Abstract
TNF-related apoptosis-inducing ligand (TRAIL) and its receptors are attractive targets for anticancer therapy owing to their ability to trigger apoptosis selectively in cancer cells but not in normal cells. To date, many combinatorial strategies, such as chemotherapy or radiotherapy, have given encouraging results for overcoming TRAIL resistance in preclinical models. In this review, we provide an overview of the molecular mechanisms underlying sensitization to TRAIL-induced apoptosis by polyphenols. These naturally occurring compounds can restore tumor cell sensitivity to TRAIL-induced cell death with no apparent toxicity towards normal cells. Both extrinsic and intrinsic pathways can be modulated by polyphenols, the activation of which largely depends on the cell type, the particular polyphenolic compound, and the conditions of treatment. The large variety of polyphenol cellular targets could prove useful in circumventing TRAIL resistance. The relevance of these combined treatments for cancer therapy is discussed in the light of recent preclinical studies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
During the last decade, the cytokine TRAIL (APO2-L or TNF-related apoptosis-inducing ligand) and agonistic antibodies targeting TRAIL receptors have gained considerable interest in cancer therapy, due to their ability to induce tumor regression in preclinical studies with no significant side-effects [1]. However, it appears that often treatment with TRAIL or agonistic antibody alone is not sufficient for an effective apoptotic response. Mapatumumab, an antibody which targets TRAIL-R1, exhibits little clinical activity as single agent in patients with refractory colorectal cancer [2]. Other clinical trials nevertheless provide encouraging results when recombinant human TRAIL or anti-TRAIL-R1 or -R2 agonistic antibodies are combined with conventional chemotherapy [3]. The problem in current cancer therapy is the occurrence of a few resistant tumor cells that cause cancer relapse. The current challenge in oncology is therefore to find a treatment able to eradicate the tumor without triggering resistance, and to limit as much as possible its toxicity against normal cells. Combined treatments seem to be the best way to reach this objective. With this in mind, since 2002 it progressively became apparent that the combination of TRAIL with naturally occurring polyphenols would represent an attractive therapeutic approach (Table 1).
TRAIL and its receptors in cancer therapy
TRAIL is a member of the tumor necrosis factor (TNF) gene superfamily that displays great apoptosis-inducing activity against cancer cells both in vitro and in vivo. Unlike FasL or TNF, which are known to cause severe toxicity to liver tissue, TRAIL was shown to be safe following in vivo administration [4–6]. However, the reason for the apparent specificity of TRAIL ligand for killing tumor cells remains largely unknown [1].
The physiological role of TRAIL is not well defined, but it has been shown to play a role in T cell memory, haematopoiesis, autoimmune diseases and many other physiological processes [7–11]. TRAIL plays a major role in the anti-tumor immune surveillance mediated by T cells and natural killer (NK) cells [12, 13]. Indeed, TRAIL was shown to contribute to the regulation of tumor onset, progression, and metastasis [14]. TRAIL is mainly expressed at the membrane level of some immune cells, but can be found in some immune privilege sites [15] or in the circulation in some pathological conditions, including viral infections [16, 17]. Soluble TRAIL, like TNF or sFasL, is less cytotoxic than the membrane-bound form [18]. However, recombinant soluble forms of TRAIL can be used for in vitro assays as tagged or non-tagged versions to allow ligand cross-linking and induction of apoptosis.
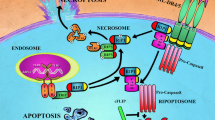
TRAIL ligand interacts with four distinct receptors at the membrane level, namely DR4 (Death Receptor 4/TRAIL-R1; see Ref. [19]), DR5 (Death Receptor 5/TRAIL-R2/Killer; see Ref. [20–23]), DcR1 (Decoy Receptor 1/TRAIL-R3/TRID/LIT [22–25]) and DcR2 (Decoy Receptor 2/TRAIL-R4/TRUNDD; see Ref. [26, 27]). The two agonistic receptors DR4 and DR5 contain an intracellular death domain (DD), which can recruit pro-Caspase-8 and -10 through the adaptor protein FADD (Fas-associated death domain) after TRAIL stimulation. The multiprotein complex formed by DR4/5, pro-Caspase-8/-10 and FADD is called the death-inducing signaling complex (DISC) and leads to the activation of effector caspases for the triggering of apoptosis (Fig. 1).
Schematic diagram of the TRAIL-induced apoptotic signaling. TRAIL binding on DR4 and/or DR5 triggers the recruitment of the adaptor protein FADD and the subsequent recruitment of the initiator Caspase-8 and/or -10, forming the DISC. The effector Caspase-3 is activated either directly by the initiator caspases (extrinsic pathway), or through the mitochondrial activation of Caspase-9 within the apoptosome (intrinsic pathway). The decoy receptors DcR1 and DcR2, as well as various anti-apoptotic proteins (in red), are involved in the regulation of the TRAIL signaling
Depending on the cell type, an amplification loop involving the mitochondria may be required to fully trigger TRAIL-induced cell death [28, 29]. This mitochondrial pathway, also called the intrinsic pathway, is activated after Caspase-8 mediated cleavage of Bid. Truncated Bid activates Bax and Bak, triggering the release of pro-apoptotic factors such as cytochrome c that lead to the formation of a soluble complex called apoptosome, in which the pro-Caspase-9 is activated. Caspase-9 in turn cleaves and activates Caspase-3, allowing execution of apoptosis. This amplification loop is negatively controlled by anti-apoptotic members of the Bcl-2 family and is heavily dependent on Bax [30], in type II cells [31]. TRAIL-induced apoptosis can also be negatively regulated by other intracellular factors including cellular inhibitor of apoptosis protein (cIAP), X-linked IAP (XIAP) or survivin, downstream of the mitochondrial pathway [32, 33]. Activation of pro-Caspase-8 and -10 within the TRAIL DISC is tightly controlled by the anti-apoptotic protein c-FLIP (cellular FLICE-like inhibitory protein) [34], but its inhibitory function also applies to Fas or TNFR1 [35].
Selective inhibition of the TRAIL pathway can be mediated by the two antagonistic TRAIL receptors, DcR1 and DcR2. Although their exact function is still unclear, these receptors lack a functional DD and are unable to induce the apoptotic program. DcR1 is a GlycosylPhosphatidylInositol (GPI)-anchored protein and does not contain the intracellular DD, whereas DcR2 contains a truncated DD that cannot induce caspases activation [22, 26]. The inhibitory effect of these two antagonistic receptors was initially attributed to their ability to sequester the ligand TRAIL, but it has recently been demonstrated that DcR1 and DcR2 act by two different mechanisms. While DcR1 competes with DR4 and DR5 for TRAIL binding, DcR2 interacts with DR5 within the DISC, and impairs efficient Caspase-8 activation [36]. Another mode of regulation has recently emerged and highlighted the importance of post-translational modifications of death receptors for TRAIL signaling, such as palmitoylation of DR4 [37] or glycosylation of both receptors [38, 39].
Defects in the intrinsic and extrinsic pathways [40, 41], including survival pathways such as NF-kB or Akt and more generally the tumor microenvironment [42], may lead to cell resistance and hamper the future clinical use of TRAIL in oncology. In all of these cases, the threshold of apoptosis induction is too high for efficient cancer therapy, and the current challenge is to decrease this threshold to restore TRAIL functionality.
Thus far, many efforts have been made to find therapeutic strategies that can eradicate cancer cells, without appearance of resistance and without toxic side-effects. Some combinatorial strategies have given interesting synergistic activities with TRAIL, for example the use of a broad range of protein inhibitors, chemotherapy, or irradiation [43]. More recently, the use of natural compounds, including polyphenols (Table 1), led to a growing interest for these combined therapeutic approaches due to their relative safety and their anti-tumor efficacy in preclinical models.
Polyphenols
Polyphenols are the products of secondary metabolism in plants. They play a role in defence mechanisms against pathogens or radiations and give plants their colors. They are found in fruits and vegetables, but also in wine, tea, coffee, chocolate, and many other plant-derived products [44]. These compounds are known for their beneficial effects against a large number of diseases, including cardiovascular or neurodegenerative diseases, osteoporosis, and cancer [45]. The biological activity of polyphenols is mainly attributed to their antioxidant properties, which is strictly related to their chemical structure [46] (Fig. 2). Polyphenols prevent reactive oxygen species (ROS)-induced DNA damage by scavenging free radicals (reactive oxygen, nitrogen, and chlorine species) and by inactivating metal catalysts by chelation, decreasing their oxidative activity. Their ability to interact with other reducing compounds and to inhibit redox-active transcription factors may also contribute to the antioxidant properties of these molecules as well as to their ability to regulate gene expression. Paradoxically, in addition to their antioxidant effects, polyphenols have also been shown to exert pro-oxidant effects that could also be responsible for their anticancer properties [47]. For example, owing to the presence of its hydroxyl groups, the flavonoid quercetin was shown to inhibit proliferation and to induce apoptosis of malignant cells through the generation of intracellular superoxide [48].
Enhancing TRAIL-induced cell death using polyphenolic compounds: molecular mechanisms
Flavonoids
This group of polyphenols has been identified in fruits, vegetables, grains, roots, flowers, wine, tea, and other related products [49]. More than 4,000 different flavonoids have been identified, many of which are responsible for the infinite color variations of flowers, leaves, and fruits. These compounds are divided into several classes on the basis of their molecular structure, namely anthocyanidins, flavanols, flavonols, flavanones, flavones, isoflavones, and chalcones.
