Abstract
Variation in pigmentation is one of the most conspicuous phenotypic traits in vertebrates. Although mammals show less variation in body pigmentation than other vertebrate groups, the genetics of colour determination and variation is best understood for them. More than 150 genes have been identified that influence pigmentation, and in many cases, the cause for variation in pigmentation has been identified down to the underlying nucleotide changes. These studies show that while some genes are often responsible for deviating pigmentation, similar or almost identical phenotypes even in the same species may be due to mutations in different genes. In this review we will first discuss the current knowledge about the genes and their functions underlying the biochemical pathways that determine pigmentation and then give examples where the mutations responsible for colour variation have been determined. Finally, we will discuss potential evolutionary causes for and consequences of differences in pigmentation between individuals.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
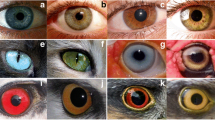
The living world is colourful; nobody having a brief look at it will doubt that. In contrast to the non-living part of our globe such as rocks or ice, which may sometimes gleam at sunset, but are mostly dull grey, organisms often show extremely bright colours, and it is difficult to imagine something more colourful than a coral reef full of fishes varying from yellow over orange, pink, violet to green and bright blue—plus all combinations. However, not only fishes are colourful, almost any animal group shows a wide range of colours (Fig. 1). Beautiful as this is, it is not only a beautiful sight, but it also raises numerous questions about the underlying biology. On the proximal level the issues focus on questions about the biochemical and developmental basis underlying colours and colour patterns, whereas on the ultimate level, it is interesting which evolutionary forces are the drivers behind this phenotypic variability. In this review we will first describe what little is known about the molecular mechanisms that underlie pigmentation, then give examples of how colouration differs between and within species, including the genes and mutations that are responsible for such differences and discuss the multitude of evolutionary forces that are the drivers behind the evolution of pigmentation. Finally, we will discuss differences in hair and skin colour in our own species. For reasons of scope and scale, we will stick with vertebrate species as indicated already in the title, but we would like to note that quite a lot is also known about the molecular basis of invertebrate pigmentation, such as differences in wing spots among butterflies [1–3] or body pigmentation in Drosophila species [4].
Colour variation among primate species. Although mammals have a very limited number of pigments to their disposal compared to other vertebrates, they are still quite variable in colouration. a Gibbon pair (Hylobates lar); b gibbon portrait (H. lar); c white-faced saki (Pithecia pithecia); d golden lion tamarind (Leontopithecus rosalia); e squirrel monkey (Saimiri sciureus); f ring-tailed lemur (Lemur catta). Pictures courtesy of Brenda Bradley
Molecular pathways involved in pigmentation
Mammals
There are two major issues that determine colouration, not only in organisms, but for anything not monochromatic. First, it is important which pigments are available and, second, how these pigments are distributed. Given the multitude of colours and the vast array of arrangements of these two traits in vertebrates, it is not surprising that more than 368 genetic loci (159 cloned genes and 209 not yet cloned genes) are so far known that are involved in pigmentation [5]. Due to this complexity and the fact that colours and their distribution are different among almost any pair of species, it is not surprising that we are far from a full understanding of how pigmentation is regulated in vertebrates. However, some patterns start to emerge.
In mammals, to start with the best known and most simple example, colour depends on two major factors: the presence of pigments at all and the balance of the black eumelanin and the yellowish pheomelanin [6]. Both are produced in melanocytes, pigment-producing cells that reside mainly in the epidermis, eye and hair follicles and are responsible for skin, eye and hair colour. Melanin is produced in specialised organelles, the melanosoms, which are then transported via dentritic processes to the growing hair or the keratinocytes [7, 8]. Although numerous genes are involved in this process (see [9] for a review), there are some key players that warrant special attention. Tyrosinase is the most important of a number of melanogenesis-related enzymes (MREs) that regulate both speed and specificity of melanogenesis. Tyrosinase, or possibly tyrosine hydroxylase, catalyses the conversion of l-tyrosine to l-DOPA, an early and important step of melanogenesis [10]. Consequently, loss-of-function mutations in tyrosinase result, among other effects, in albinism. Eumelanin synthesis is further stimulated by two tyrosinase-related proteins, TRP1 and TRP2, and also depends on more general factors such as pH value, metal ions, reducing agents and oxygen.
In addition, there are numerous regulators of melanogenesis functioning upstream of tyrosinase and other MREs. The promoter of the tyrosinase gene contains a wide variety of binding sites, including UV response elements and several potential microphtalmia-associated transcription factor (MITF) binding sites. In addition to tyrosinase transcription, MITF also stimulates the transcription of TRP1 and TRP2. MITF transcription is itself under control of cAMP via cAMP response elements (CRE). Therefore, MITF plays a key role in melanogenesis, as cAMP is the major second messenger-stimulating melanogenesis [11]. In addition to cAMP, Ca2+ plays an important role as second messenger in melanogenesis by stimulating the synthesis of L-tyrosine, the starting point of melanin synthesis, from l-phenylalanine [12].
