2 in the kidney by antagonizing miR-17 activity. Development 137(7):1107–1116. doi:10.1242/dev.046045
Mitchell KA (2013) Isolation of primary cilia by shear force. Current protocols in cell biology/editorial board, Juan S Bonifacino [et al] Chapter 3:Unit 3 42 41–49. doi:10.1002/0471143030.cb0342s59
Berselli P, Zava S, Sottocornola E, Milani S, Berra B, Colombo I (2006) Human GM3 synthase: a new mRNA variant encodes an NH2-terminal extended form of the protein. Biochim Biophys Acta 1759(7):348–358. doi:10.1016/j.bbaexp.2006.07.001
Koulen P, Cai Y, Geng L, Maeda Y, Nishimura S, Witzgall R, Ehrlich BE, Somlo S (2002) Polycystin-2 is an intracellular calcium release channel. Nat Cell Biol 4(3):191–197. doi:10.1038/ncb754
Elofsson R, Andersson A, Falck B, Sjoborg S (1984) The ciliated human keratinocyte. J Ultrastruct Res 87(3):212–220
Dalen H (1981) An ultrastructural study of primary cilia, abnormal cilia and ciliary knobs from the ciliated cells of the guinea-pig trachea. Cell Tissue Res 220(4):685–697
Albrecht-Buehler G, Bushnell A (1980) The ultrastructure of primary cilia in quiescent 3T3 cells. Exp Cell Res 126(2):427–437
Jensen CG, Davison EA, Bowser SS, Rieder CL (1987) Primary cilia cycle in PtK1 cells: effects of colcemid and taxol on cilia formation and resorption. Cell Motil Cytoskelet 7(3):187–197. doi:10.1002/cm.970070302
Jensen CG, Jensen LC, Rieder CL (1979) The occurrence and structure of primary cilia in a subline of Potorous tridactylus. Exp Cell Res 123(2):444–449
Wood CR, Huang K, Diener DR, Rosenbaum JL (2013) The cilium secretes bioactive ectosomes. Curr Biol 23(10):906–911. doi:10.1016/j.cub.2013.04.019
Hogan MC, Manganelli L, Woollard JR, Masyuk AI, Masyuk TV, Tammachote R, Huang BQ, Leontovich AA, Beito TG, Madden BJ, Charlesworth MC, Torres VE, LaRusso NF, Harris PC, Ward CJ (2009) Characterization of PKD protein-positive exosome-like vesicles. J Am Soc Nephrol 20(2):278–288. doi:10.1681/ASN.2008060564
Wang J, Silva M, Haas LA, Morsci NS, Nguyen KC, Hall DH, Barr MM (2014) C. elegans ciliated sensory neurons release extracellular vesicles that function in animal communication. Curr Biol 24(5):519–525. doi:10.1016/j.cub.2014.01.002
Kaimori JY, Nagasawa Y, Menezes LF, Garcia-Gonzalez MA, Deng J, Imai E, Onuchic LF, Guay-Woodford LM, Germino GG (2007) Polyductin undergoes notch-like processing and regulated release from primary cilia. Hum Mol Genet 16(8):942–956. doi:10.1093/hmg/ddm039
Hidaka S, Konecke V, Osten L, Witzgall R (2004) PIGEA-14, a novel coiled-coil protein affecting the intracellular distribution of polycystin-2. J Biol Chem 279(33):35009–35016. doi:10.1074/jbc.M314206200
Cai Y, Maeda Y, Cedzich A, Torres VE, Wu G, Hayashi T, Mochizuki T, Park JH, Witzgall R, Somlo S (1999) Identification and characterization of polycystin-2, the PKD2 gene product. J Biol Chem 274(40):28557–28565
Wang L, Eckmann CR, Kadyk LC, Wickens M, Kimble J (2002) A regulatory cytoplasmic poly(A) polymerase in Caenorhabditis elegans. Nature 419(6904):312–316. doi:10.1038/nature01039
Saffman EE, Styhler S, Rother K, Li W, Richard S, Lasko P (1998) Premature translation of oskar in oocytes lacking the RNA-binding protein bicaudal-C. Mol Cell Biol 18(8):4855–4862
Mahone M, Saffman EE, Lasko PF (1995) Localized Bicaudal-C RNA encodes a protein containing a KH domain, the RNA binding motif of FMR1. EMBO J 14(9):2043–2055
Acknowledgments
This work partially fulfills the requirements of a PhD degree in Medicinal and Biological Chemistry for Ashraf M. Mohieldin. This work was supported by the Department of Defense PR130153 (SMN) and in part by the National Institute of Health R01DK080640 (SMN), R01DK080745 (OW) and F31DK096870 (AMM).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 8 (AVI 553 kb)
Supplementary material 9 (AVI 1498 kb)
Rights and permissions
About this article
Cite this article
Mohieldin, A.M., Haymour, H.S., Lo, S.T. et al. Protein composition and movements of membrane swellings associated with primary cilia. Cell. Mol. Life Sci. 72, 2415–2429 (2015). https://doi.org/10.1007/s00018-015-1838-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-015-1838-x