Anthocyanidins
Anthocyanidins and flavanols are the most common flavonoids found in the diet. They are generally found in nature as glycosides, called anthocyanins, and are responsible for the blue, red, or purple colors of plants. Anthocyanin-rich extracts demonstrated chemopreventive activities against cancer in animal models of carcinogenesis [50]. Single agents have been tested in in vitro models and have shown interesting anti-proliferative and pro-apoptotic properties, acting by modulating survival pathways such as NFκB [51] or MAPK [52]. So far, their association with TRAIL has not been documented, albeit the pro-apoptotic activity of prodelphinidin B-2 3,3′-di-O-gallate, a proanthocyanidin, was proposed to proceed through the concurrent upregulation of Fas and FasL [53]. It remains to be demonstrated whether this group of flavonoids might prove useful to sensitize resistant cancer cells to TRAIL. Since anthocyanins are more stable than their aglycone anthocyanidins [54], the former should be preferred to assess their suitability in preclinical settings before considering these compounds for future clinical use.
Flavanols
The richest sources of flavanols are green tea, chocolate, red wine, and many types of fruits. They are found as monomers, called catechins, or as polymers, commonly called condensed tannins. The only compound tested in association with TRAIL is epigallocatechin-3-gallate (EGCG) (Fig. 2a). Nishikawa et al. [55] were the first to show the synergistic effect of the association of EGCG with TRAIL in human hepatocellular carcinomas. The authors attributed this effect to the inhibition of Bcl-2 and Bcl-XL by EGCG, probably through NFκB inhibition. In this study, no apparent change in the survivin, XIAP, c-IAP1, or c-FLIP expression levels were observed. Of note, the amount of EGCG used in this work was high (100 μg/ml) and the safety of such a concentration on normal cells was not addressed. Later, two different teams demonstrated the efficiency of EGCG, used at lower concentrations, on glioblastomas [56] or prostate carcinomas [57]. Siegelin et al. [56] observed that sensitization of glioblastomas to TRAIL-induced apoptosis occurred through EGCG-mediated Akt inhibition, leading to the downregulation of survivin and the death effector-domain (DED)-containing protein PEA15. DR4 and DR5 were not regulated by EGCG at the protein level, but since their expression at the membrane level was not assessed, it cannot be excluded that these receptors might partially contribute to the synergy. On the contrary, another study by Siddiqui et al. [57] demonstrated that similar concentrations of EGCG induced an increase in DR4 expression on prostate carcinomas. Many other proteins were modulated in this model, such as Bcl-2, Bcl-XL, IAP proteins, survivin, c-FLIP, Bad, Bax, Bak, and Smac/Diablo, but their relative requirement regarding cell sensitization to TRAIL was not assessed. Another paper recently revealed that EGCG upregulated DR4 and DR5 and promoted TRAIL-induced cell death of pancreatic carcinomas [58]. The human melanoma A375 cell line was also shown to be responsive to the combinatorial treatment, but the molecular mechanisms were not clarified [59].
Flavonols
This flavonoid subgroup is abundant in a variety of foods including onion, broccoli, apple, curly kale, leek, and tea. To date, flavonols that have been described to exhibit TRAIL-sensitizing properties are quercetin, kaempferol, and myricetin (Fig. 2a).
Quercetin has been widely studied for its anti-oxidative action and its effect on the expression of many genes. It has been reported to confer protection against disorders such as neurodegeneration, cardiovascular diseases, or cancers [60]. In 1996, a phase I clinical trial showed that quercetin can be safely administered by intravenous injection [61]. Several studies have been performed investigating the effects of associating TRAIL with quercetin. Kim et al. [62] showed that this co-treatment efficiently killed prostate carcinomas but not normal prostate cells, though the concentrations of quercetin used in this study were very high. Quercetin’s sensitizing activity was associated with its ability to inhibit the Akt pathway. The authors subsequently demonstrated that quercetin enhanced TRAIL-induced apoptosis by downregulating survivin through a mechanism involving ERK-MSK1-mediated deacetylation of histone H3, independently of the MAPK and the JNK pathways [63]. They also showed that quercetin did not change the protein levels of the TRAIL receptors, c-FLIP, IAP proteins, Bcl-2, Bcl-XL, and Bax. Russo et al. [64] reported that leukemia cell lines were efficiently sensitized by quercetin and TRAIL co-treatment, except for the T cell acute lymphoblastic leukemia HPB-ALL. At the same time, Chen et al. [65] elegantly demonstrated that pre-treatment with quercetin sensitized non-small cell lung cancer cells to TRAIL-induced apoptosis via two distinct mechanisms. On one hand, quercetin acted on the Akt pathway to downregulate survivin expression and, on the other hand, quercetin targeted the PKC kinase, leading to an increase in DR5 expression. In their settings, neither c-FLIP nor IAP family members were modulated, and interestingly, normal bronchial epithelial cells were not affected by this treatment. Another report showed that quercetin stimulated DR5 expression and synergized with TRAIL to kill six different hepatoma cell lines [66]. Quercetin-mediated DR5 upregulation was triggered by Sp1. Supporting Chen’s results, this transcription factor was shown to be itself under the control of PKC [67]. Moreover, this study is probably the only instance in the literature to report a diminution of the short isoform of c-FLIP by quercetin, through proteasomal degradation. The synergistic activation of the TRAIL pathway by quercetin has also been associated with the cell surface redistribution of TRAIL receptors. Indeed, while Psahoulia et al. [68] observed no particular change in DR4 and DR5 expression levels on colon adenocarcinomas after quercetin pre-treatment, they found out that these receptors were redistributed into lipid rafts, thus facilitating TRAIL DISC formation and initiator caspases processing. However, as far as TRAIL receptor partitioning to lipid rafts is concerned, conclusions regarding enhanced DISC formation in these particular membrane compartments should be moderated, as DISC analysis was performed using a detergent that is unable to solubilize lipid rafts. Alternatively, mitochondrial activation or post-mitochondrial events were also shown to contribute to the synergy. Siegelin et al. [69] supported the importance of survivin in quercetin-induced sensitization to TRAIL on various glioma cell lines. In four glioma cell lines that displayed good synergistic apoptotic activities upon TRAIL and quercetin treatment, the authors observed a proteasomal degradation of survivin mediated by inhibition of Akt, whereas in gliomas resistant to the combined treatment, survivin levels remained unchanged. In this model, the ability of quercetin to regulate survivin expression appeared to be the major event governing the efficiency of the combined treatment. Finally, Hasegawa et al. [70] assessed the efficiency of methyldihydroquercetin, a methylated version of quercetin called BB-1, extracted from the Asian medicinal plant Blumea balsamifera. Similar to quercetin, BB-1 enhanced TRAIL-induced apoptosis in six different leukemia cell lines. Upon methyldihydroquercetin pre-treatment, DR5 was upregulated at the membrane level, but contrary to the observation of Chen et al., PKC was not required for this regulation. In addition, while a downregulation of active Akt and a modulation of transcription factors such as c-Rel and p52 were observed, the levels of survivin, Bax, Bak, Bcl-2, Bcl-XL, XIAP, c-IAP1, c-IAP2, and c-FLIPs remained unaffected in this study. Interestingly, this treatment was not toxic against normal peripheral blood mononuclear cells (PBMC).
Kaempferol is another flavonol that has been described to facilitate TRAIL-induced apoptosis. Resistant colon carcinomas were efficiently eradicated by this co-treatment through a mechanism involving DR4 and DR5 upregulation, but in the absence of XIAP, survivin, Bcl-XL, or Bax regulation [71]. Interestingly, while silencing of DR5 blocked the synergy, silencing of DR4 was unable to protect tumor cells to the combination. However, it should be noted that the membrane levels of DR5 or DR4 were not assessed in this study, so the absence of blockade after DR4 silencing could be explained by a lack of DR4 at the cell surface. Consistent with a previous study [72], Bcl-2 was surprisingly upregulated by kaempferol without compromising TRAIL-induced cell death. Association of kaempferol with TRAIL was also effective in the prostate carcinoma cell line PC-3 [71], and in three out of seven gliomas [73]. The sensitization was associated in gliomas with a decrease in XIAP, Bcl-2 (but not Bcl-XL), Mcl-1, and with the proteasomal-mediated degradation of survivin, due to Akt inhibition. Kaempferol and TRAIL co-treatments were unable to engage the apoptotic machinery in normal hepatocytes [71].
Another flavonol of interest for combination with TRAIL therapy is myricetin. It has been revealed to exert synergistic pro-apoptotic effects when combined with TRAIL at subtoxic doses on glioma cells, but not on normal astrocytes [74]. During synergy, both c-FLIPS and c-FLIPL were downregulated, as well as Bcl-2. Interestingly, overexpression of Bcl-2 or c-FLIPS (but not c-FLIPL) attenuated TRAIL-induced cell death in cells co-treated with myricetin, indicating that these inhibitory proteins are relevant for the control of the synergy. Here, sensitization to TRAIL was independent of the tumor suppressor P53 because P53-mutant gliomas were as responsive as wild-type P53 expressing gliomas.
Flavanones
Hesperetin, naringenin, and eriodictyol are the most abundant flavanones found in plants, mainly in citrus fruits. The validity of this kind of flavonoid in combination with TRAIL remains to be assessed.
Flavones
The flavones subgroup of flavonoids is not widely distributed in plants but is abundant in celery, parsley, and some herbs. Apigenin, luteolin, wogonin, and baicalein were studied in association with TRAIL and were shown to exhibit interesting combinatorial properties (Fig. 2a).
Apigenin was the first flavone revealed to sensitize breast cancer cells to TRAIL-induced apoptosis [75]. This phytochemical was shown to inhibit Casein kinase II, leading to inhibition of NFκB-mediated expression of Bcl-XL and c-FLIP. The synergy appeared to be largely dependent on Bax but independent of p53, as demonstrated on colon carcinomas deficient either for Bax or for p53. Later, Horinaka et al. [76] reported the suitability of this co-treatment on lymphoblastic leukemia (Jurkat), metastatic prostate carcinoma (DU145), and colon carcinoma (DLD-1). They described a new mechanism of action of apigenin, based on DR5 stabilization independent of p53. The co-treatment was safe toward normal PBMC in vitro, and DR5 was not modulated by apigenin on these non-cancerous cells.
Horinaka et al. [77] also studied the flavone luteolin, in human cervical cancer HeLa cells. Cleavage of Bid was observed during co-treatment with TRAIL and luteolin, as well as an increase in the expression of the TRAIL receptor DR5. While the synergy was blocked by DR5 silencing, underlying the importance of this receptor, the implication of DR4 was not analyzed in this study. However, the authors demonstrated that the combination of luteolin and TRAIL is safe for normal PBMC. Concurrently, Shi et al. [78] demonstrated another mechanism of sensitization, independent of any regulation of the TRAIL receptors or Bcl-2, Bcl-XL, c-FLIP, c-IAP1, c-IAP2, or NFκB. In their study, cervical, colon, liver, or nasopharyngeal carcinomas were greatly sensitized to TRAIL-induced cell death upon a short exposure to luteolin. Sensitization was attributed to the inhibition of PKC, which led to increased XIAP ubiquitination and degradation. Thus, the authors proposed that luteolin could be a potent compound to overcome TRAIL resistance in cancers that exhibit elevated PKC activity.
Wogonin is another flavone shown to sensitize different lymphomas to TRAIL or TNF-α-induced cell death, without having effects on normal peripheral blood T cells [79]. Wogonin was shown to inhibit TNF-α-induced NFκB activation in a ROS-independent manner, but it was not confirmed for TRAIL. More recently, Lee et al. [80] underlined the importance of P53, Puma, and Bax during co-treatment with TRAIL and wogonin, using prostate and colon carcinomas deficient for each of these proteins. A schematic model of action was proposed, where wogonin induced ROS production and subsequent P53 activation. The histone H2A.X was also phosphorylated, which is a typical feature of DNA damage. These events led to the upregulation of Puma and the activation of the mitochondrial pathway [81]. In another study, wogonin was shown to be unable to sensitize four different pancreatic carcinomas to the combined treatment with TRAIL [82], but these cells are known to bear p53 mutations. This fact could explain, at least in part, the relative cell specificity of the combined treatment. Finally, wogonin has been shown to be safe when administered intravenously to dogs [83], and could be an interesting therapeutic candidate in association with TRAIL for tumors harboring a functional P53 protein.
Baicalein is a flavone originally isolated from the roots of the Asian medicinal herb Scutellaria baicalensis. This molecule was studied for its anticancer potential when associated with TRAIL on prostate, colon, T cell leukemia, and hepatoma cancer cell lines [84], and the combination was not toxic against normal blood cells and hepatocytes. Baicalein-induced sensitization to TRAIL was shown to be mediated via two different mechanisms, namely ROS induction or upregulation of CHOP transcription factor, depending on the cell type. Both pathways led to an increased expression of DR5 that could explain the synergistic effect.
Isoflavones
Isoflavones are commonly referred to as phytoestrogens because of their structural similarities with estrogens. Particularly, these molecules can bind estrogen receptors and mimic their effects [85]. Leguminous plants are almost the exclusive source of isoflavones, such as genistein or daidzein (Fig. 2a).
The ability of genistein to enhance TRAIL-induced cell death was first demonstrated on lung carcinomas, through downregulation of both Akt and Bcl-XL [86]. An in vivo study in mice transplanted with human pancreatic cancer cells revealed that genistein co-treatment with TRAIL triggered a significant reduction of tumor volume, without toxic side-effects [87]. The effectiveness of genistein was explained in cervical cancer HeLa cells by its ability to inhibit the ERK pathway [88]. Genistein was reported to enhance TRAIL killing of hepatocellular and lung carcinomas, via inactivation of the p38 MAPK signaling and activation of the mitochondrial pathway [89, 90]. Gastric adenocarcinomas were also greatly sensitized, as demonstrated by Jin et al. [91], in a way involving mitochondrial activation, DR5 upregulation, and Bcl-XL downregulation, without affecting the levels of Bcl-2 and IAP proteins. On the contrary, Siegelin et al. [92] showed that genistein decreased the levels of Bcl-2, XIAP, survivin, and active Akt, and enhanced the proteasomal degradation of c-FLIPS, in glioblastoma cells. Interestingly, normal human astrocytes were not sensitized to TRAIL-induced cell death by genistein. While these findings are interesting in vitro for the treatment of glioblastomas, it remains to be determined whether genistein can cross the blood–brain barrier.
Only one study has reported the synergistic effect of the isoflavone daidzein in association with TRAIL against glioblastoma cancer cells [93]. Siegelin et al. demonstrated that this synergy was essentially mediated via Bcl-2 downregulation, whereas c-FLIP, XIAP, survivin, or the TRAIL receptors were not modulated.
Chalcones
Chalcone is the first compound in flavonoid biosynthesis and thus is widely found in all kinds of plants. Two chalcone derivatives have been tested in combination with TRAIL, namely butein and isoliquiritigenin (Fig. 2a).
Butein, a chalcone purified from the barks of the lacquer tree Rhus verniciflua, was reported to enhance TRAIL-promoting effects on monoblastoma and leukemia cell lines, through an increase in DR5 expression at the membrane level [94]. This compound could be interesting for future clinical protocols because no toxicity was observed in normal lymphocytes and CD34+ cells from healthy donors.
Isoliquiritigenin is a chalcone derivative found abundantly in licorice. Co-treatment with subtoxic doses of isoliquiritigenin and TRAIL efficiently killed colorectal HT29 cells [95]. Expression of Bcl-2 and Bcl-XL was not changed, but a slight increase of DR5 was reported. However, the importance of DR5 during the synergy was not assessed and the mechanism of action need to be further explored.
Stilbenes
Stilbenes are produced by plants that are subjected to various stressful conditions. Resveratrol has been widely studied for several years due to its anti-carcinogenic properties (Fig. 2b). Since 2003, ten different studies have assessed the potential of resveratrol to sensitize resistant cancer cells to TRAIL-induced apoptosis (Table 1). Resveratrol, at a concentration of 10 μM, was initially shown to be unable to sensitize prostate LNCaP cells to TRAIL [96]. In two other studies using ten-fold higher concentrations, Fulda and Debatin [97, 98] reported that resveratrol sensitized various cancer cells to TRAIL-induced apoptosis, including neuroblastoma, medulloblastoma, glioblastoma, melanoma, T cell leukemia, as well as pancreatic, breast, and colon carcinomas. Sensitization was associated with a p21-mediated cell-cycle arrest and concomitant survivin depletion, independent of P53. Untransformed human fibroblasts remained insensitive to the co-treatment. Delmas et al. [99] demonstrated that, while their expression levels remained the same, partitioning of the TRAIL receptors into lipid rafts was responsible for resveratrol’s sensitizing activity in two carcinoma cell lines. Later, Shankar et al. [100, 101] reported that resveratrol sensitized prostate carcinomas to TRAIL by way of the modulation of many molecular targets. The expression of survivin, Bcl-2, Bcl-XL, and XIAP was inhibited while the expression of DR4, DR5, Bim, Bax, Bak, Noxa, and PUMA was increased. Of note, sequential treatments were more efficient than co-treatment in triggering cell death in this study. Importantly, the combination was not toxic against normal prostate epithelial cells. Contrary to these results, Gill et al. [102] observed no major changes regarding DR4, DR5, Bcl-2, Bcl-XL, survivin, and XIAP on the same prostate cancer cell lines. In their hands, c-IAP1 was downregulated but seemed to be only partially involved, because cIAP-1 siRNA alone did not restore the full sensitivity to TRAIL. The synergy could also be explained by the increased expression of Bax that could change the Bax/Bcl-2/Bcl-XL ratio, or upstream by the observed inactivation of the Akt pathway. Using the same prostate cells, Sallman et al. [103] proposed that the combined effect of resveratrol and TRAIL is mediated by the inhibition of the src/jak-Stat1 pathway. According to their study, resveratrol decreased Clusterin expression by inhibiting the phosphorylation of the Src and Jak kinases, resulting in loss of Stat1 activation. Using different melanoma cell lines, Ivanov et al. [104] also demonstrated the efficacy of resveratrol associated with TRAIL. They observed that the JNK-cJun and MAPK p38-ATF2 pathways were activated upon resveratrol pre-treatment, as well as many downstream targets such as c-FLIP, Bcl-XL, survivin, and Cyclin D1. Moreover, normal human lung fibroblasts and melanocytes remained protected from apoptosis. Despite many promising investigations regarding the effect of resveratrol, the TRAIL-enhancing properties of this phytochemical might depend on the cellular context. For example, researchers have shown that three pancreatic carcinomas were resistant to the combined treatment, whereas one other cell line exhibited only a slight additive effect [82].
Lignans and flavonolignans
Plant lignans are polyphenolic compounds that are referred to as phytoestrogens, like isoflavones. Flavonolignans, such as silibinin, are heterodimers formed through the coupling of a flavonoid and a lignan component (Fig. 2c).
Nordihydroguaiaretic acid (NDGA) is a phenolic lignan that has been considered as a lipoxygenase inhibitor. This molecule was shown to enhance the pro-apoptotic effect of TRAIL in leukemia Jurkat cells, SW480 colon carcinomas, and prostate cancer DU145 cells, without affecting normal PBMC [105]. The increased expression of DR5 at the membrane level was proposed to explain the synergy because most of the major apoptosis-related proteins such as Bcl-2, Bcl-XL, survivin, c-IAP1, and XIAP remained unaffected.
Honokiol is a lignan originally isolated from Magnolia officinalis. Raja et al. [106] reported an interesting synergy with TRAIL on non-small cell lung cancer cell lines, which was accompanied by the modulation of proteins such as DR4, DR5, survivin, and c-FLIP. However, some clues highlighted the prevalence of c-FLIP regulation during honokiol and TRAIL co-treatment. Actually, c-FLIP inhibition appeared to be the earlier event, and its overexpression strongly abolished the synergy. The researchers went a step further in the experiment by testing different honokiol derivatives. They observed a correlation between the efficacy of these molecules and their ability to inhibit c-FLIP. Therefore, c-FLIP appeared to be critical for honokiol-mediated sensitization to TRAIL in this model.
Silibinin from the herb milk thistle was tested with TRAIL on glioma cells [107]. This flavonolignan exerted significant TRAIL-enhancing effects on gliomas but not on normal astrocytes. During synergy, DR5 appeared to be upregulated via the transcription factor CHOP, whereas survivin and c-FLIP were downregulated.
Phenolic acids and derivatives
Plant phenolic acids are derived either from benzoic acid or cinnamic acid (Fig. 2d). Curcumin is a hydroxycinnamic acid derivative responsible for the yellow color of the spice turmeric. Anticancer action of curcumin has given rise to many publications that have revealed a broad spectrum of molecular targets [108, 109]. Co-treatment with TRAIL has also been well studied since 2003 (Table 1). Deeb et al. demonstrated in human prostate carcinomas that curcumin-mediated sensitization to TRAIL involved both the activation of mitochondria [96] and the inhibition of NFκB signaling [110]. Curcumin was shown to block IkBα phosphorylation, thus allowing sustained sequestration of NFκB into the cytosol [111]. The combination of curcumin with TRAIL appeared to be safe for non-tumorigenic prostate epithelial cells. The authors further pointed out that in prostate cancer cells with a high level of active Akt (LNCap and PC-3), sensitization to TRAIL by curcumin was mediated by the suppression of NFκB via inactivation of the Akt pathway. In prostate cancer cells that lack basal active Akt (DU145), sensitization was also mediated by inhibition of NFκB through the suppression of IκBα phosphorylation, but independently of Akt [112]. In both cases, NFκB inhibition by curcumin resulted in the inhibition of Bcl-2, Bcl-XL, and XIAP expression. These results were confirmed in vivo, as prostate PC3 cells xenografted in mice were efficiently killed upon co-treatment [113]. Shankar et al. also studied the effect of curcumin and TRAIL on prostate carcinomas. They confirmed the observations of Deeb et al. concerning the downregulation of XIAP, Bcl-2, and Bcl-XL, but showed in addition that survivin and Noxa were also inhibited whereas the levels of Bax, Bak, Bim, PUMA, DR4, and DR5 were augmented [114]. In vivo experiments performed on nude mice xenografted with prostate LNCAP cells revealed that treatment with a combination of curcumin and TRAIL inhibited tumor growth by apoptosis triggering, but also through the activation of anti-proliferative, anti-angiogenic, and anti-metastatic mechanisms [115]. Many proteins were positively regulated, such as DR4, DR5, Bax, Bak, p21, and p27, whereas NFκB was negatively regulated as well as its various gene products, including Bcl-2, Bcl-XL, and Cyclin D1. In contrast, Jung et al. [116] demonstrated that in renal and hepatocellular carcinomas, curcumin and TRAIL-mediated apoptosis occurred with no change in expression of Bcl-2, Bax, c-IAP2, or XIAP. The synergy was proposed to be induced via a ROS-mediated DR5 upregulation, and the combination was safe toward non-malignant mesangial cells [117]. However, in these settings, the transcription factor CHOP, although activated by ROS, was neither associated with curcumin-mediated DR5 upregulation nor with the synergistic activity of the combination. The mitochondrial pathway was proposed to play a minor role because Bcl-2 overexpression in hepatocarcinomas failed to interfere with the sensitization. Using Burkitt’s lymphoma cells lacking a functional Bax protein, Hussain et al. [118] came to similar conclusions. However, the mitochondrial pathway was still activated upon co-treatment in Bax-deficient lymphomas, suggesting that Bak may compensate for the loss of Bax. Sensitization to TRAIL in these cells was also associated with the upregulation of DR5 and the inactivation of NFκB in a ROS-dependent manner. The efficacy of curcumin and TRAIL association was also demonstrated in gliomas [119], bladder [120], and ovarian carcinomas [121]. Thereby, curcumin could be a potent phytochemical in order to overcome TRAIL resistance in diverse cancer types.
Recently, another phenolic compound called cycloartenyl ferulate, extracted from rice bran oil, has been shown to potentiate TRAIL-induced cell death, which is associated with the upregulation of DR4, DR5, and Bak and the decrease in Bcl-2 expression [122].
Synthetic flavonoids
Given that naturally occurring flavonoids are interesting compounds for anticancer therapy or chemoprevention, researchers aimed to design synthetic versions that can be produced in large amounts. Thus, a semi-synthetic flavone called flavopiridol has been developed by Aventis (Fig. 3), and has been shown to display both in vitro and in vivo selective antitumor activity [123]. Phase I and II trials have demonstrated the absence of cytotoxicity of this molecule [124]. Flavopiridol was shown to efficiently synergize with TRAIL in several cancer types including cholangiocarcinomas [125] and leukemia cell lines [126] through the respective downregulation of Mcl-1 or XIAP. In hepatocarcinomas, co-administration of flavopiridol and TRAIL resulted in a decline of survivin expression and an increase of DR4 and DR5 at the membrane level [127]. In contrast, breast cancer cells were mainly sensitized by an enhanced formation of the TRAIL DISC and a downregulation of c-FLIP [128]. Likewise, a deregulation of c-FLIP was observed in myeloma and breast cancer cells [129]. Flavopiridol antitumor activity with TRAIL has also been reported in lung carcinomas [130].
Concluding remarks
The interest in natural substances for the treatment of cancer is not recent [131]. Many existing drugs have been discovered from plants used in traditional medicine. For the moment, polyphenols are not yet used in anticancer therapy, but they are currently used as dietary supplements because of their known preventive properties against ageing, neurodegeneration, cardiovascular risk, and cancer. The anticancer efficacy of polyphenols in association with chemotherapy, radiotherapy [132], or in combination with TRAIL [133, 134] in preclinical models is now well demonstrated. The molecular events driving tumor cell sensitization to TRAIL-induced cell death generally target both the intrinsic and the extrinsic pathways. In most cases, polyphenols are able to lower the threshold of caspase activation, thus allowing TRAIL-induced apoptosis triggering. Considering all the data published so far, it is difficult to define a general rule concerning the molecular action of each polyphenol. Rather, it appears that molecular events that allow engagement of the apoptotic machinery upon combined treatments largely depend on the cancer type, and on the signaling pathways engaged in controlling cell resistance to apoptosis. For example, in cancer cells that are resistant to TRAIL through a blockage of the intrinsic pathway, polyphenolic compounds are generally able to inhibit anti-apoptotic proteins of the Bcl-2 family such as Bcl-XL, Bcl-2, or Mcl-1, or to upregulate pro-apoptotic members such as Bax or Bak (Fig. 4). Likewise, inhibition of the Akt pathway by polyphenols can lead to the restoration of the mitochondrial pathway, affording an enhanced sensitivity of the tumor cells to TRAIL-induced cell death (Fig. 4). On the other hand, when the resistance occurs at the level of the extrinsic pathway, the synergistic activity of these polyphenols is often associated with the upregulation of the agonistic TRAIL receptors, the downregulation of c-FLIP, or through a facilitation of TRAIL receptor aggregation and DISC formation (Fig. 4). Interestingly, most of these synergistic activities appear to occur independently of p53, a tumor suppressor gene that confers resistance to anticancer treatments if mutated or deleted. Aside from polyphenols, it is relevant to mention that other agents with related structures, although not polyphenolic, could also sensitize cells to TRAIL by similar mechanisms of action, such as the triterpenoids celastrol [135, 136] and lupeol [137].
Schematic model of action of polyphenols on the TRAIL signaling pathway. Polyphenols modulate both the extrinsic and intrinsic pathways, and also tumor suppressors such as P53 and survival proteins such as Akt. The molecular targets vary depending on the structure of the polyphenolic compound and the cell type. This model reports only the main molecular targets of polyphenols
Overall, the finding that polyphenols exert multimodal sensitizing activities is very encouraging for future combined treatments with TRAIL or TRAIL derivatives. However, it is crucial to be cautious about the clinical relevance of these in vitro studies. Most in vitro studies are likely to be relevant for intravenous administration protocols in future clinical uses, but not for nutritional intake of a polyphenol-rich food. Indeed, in vitro studies are often performed with high concentrations of polyphenol that in no way mimic the concentration at the tumor site following oral delivery [138]. Moreover, the compounds tested in these studies are almost always native polyphenols, but these non-conjugated forms are found at low levels after oral administration due to fact that polyphenols are rapidly absorbed and metabolized, mainly as sulfates, glucuronates, or methylated forms [139]. Thus, almost all the data collected correspond to a direct injection of polyphenols into the blood, but not to a dietary supplementation. The poor stability of polyphenolic compounds within the blood, although problematic, could nonetheless be resolved using various galenic formulations, such as calcium-pectinate beads or liposome-based delivery strategies [140, 141]. For example, intratumor injection of EGCG after encapsulation into liposomes provided interesting results in a mouse model of human cancer [142]. Liposomes protected EGCG from degradation and increased cell death in melanomas and colon carcinomas compared to free EGCG. Thus, liposomes could allow the use of lower doses of polyphenol and possibly reduce side-effects without reducing their anticancer efficacy. Accordingly, it has been demonstrated that encapsulation of quercetin into liposomes drastically increases its lifetime in plasma, and allows increased quercetin delivery to the tumor, leading to cancer growth inhibition in tumor-bearing mice after intravenous injection [143]. Interestingly, liposome-mediated quercetin delivery into the kidney and the lungs was lower as compared to free quercetin, a finding that may prove useful to limit treatment side-effects. According to the authors, the tumor-selectivity of these liposomes could be explained by the presence of defects in the capillary endothelium of tumoral tissues, which allow their extravasion from the blood to the tumor cells [143]. Therefore, the use of liposomes for intravenous administration of polyphenols could be relevant for the treatment of solid tumors.
Alternatively, direct administration by application onto the skin may be considered in the case of skin cancers, including melanomas. Indeed, topically applied resveratrol was shown to be a safe and efficient approach for transdermal delivery of active polyphenols into the skin [144].
These preliminary in vitro studies are very promising and encouraging for the use of polyphenols and TRAIL for future therapeutic protocols. Both compounds exert specific antitumor activity, and the combination of the two molecules is proven safe for normal cells, at least in vitro. Thus, future work in vivo should help our understanding of the different mechanisms for synergy, and ultimately clinical trials will reveal whether a combination treatment utilizing TRAIL and natural polyphenols represents a realistic and effective anticancer therapy.
References
Ashkenazi A, Holland P, Eckhardt SG (2008) Ligand-based targeting of apoptosis in cancer: the potential of recombinant human apoptosis ligand 2/tumor necrosis factor-related apoptosis-inducing ligand (rhApo2L/TRAIL). J Clin Oncol 26:3621–3630
Trarbach T, Moehler M, Heinemann V, Kohne CH, Przyborek M, Schulz C, Sneller V, Gallant G, Kanzler S (2010) Phase II trial of mapatumumab, a fully human agonistic monoclonal antibody that targets and activates the tumour necrosis factor apoptosis-inducing ligand receptor-1 (TRAIL-R1), in patients with refractory colorectal cancer. Br J Cancer 102:506–512
Newsom-Davis T, Prieske S, Walczak H (2009) Is TRAIL the holy grail of cancer therapy? Apoptosis 14:607–623
Ashkenazi A, Pai RC, Fong S, Leung S, Lawrence DA, Marsters SA, Blackie C, Chang L, McMurtrey AE, Hebert A, DeForge L, Koumenis IL, Lewis D, Harris L, Bussiere J, Koeppen H, Shahrokh Z, Schwall RH (1999) Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Investig 104:155–162
Kelley SK, Harris LA, Xie D, Deforge L, Totpal K, Bussiere J, Fox JA (2001) Preclinical studies to predict the disposition of Apo2L/tumor necrosis factor-related apoptosis-inducing ligand in humans: characterization of in vivo efficacy, pharmacokinetics, and safety. J Pharmacol Exp Ther 299:31–38
Lawrence D, Shahrokh Z, Marsters S, Achilles K, Shih D, Mounho B, Hillan K, Totpal K, DeForge L, Schow P, Hooley J, Sherwood S, Pai R, Leung S, Khan L, Gliniak B, Bussiere J, Smith CA, Strom SS, Kelley S, Fox JA, Thomas D, Ashkenazi A (2001) Differential hepatocyte toxicity of recombinant Apo2L/TRAIL versions. Nat Med 7:383–385
Zauli G, Secchiero P (2006) The role of the TRAIL/TRAIL receptors system in hematopoiesis and endothelial cell biology. Cytokine Growth Factor Rev 17:245–257
Boehrer S, Nowak D, Hoelzer D, Mitrou PS, Chow KU (2006) The molecular biology of TRAIL-mediated signaling and its potential therapeutic exploitation in hematopoietic malignancies. Curr Med Chem 13:2091–2100
Cretney E, Shanker A, Yagita H, Smyth MJ, Sayers TJ (2006) TNF-related apoptosis-inducing ligand as a therapeutic agent in autoimmunity and cancer. Immunol Cell Biol 84:87–98
Diehl GE, Yue HH, Hsieh K, Kuang AA, Ho M, Morici LA, Lenz LL, Cado D, Riley LW, Winoto A (2004) TRAIL-R as a negative regulator of innate immune cell responses. Immunity 21:877–889
Janssen EM, Droin NM, Lemmens EE, Pinkoski MJ, Bensinger SJ, Ehst BD, Griffith TS, Green DR, Schoenberger SP (2005) CD4+ T-cell help controls CD8+ T-cell memory via TRAIL-mediated activation-induced cell death. Nature 434:88–93
Cretney E, Takeda K, Yagita H, Glaccum M, Peschon JJ, Smyth MJ (2002) Increased susceptibility to tumor initiation and metastasis in TNF-related apoptosis-inducing ligand-deficient mice. J Immunol 168:1356–1361
Schmaltz C, Alpdogan O, Kappel BJ, Muriglan SJ, Rotolo JA, Ongchin J, Willis LM, Greenberg AS, Eng JM, Crawford JM, Murphy GF, Yagita H, Walczak H, Peschon JJ, van den Brink MR (2002) T cells require TRAIL for optimal graft-versus-tumor activity. Nat Med 8:1433–1437
Grosse-Wilde A, Kemp CJ (2008) Metastasis suppressor function of tumor necrosis factor-related apoptosis-inducing ligand-R in mice: implications for TRAIL-based therapy in humans? Cancer Res 68:6035–6037
Secchiero P, Lamberti G, Corallini F, Melloni E, Guarnotta C, Sebastiani A, Zauli G (2009) Conjunctival sac fluid contains elevated levels of soluble TRAIL: implications for the anti-tumoral surveillance of the anterior surface of the eye. J Cell Physiol 218:199–204
Bem RA, Bos AP, Wosten-van Asperen RM, Bruijn M, Lutter R, Sprick MR, van Woensel JB (2009) Potential role of soluble TRAIL in epithelial injury in children with severe RSV infection. Am J Respir Cell Mol Biol. doi:10.1165/rcmb.2009-0100OC
Han LH, Sun WS, Ma CH, Zhang LN, Liu SX, Zhang Q, Gao LF, Chen YH (2002) Detection of soluble TRAIL in HBV infected patients and its clinical implications. World J Gastroenterol 8:1077–1080
Schneider P, Holler N, Bodmer JL, Hahne M, Frei K, Fontana A, Tschopp J (1998) Conversion of membrane-bound Fas(CD95) ligand to its soluble form is associated with downregulation of its proapoptotic activity and loss of liver toxicity. J Exp Med 187:1205–1213
Pan G, O’Rourke K, Chinnaiyan AM, Gentz R, Ebner R, Ni J, Dixit VM (1997) The receptor for the cytotoxic ligand TRAIL. Science 276:111–113
Chaudhary PM, Eby M, Jasmin A, Bookwalter A, Murray J, Hood L (1997) Death receptor 5, a new member of the TNFR family, and DR4 induce FADD-dependent apoptosis and activate the NF-kappaB pathway. Immunity 7:821–830
Walczak H, Degli-Esposti MA, Johnson RS, Smolak PJ, Waugh JY, Boiani N, Timour MS, Gerhart MJ, Schooley KA, Smith CA, Goodwin RG, Rauch CT (1997) TRAIL-R2: a novel apoptosis-mediating receptor for TRAIL. EMBO J 16:5386–5397
Schneider P, Bodmer JL, Thome M, Hofmann K, Holler N, Tschopp J (1997) Characterization of two receptors for TRAIL. FEBS Lett 416:329–334
MacFarlane M, Ahmad M, Srinivasula SM, Fernandes-Alnemri T, Cohen GM, Alnemri ES (1997) Identification and molecular cloning of two novel receptors for the cytotoxic ligand TRAIL. J Biol Chem 272:25417–25420
Degli-Esposti MA, Smolak PJ, Walczak H, Waugh J, Huang CP, DuBose RF, Goodwin RG, Smith CA (1997) Cloning and characterization of TRAIL-R3, a novel member of the emerging TRAIL receptor family. J Exp Med 186:1165–1170
Pan G, Ni J, Wei YF, Yu G, Gentz R, Dixit VM (1997) An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science 277:815–818
Degli-Esposti MA, Dougall WC, Smolak PJ, Waugh JY, Smith CA, Goodwin RG (1997) The novel receptor TRAIL-R4 induces NF-kappaB and protects against TRAIL-mediated apoptosis, yet retains an incomplete death domain. Immunity 7:813–820
Marsters SA, Sheridan JP, Pitti RM, Huang A, Skubatch M, Baldwin D, Yuan J, Gurney A, Goddard AD, Godowski P, Ashkenazi A (1997) A novel receptor for Apo2L/TRAIL contains a truncated death domain. Curr Biol 7:1003–1006
Scaffidi C, Fulda S, Srinivasan A, Friesen C, Li F, Tomaselli KJ, Debatin KM, Krammer PH, Peter ME (1998) Two CD95 (APO-1/Fas) signaling pathways. EMBO J 17:1675–1687
Ozoren N, El-Deiry WS (2002) Defining characteristics of types I and II apoptotic cells in response to TRAIL. Neoplasia 4:551–557
LeBlanc H, Lawrence D, Varfolomeev E, Totpal K, Morlan J, Schow P, Fong S, Schwall R, Sinicropi D, Ashkenazi A (2002) Tumor-cell resistance to death receptor–induced apoptosis through mutational inactivation of the proapoptotic Bcl-2 homolog Bax. Nat Med 8:274–281
Scaffidi C, Schmitz I, Zha J, Korsmeyer SJ, Krammer PH, Peter ME (1999) Differential modulation of apoptosis sensitivity in CD95 type I and type II cells. J Biol Chem 274:22532–22538
Aggarwal BB, Bhardwaj U, Takada Y (2004) Regulation of TRAIL-induced apoptosis by ectopic expression of antiapoptotic factors. Vitam Horm 67:453–483
LaCasse EC, Mahoney DJ, Cheung HH, Plenchette S, Baird S, Korneluk RG (2008) IAP-targeted therapies for cancer. Oncogene 27:6252–6275
Ricci MS, Jin Z, Dews M, Yu D, Thomas-Tikhonenko A, Dicker DT, El-Deiry WS (2004) Direct repression of FLIP expression by c-myc is a major determinant of TRAIL sensitivity. Mol Cell Biol 24:8541–8555
Micheau O, Tschopp J (2003) Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell 114:181–190
Merino D, Lalaoui N, Morizot A, Schneider P, Solary E, Micheau O (2006) Differential inhibition of TRAIL-mediated DR5-DISC formation by decoy receptors 1 and 2. Mol Cell Biol 26:7046–7055
Rossin A, Derouet M, Abdel-Sater F, Hueber AO (2009) Palmitoylation of the TRAIL receptor DR4 confers an efficient TRAIL-induced cell death signalling. Biochem J 419:185–192 (2 p following 192)
Wagner KW, Punnoose EA, Januario T, Lawrence DA, Pitti RM, Lancaster K, Lee D, von Goetz M, Yee SF, Totpal K, Huw L, Katta V, Cavet G, Hymowitz SG, Amler L, Ashkenazi A (2007) Death-receptor O-glycosylation controls tumor-cell sensitivity to the proapoptotic ligand Apo2L/TRAIL. Nat Med 13:1070–1077
Yoshida T, Shiraishi T, Horinaka M, Wakada M, Sakai T (2007) Glycosylation modulates TRAIL-R1/death receptor 4 protein: different regulations of two pro-apoptotic receptors for TRAIL by tunicamycin. Oncol Rep 18:1239–1242
Thorburn A, Behbakht K, Ford H (2008) TRAIL receptor-targeted therapeutics: resistance mechanisms and strategies to avoid them. Drug Resist Updat 11:17–24
Zhang L, Fang B (2005) Mechanisms of resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Ther 12:228–237
Kim M, Park SY, Pai HS, Kim TH, Billiar TR, Seol DW (2004) Hypoxia inhibits tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by blocking Bax translocation. Cancer Res 64:4078–4081
Mahalingam D, Szegezdi E, Keane M, Jong S, Samali A (2009) TRAIL receptor signalling and modulation: are we on the right TRAIL? Cancer Treat Rev 35:280–288
D’Archivio M, Filesi C, Di Benedetto R, Gargiulo R, Giovannini C, Masella R (2007) Polyphenols, dietary sources and bioavailability. Ann Ist Super Sanita 43:348–361
Ramos S (2008) Cancer chemoprevention and chemotherapy: dietary polyphenols and signalling pathways. Mol Nutr Food Res 52:507–526
Depeint F, Gee JM, Williamson G, Johnson IT (2002) Evidence for consistent patterns between flavonoid structures and cellular activities. Proc Nutr Soc 61:97–103
Murzakhmetova M, Moldakarimov S, Tancheva L, Abarova S, Serkedjieva J (2008) Antioxidant and prooxidant properties of a polyphenol-rich extract from Geranium sanguineum L. in vitro and in vivo. Phytother Res 22:746–751
Sakao K, Fujii M, Hou DX (2009) Clarification of the role of quercetin hydroxyl groups in superoxide generation and cell apoptosis by chemical modification. Biosci Biotechnol Biochem 73:2048–2053
Harborne JB, Williams CA (2000) Advances in flavonoid research since 1992. Phytochemistry 55:481–504
Thomasset S, Teller N, Cai H, Marko D, Berry DP, Steward WP, Gescher AJ (2009) Do anthocyanins and anthocyanidins, cancer chemopreventive pigments in the diet, merit development as potential drugs? Cancer Chemother Pharmacol 64:201–211
Hafeez BB, Siddiqui IA, Asim M, Malik A, Afaq F, Adhami VM, Saleem M, Din M, Mukhtar H (2008) A dietary anthocyanidin delphinidin induces apoptosis of human prostate cancer PC3 cells in vitro and in vivo: involvement of nuclear factor-kappaB signaling. Cancer Res 68:8564–8572
Ding M, Feng R, Wang SY, Bowman L, Lu Y, Qian Y, Castranova V, Jiang BH, Shi X (2006) Cyanidin-3-glucoside, a natural product derived from blackberry, exhibits chemopreventive and chemotherapeutic activity. J Biol Chem 281:17359–17368
Kuo PL, Hsu YL, Lin TC, Lin LT, Lin CC (2004) Induction of apoptosis in human breast adenocarcinoma MCF-7 cells by prodelphinidin B-2 3, 3′-di-O-gallate from Myrica rubra via Fas-mediated pathway. J Pharm Pharmacol 56:1399–1406
Fleschhut J, Kratzer F, Rechkemmer G, Kulling SE (2006) Stability and biotransformation of various dietary anthocyanins in vitro. Eur J Nutr 45:7–18
Nishikawa T, Nakajima T, Moriguchi M, Jo M, Sekoguchi S, Ishii M, Takashima H, Katagishi T, Kimura H, Minami M, Itoh Y, Kagawa K, Okanoue T (2006) A green tea polyphenol, epigalocatechin-3-gallate, induces apoptosis of human hepatocellular carcinoma, possibly through inhibition of Bcl-2 family proteins. J Hepatol 44:1074–1082
Siegelin MD, Habel A, Gaiser T (2008) Epigalocatechin-3-gallate (EGCG) downregulates PEA15 and thereby augments TRAIL-mediated apoptosis in malignant glioma. Neurosci Lett 448:161–165
Siddiqui IA, Malik A, Adhami VM, Asim M, Hafeez BB, Sarfaraz S, Mukhtar H (2008) Green tea polyphenol EGCG sensitizes human prostate carcinoma LNCaP cells to TRAIL-mediated apoptosis and synergistically inhibits biomarkers associated with angiogenesis and metastasis. Oncogene 27:2055–2063
Basu A, Haldar S (2009) Combinatorial effect of epigallocatechin-3-gallate and TRAIL on pancreatic cancer cell death. Int J Oncol 34:281–286
Shen Q, Tian F, Jiang P, Li Y, Zhang L, Lu J, Li J (2009) EGCG enhances TRAIL-mediated apoptosis in human melanoma A375 cell line. J Huazhong Univ Sci Technol Med Sci 29:771–775
Bischoff SC (2008) Quercetin: potentials in the prevention and therapy of disease. Curr Opin Clin Nutr Metab Care 11:733–740
Ferry DR, Smith A, Malkhandi J, Fyfe DW, deTakats PG, Anderson D, Baker J, Kerr DJ (1996) Phase I clinical trial of the flavonoid quercetin: pharmacokinetics and evidence for in vivo tyrosine kinase inhibition. Clin Cancer Res 2:659–668
Kim YH, Lee YJ (2007) TRAIL apoptosis is enhanced by quercetin through Akt dephosphorylation. J Cell Biochem 100:998–1009
Kim YH, Lee DH, Jeong JH, Guo ZS, Lee YJ (2008) Quercetin augments TRAIL-induced apoptotic death: involvement of the ERK signal transduction pathway. Biochem Pharmacol 75:1946–1958
Russo M, Nigro P, Rosiello R, D’Arienzo R, Russo GL (2007) Quercetin enhances CD95- and TRAIL-induced apoptosis in leukemia cell lines. Leukemia 21:1130–1133
Chen W, Wang X, Zhuang J, Zhang L, Lin Y (2007) Induction of death receptor 5 and suppression of survivin contribute to sensitization of TRAIL-induced cytotoxicity by quercetin in non-small cell lung cancer cells. Carcinogenesis 28:2114–2121
Kim JY, Kim EH, Park SS, Lim JH, Kwon TK, Choi KS (2008) Quercetin sensitizes human hepatoma cells to TRAIL-induced apoptosis via Sp1-mediated DR5 up-regulation and proteasome-mediated c-FLIPS down-regulation. J Cell Biochem 105:1386–1398
You HL, Eng HL, Hsu SF, Chen CM, Ye TC, Liao WT, Huang MY, Baer R, Cheng JT (2007) A PKC-Sp1 signaling pathway induces early differentiation of human keratinocytes through upregulation of TSG101. Cell Signal 19:1201–1211
Psahoulia FH, Drosopoulos KG, Doubravska L, Andera L, Pintzas A (2007) Quercetin enhances TRAIL-mediated apoptosis in colon cancer cells by inducing the accumulation of death receptors in lipid rafts. Mol Cancer Ther 6:2591–2599
Siegelin MD, Reuss DE, Habel A, Rami A, von Deimling A (2009) Quercetin promotes degradation of survivin and thereby enhances death-receptor-mediated apoptosis in glioma cells. Neuro Oncol 11:122–131
Hasegawa H, Yamada Y, Komiyama K, Hayashi M, Ishibashi M, Yoshida T, Sakai T, Koyano T, Kam TS, Murata K, Sugahara K, Tsuruda K, Akamatsu N, Tsukasaki K, Masuda M, Takasu N, Kamihira S (2006) Dihydroflavonol BB-1, an extract of natural plant Blumea balsamifera, abrogates TRAIL resistance in leukemia cells. Blood 107:679–688
Yoshida T, Konishi M, Horinaka M, Yasuda T, Goda AE, Taniguchi H, Yano K, Wakada M, Sakai T (2008) Kaempferol sensitizes colon cancer cells to TRAIL-induced apoptosis. Biochem Biophys Res Commun 375:129–133
Leung LK, Po LS, Lau TY, Yuen YM (2004) Effect of dietary flavonols on oestrogen receptor transactivation and cell death induction. Br J Nutr 91:831–839
Siegelin MD, Reuss DE, Habel A, Herold-Mende C, von Deimling A (2008) The flavonoid kaempferol sensitizes human glioma cells to TRAIL-mediated apoptosis by proteasomal degradation of survivin. Mol Cancer Ther 7:3566–3574
Siegelin MD, Gaiser T, Habel A, Siegelin Y (2009) Myricetin sensitizes malignant glioma cells to TRAIL-mediated apoptosis by down-regulation of the short isoform of FLIP and bcl-2. Cancer Lett 283:230–238
Ravi R, Bedi A (2002) Sensitization of tumor cells to Apo2 ligand/TRAIL-induced apoptosis by inhibition of casein kinase II. Cancer Res 62:4180–4185
Horinaka M, Yoshida T, Shiraishi T, Nakata S, Wakada M, Sakai T (2006) The dietary flavonoid apigenin sensitizes malignant tumor cells to tumor necrosis factor-related apoptosis-inducing ligand. Mol Cancer Ther 5:945–951
Horinaka M, Yoshida T, Shiraishi T, Nakata S, Wakada M, Nakanishi R, Nishino H, Sakai T (2005) The combination of TRAIL and luteolin enhances apoptosis in human cervical cancer HeLa cells. Biochem Biophys Res Commun 333:833–838
Shi RX, Ong CN, Shen HM (2005) Protein kinase C inhibition and x-linked inhibitor of apoptosis protein degradation contribute to the sensitization effect of luteolin on tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in cancer cells. Cancer Res 65:7815–7823
Fas SC, Baumann S, Zhu JY, Giaisi M, Treiber MK, Mahlknecht U, Krammer PH, Li-Weber M (2006) Wogonin sensitizes resistant malignant cells to TNFalpha- and TRAIL-induced apoptosis. Blood 108:3700–3706
Lee DH, Rhee JG, Lee YJ (2009) Reactive oxygen species up-regulate p53 and Puma; a possible mechanism for apoptosis during combined treatment with TRAIL and wogonin. Br J Pharmacol 157:1189–1202
Rushworth SA, Micheau O (2009) Molecular crosstalk between TRAIL and natural antioxidants in the treatment of cancer. Br J Pharmacol 157:1186–1188
Kallifatidis G, Rausch V, Baumann B, Apel A, Beckermann BM, Groth A, Mattern J, Li Z, Kolb A, Moldenhauer G, Altevogt P, Wirth T, Werner J, Schemmer P, Buchler MW, Salnikov AV, Herr I (2009) Sulforaphane targets pancreatic tumour-initiating cells by NF-kappaB-induced antiapoptotic signalling. Gut 58:949–963
Peng J, Qi Q, You Q, Hu R, Liu W, Feng F, Wang G, Guo Q (2009) Subchronic toxicity and plasma pharmacokinetic studies on wogonin, a natural flavonoid, in Beagle dogs. J Ethnopharmacol 124:257–262
Taniguchi H, Yoshida T, Horinaka M, Yasuda T, Goda AE, Konishi M, Wakada M, Kataoka K, Yoshikawa T, Sakai T (2008) Baicalein overcomes tumor necrosis factor-related apoptosis-inducing ligand resistance via two different cell-specific pathways in cancer cells but not in normal cells. Cancer Res 68:8918–8927
Messina MJ, Wood CE (2008) Soy isoflavones, estrogen therapy, and breast cancer risk: analysis and commentary. Nutr J 7:17
Park SY, Seol DW (2002) Regulation of Akt by EGF-R inhibitors, a possible mechanism of EGF-R inhibitor-enhanced TRAIL-induced apoptosis. Biochem Biophys Res Commun 295:515–518
Nozawa F, Itami A, Saruc M, Kim M, Standop J, Picha KS, Cowan KH, Pour PM (2004) The combination of tumor necrosis factor-related apoptosis-inducing ligand (TRAIL/Apo2L) and Genistein is effective in inhibiting pancreatic cancer growth. Pancreas 29:45–52
Lee MW, Bach JH, Lee HJ, Lee DY, Joo WS, Kim YS, Park SC, Kim KY, Lee WB, Kim SS (2005) The activation of ERK1/2 via a tyrosine kinase pathway attenuates trail-induced apoptosis in HeLa cells. Cancer Investig 23:586–592
Jin CY, Park C, Kim GY, Lee SJ, Kim WJ, Choi YH (2009) Genistein enhances TRAIL-induced apoptosis through inhibition of p38 MAPK signaling in human hepatocellular carcinoma Hep3B cells. Chem Biol Interact 180:143–150
Jin CY, Park C, Moon SK, Kim GY, Kwon TK, Lee SJ, Kim WJ, Choi YH (2009) Genistein sensitizes human hepatocellular carcinoma cells to TRAIL-mediated apoptosis by enhancing Bid cleavage. Anticancer Drugs 20:713–722
Jin CY, Park C, Cheong J, Choi BT, Lee TH, Lee JD, Lee WH, Kim GY, Ryu CH, Choi YH (2007) Genistein sensitizes TRAIL-resistant human gastric adenocarcinoma AGS cells through activation of caspase-3. Cancer Lett 257:56–64
Siegelin MD, Siegelin Y, Habel A, Gaiser T (2009) Genistein enhances proteasomal degradation of the short isoform of FLIP in malignant glioma cells and thereby augments TRAIL-mediated apoptosis. Neurosci Lett 453:92–97
Siegelin MD, Gaiser T, Habel A, Siegelin Y (2009) Daidzein overcomes TRAIL-resistance in malignant glioma cells by modulating the expression of the intrinsic apoptotic inhibitor, Bcl-2. Neurosci Lett 454:223–228
Kim N (2008) Butein sensitizes human leukemia cells to apoptosis induced by tumor necrosis factor-related apoptosis inducing ligand (TRAIL). Arch Pharm Res 31:1179–1186
Yoshida T, Horinaka M, Takara M, Tsuchihashi M, Mukai N, Wakada M, Sakai T (2008) Combination of isoliquiritigenin and tumor necrosis factor-related apoptosis-inducing ligand induces apoptosis in colon cancer HT29 cells. Environ Health Prev Med 13:281–287
Deeb D, Xu YX, Jiang H, Gao X, Janakiraman N, Chapman RA, Gautam SC (2003) Curcumin (diferuloyl-methane) enhances tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis in LNCaP prostate cancer cells. Mol Cancer Ther 2:95–103
Fulda S, Debatin KM (2004) Sensitization for tumor necrosis factor-related apoptosis-inducing ligand-induced apoptosis by the chemopreventive agent resveratrol. Cancer Res 64:337–346
Fulda S, Debatin KM (2005) Resveratrol-mediated sensitisation to TRAIL-induced apoptosis depends on death receptor and mitochondrial signalling. Eur J Cancer 41:786–798
Delmas D, Rebe C, Micheau O, Athias A, Gambert P, Grazide S, Laurent G, Latruffe N, Solary E (2004) Redistribution of CD95, DR4 and DR5 in rafts accounts for the synergistic toxicity of resveratrol and death receptor ligands in colon carcinoma cells. Oncogene 23:8979–8986
Shankar S, Chen Q, Siddiqui I, Sarva K, Srivastava RK (2007) Sensitization of TRAIL-resistant LNCaP cells by resveratrol (3, 4′, 5 tri-hydroxystilbene): molecular mechanisms and therapeutic potential. J Mol Signal 2:7
Shankar S, Siddiqui I, Srivastava RK (2007) Molecular mechanisms of resveratrol (3, 4, 5-trihydroxy-trans-stilbene) and its interaction with TNF-related apoptosis inducing ligand (TRAIL) in androgen-insensitive prostate cancer cells. Mol Cell Biochem 304:273–285
Gill C, Walsh SE, Morrissey C, Fitzpatrick JM, Watson RW (2007) Resveratrol sensitizes androgen independent prostate cancer cells to death-receptor mediated apoptosis through multiple mechanisms. Prostate 67:1641–1653
Sallman DA, Chen X, Zhong B, Gilvary DL, Zhou J, Wei S, Djeu JY (2007) Clusterin mediates TRAIL resistance in prostate tumor cells. Mol Cancer Ther 6:2938–2947
Ivanov VN, Partridge MA, Johnson GE, Huang SX, Zhou H, Hei TK (2008) Resveratrol sensitizes melanomas to TRAIL through modulation of antiapoptotic gene expression. Exp Cell Res 314:1163–1176
Yoshida T, Shiraishi T, Horinaka M, Nakata S, Yasuda T, Goda AE, Wakada M, Mizutani Y, Miki T, Nishikawa A, Sakai T (2007) Lipoxygenase inhibitors induce death receptor 5/TRAIL-R2 expression and sensitize malignant tumor cells to TRAIL-induced apoptosis. Cancer Sci 98:1417–1423
Raja SM, Chen S, Yue P, Acker TM, Lefkove B, Arbiser JL, Khuri FR, Sun SY (2008) The natural product honokiol preferentially inhibits cellular FLICE-inhibitory protein and augments death receptor-induced apoptosis. Mol Cancer Ther 7:2212–2223
Son YG, Kim EH, Kim JY, Kim SU, Kwon TK, Yoon AR, Yun CO, Choi KS (2007) Silibinin sensitizes human glioma cells to TRAIL-mediated apoptosis via DR5 up-regulation and down-regulation of c-FLIP and survivin. Cancer Res 67:8274–8284
Reuter S, Eifes S, Dicato M, Aggarwal BB, Diederich M (2008) Modulation of anti-apoptotic and survival pathways by curcumin as a strategy to induce apoptosis in cancer cells. Biochem Pharmacol 76:1340–1351
Strimpakos AS, Sharma RA (2008) Curcumin: preventive and therapeutic properties in laboratory studies and clinical trials. Antioxid Redox Signal 10:511–545
Deeb DD, Jiang H, Gao X, Divine G, Dulchavsky SA, Gautam SC (2005) Chemosensitization of hormone-refractory prostate cancer cells by curcumin to TRAIL-induced apoptosis. J Exp Ther Oncol 5:81–91
Deeb D, Jiang H, Gao X, Hafner MS, Wong H, Divine G, Chapman RA, Dulchavsky SA, Gautam SC (2004) Curcumin sensitizes prostate cancer cells to tumor necrosis factor-related apoptosis-inducing ligand/Apo2L by inhibiting nuclear factor-kappaB through suppression of IkappaBalpha phosphorylation. Mol Cancer Ther 3:803–812
Deeb D, Jiang H, Gao X, Al-Holou S, Danyluk AL, Dulchavsky SA, Gautam SC (2007) Curcumin [1, 7-bis(4-hydroxy-3-methoxyphenyl)-1-6-heptadine-3, 5-dione; C21H20O6] sensitizes human prostate cancer cells to tumor necrosis factor-related apoptosis-inducing ligand/Apo2L-induced apoptosis by suppressing nuclear factor-kappaB via inhibition of the prosurvival Akt signaling pathway. J Pharmacol Exp Ther 321:616–625
Andrzejewski T, Deeb D, Gao X, Danyluk A, Arbab AS, Dulchavsky SA, Gautam SC (2008) Therapeutic efficacy of curcumin/TRAIL combination regimen for hormone-refractory prostate cancer. Oncol Res 17:257–267
Shankar S, Chen Q, Sarva K, Siddiqui I, Srivastava RK (2007) Curcumin enhances the apoptosis-inducing potential of TRAIL in prostate cancer cells: molecular mechanisms of apoptosis, migration and angiogenesis. J Mol Signal 2:10
Shankar S, Ganapathy S, Chen Q, Srivastava RK (2008) Curcumin sensitizes TRAIL-resistant xenografts: molecular mechanisms of apoptosis, metastasis and angiogenesis. Mol Cancer 7:16
Jung EM, Park JW, Choi KS, Lee HI, Lee KS, Kwon TK (2006) Curcumin sensitizes tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-mediated apoptosis through CHOP-independent DR5 upregulation. Carcinogenesis 27:2008–2017
Jung EM, Lim JH, Lee TJ, Park JW, Choi KS, Kwon TK (2005) Curcumin sensitizes tumor necrosis factor-related apoptosis-inducing ligand (TRAIL)-induced apoptosis through reactive oxygen species-mediated upregulation of death receptor 5 (DR5). Carcinogenesis 26:1905–1913
Hussain AR, Ahmed M, Al-Jomah NA, Khan AS, Manogaran P, Sultana M, Abubaker J, Platanias LC, Al-Kuraya KS, Uddin S (2008) Curcumin suppresses constitutive activation of nuclear factor-kappa B and requires functional Bax to induce apoptosis in Burkitt’s lymphoma cell lines. Mol Cancer Ther 7:3318–3329
Gao X, Deeb D, Jiang H, Liu YB, Dulchavsky SA, Gautam SC (2005) Curcumin differentially sensitizes malignant glioma cells to TRAIL/Apo2L-mediated apoptosis through activation of procaspases and release of cytochrome c from mitochondria. J Exp Ther Oncol 5:39–48
Kamat AM, Sethi G, Aggarwal BB (2007) Curcumin potentiates the apoptotic effects of chemotherapeutic agents and cytokines through down-regulation of nuclear factor-kappaB and nuclear factor-kappaB-regulated gene products in IFN-alpha-sensitive and IFN-alpha-resistant human bladder cancer cells. Mol Cancer Ther 6:1022–1030
Wahl H, Tan L, Griffith K, Choi M, Liu JR (2007) Curcumin enhances Apo2L/TRAIL-induced apoptosis in chemoresistant ovarian cancer cells. Gynecol Oncol 105:104–112
Kong CK, Lam WS, Chiu LC, Ooi VE, Sun SS, Wong YS (2009) A rice bran polyphenol, cycloartenyl ferulate, elicits apoptosis in human colorectal adenocarcinoma SW480 and sensitizes metastatic SW620 cells to TRAIL-induced apoptosis. Biochem Pharmacol 77:1487–1496
Drees M, Dengler WA, Roth T, Labonte H, Mayo J, Malspeis L, Grever M, Sausville EA, Fiebig HH (1997) Flavopiridol (L86-8275): selective antitumor activity in vitro and activity in vivo for prostate carcinoma cells. Clin Cancer Res 3:273–279
Shapiro GI (2004) Preclinical and clinical development of the cyclin-dependent kinase inhibitor flavopiridol. Clin Cancer Res 10:4270s–4275s
Taniai M, Grambihler A, Higuchi H, Werneburg N, Bronk SF, Farrugia DJ, Kaufmann SH, Gores GJ (2004) Mcl-1 mediates tumor necrosis factor-related apoptosis-inducing ligand resistance in human cholangiocarcinoma cells. Cancer Res 64:3517–3524
Rosato RR, Dai Y, Almenara JA, Maggio SC, Grant S (2004) Potent antileukemic interactions between flavopiridol and TRAIL/Apo2L involve flavopiridol-mediated XIAP downregulation. Leukemia 18:1780–1788
Miyashita K, Shiraki K, Fuke H, Inoue T, Yamanaka Y, Yamaguchi Y, Yamamoto N, Ito K, Sugimoto K, Nakano T (2006) The cyclin-dependent kinase inhibitor flavopiridol sensitizes human hepatocellular carcinoma cells to TRAIL-induced apoptosis. Int J Mol Med 18:249–256
Palacios C, Yerbes R, Lopez-Rivas A (2006) Flavopiridol induces cellular FLICE-inhibitory protein degradation by the proteasome and promotes TRAIL-induced early signaling and apoptosis in breast tumor cells. Cancer Res 66:8858–8869
Fandy TE, Ross DD, Gore SD, Srivastava RK (2007) Flavopiridol synergizes TRAIL cytotoxicity by downregulation of FLIPL. Cancer Chemother Pharmacol 60:313–319
Kim DM, Koo SY, Jeon K, Kim MH, Lee J, Hong CY, Jeong S (2003) Rapid induction of apoptosis by combination of flavopiridol and tumor necrosis factor (TNF)-alpha or TNF-related apoptosis-inducing ligand in human cancer cell lines. Cancer Res 63:621–626
Newman DJ, Cragg GM (2007) Natural products as sources of new drugs over the last 25 years. J Nat Prod 70:461–477
Garg AK, Buchholz TA, Aggarwal BB (2005) Chemosensitization and radiosensitization of tumors by plant polyphenols. Antioxid Redox Signal 7:1630–1647
Ishibashi M, Ohtsuki T (2008) Studies on search for bioactive natural products targeting TRAIL signaling leading to tumor cell apoptosis. Med Res Rev 28:688–714
Szliszka E, Czuba ZP, Domino M, Mazur B, Zydowicz G, Krol W (2009) Ethanolic extract of propolis (EEP) enhances the apoptosis-inducing potential of TRAIL in cancer cells. Molecules 14:738–754
Sung B, Park B, Yadav VR, Aggarwal BB (2010) Celastrol, a triterpene, enhances TRAIL-induced apoptosis through the down-regulation of cell survival proteins and up-regulation of death receptors. J Biol Chem 285:11498–11507
Zhu H, Ding WJ, Wu R, Weng QJ, Lou JS, Jin RJ, Lu W, Yang B, He QJ (2010) Synergistic anti-cancer activity by the combination of TRAIL/APO-2L and celastrol. Cancer Investig 28:23–32
Murtaza I, Saleem M, Adhami VM, Hafeez BB, Mukhtar H (2009) Suppression of cFLIP by lupeol, a dietary triterpene, is sufficient to overcome resistance to TRAIL-mediated apoptosis in chemoresistant human pancreatic cancer cells. Cancer Res 69:1156–1165
Scalbert A, Williamson G (2000) Dietary intake and bioavailability of polyphenols. J Nutr 130:2073S–2085S
Kroon PA, Clifford MN, Crozier A, Day AJ, Donovan JL, Manach C, Williamson G (2004) How should we assess the effects of exposure to dietary polyphenols in vitro? Am J Clin Nutr 80:15–21
Das S, Ng KY (2010) Resveratrol-loaded calcium-pectinate beads: effects of formulation parameters on drug release and bead characteristics. J Pharm Sci 99:840–860
Narayanan NK, Nargi D, Randolph C, Narayanan BA (2009) Liposome encapsulation of curcumin and resveratrol in combination reduces prostate cancer incidence in PTEN knockout mice. Int J Cancer 125:1–8
Fang JY, Lee WR, Shen SC, Huang YL (2006) Effect of liposome encapsulation of tea catechins on their accumulation in basal cell carcinomas. J Dermatol Sci 42:101–109
Yuan ZP, Chen LJ, Fan LY, Tang MH, Yang GL, Yang HS, Du XB, Wang GQ, Yao WX, Zhao QM, Ye B, Wang R, Diao P, Zhang W, Wu HB, Zhao X, Wei YQ (2006) Liposomal quercetin efficiently suppresses growth of solid tumors in murine models. Clin Cancer Res 12:3193–3199
Hung CF, Lin YK, Huang ZR, Fang JY (2008) Delivery of resveratrol, a red wine polyphenol, from solutions and hydrogels via the skin. Biol Pharm Bull 31:955–962
Acknowledgments
This work was supported by grants of the Conseil Regional de Bourgogne, the INCa (Institut National du Cancer) Canceropôle Grand-Est, ANR (Agence Nationale de la Recherche, ANR-06-JCJC-0103 and 07-PCV-0031) and the European Community (ApopTrain Marie Curie RTN) (O.M.). G.J. is supported by a fellowship from the Ligue Nationale Contre le Cancer. S.S. is supported by the INCa (POLYNOM-174).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jacquemin, G., Shirley, S. & Micheau, O. Combining naturally occurring polyphenols with TNF-related apoptosis-inducing ligand: a promising approach to kill resistant cancer cells?. Cell. Mol. Life Sci. 67, 3115–3130 (2010). https://doi.org/10.1007/s00018-010-0407-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-010-0407-6