The cAMP level in melanocytes is mainly controlled by melanocyte-stimulating hormones (MSH). MSH and several other bioactive peptide hormones are processed from the prohormone propiomelanocortin (POMC). α-MSH but also other structurally related peptides such as β-MSH and the adrenocorticotrophic hormone (ACTH) are endogenous agonists of the melanocortin 1 receptor (MC1R) [13], a rhodopsin-like G protein-coupled receptor (GPCR) [14] and one of the key regulators in melanogenesis. Upon binding of MSH to MC1R, conformational changes within this plasma membrane receptor activate the heterotrimeric Gs protein. The Gs α-subunit stimulates adenylyl cyclases, which convert ATP to cAMP, thereby stimulating the synthesis of dark eumelanin. Failure of MC1R to induce cAMP formation accordingly results in a reduced or diminished eumelanin production, and the pigmentation is then mainly determined by pheomelanin [15, 16]. Therefore, gain-of-function mutations in MC1R result in melanism, e.g., in mouse, pig, fox, sheep and chicken [17–21], while loss-of-function mutations usually lead to lighter, reddish or yellowish pigmentation, including red hair and pale skin in humans [22, 23]. Numerous other cases of MC1R as the cause of colour variation are known from both wild and domestic species, and it probably represents the most studied of all proteins involved in pigmentation (see below). MC1R is furthermore regulated by agouti signalling protein (ASIP), which works as an antagonist/inverse agonist to MC1R function. The current model of GPCR activation, the so-called allosteric ternary complex model, proposes that a receptor, independent of ligand binding, is in equilibrium between an inactive and active conformation [24]. Agonist binding stabilises the active conformation of a given receptor, whereas inverse agonists, such as ASIP, shift the equilibrium to the inactive conformation. Many MC1R orthologs display high basal activity and, therefore, ASIP acts not only by displacing MSH but also by reducing MC1R basal activity [25]. Consequently, yellow/light coat colour in mammals may be caused by either loss of function mutations in MC1R or increased production of ASIP due to gene duplication or mutations within the promoter region of the ASIP gene [26–28]. It has also been shown that loss-of-function mutations in agouti result in melanism [21, 29]. Interestingly, this is the case even in POMC knock-out mice [30], showing that apart from MC1R, other GPCR or factors are able to provide the cAMP required to trigger eumelanogenesis [12]. One of the factors that may help to explain the apparent paradox that mutations of POMC have a relatively minor effect on pigmentation are probably β-defensins. For example, β-defensin 103 is a high-affinity ligand of MC1R and has effects on pigment type-switching in wolves, domestic dogs and transgenic mice [31, 32].
There are numerous additional genes influencing aspects of pigmentation. A specific form of ocular albinism (ocular albinism type 1, OA1) has been associated with an x-chromosomally encoded protein [33]. The OA1 protein has been identified as an intracellular GPCR (GPR143) regulating melanosome transport in pigment cells. Recently, l-DOPA, a basic substrate in pigment biosynthese, was determined as the endogenous agonist for GPR143 [34]. Inactivating mutations in GPR143 are responsible for OA1 [35]. So far, this type of hypopigmentation appears to be restricted to the ocular fundus in mammals, including humans [36]. There are also several forms of oculocutaneous albinisms (OCA1-4). The oculocutaneous albinisms type 1A and type 1B (OCA1A and OCA1B) are caused by inactivating and partially inactivating, respectively, mutations in the tyrosinase gene. OCA2, OCA3 and OCA4 have somewhat milder phenotypes and are caused by mutations in the OCA2 (its gene product has structural features of a transporter), the tyrosinase-related protein, and transporter MATP (SLC45A2) genes, respectively. Loss-of-function mutations in OCA2 cause albinism in populations of Mexican cavefish tetra [37], while mutations in the fish MATP homolog reduce melanin content in medaka fish [38].
Finally, p53 seems to play a major role in eumelanogenesis, especially as a reaction to UV exposure via both the tyrosinase and the POMC pathway. Thus, melanogenesis represents a complex and integrated pathway, including both stimulatory and inhibitory interactions (for further details on melanogenesis, see [11, 12]).
Apart from the production of melanin, the migration of melanoblasts/melanocytes from the neural crest during embryogenesis and into the developing skin and hair follicles is obligatory for pigmentation. The major factors during this process are the c-kit receptor/steel factor system, which is critical for melanocyte survival. Consequently, c-kit mutations are associated with white coat colour in a number of species, including mice, rats, pigs and horses [39–42].
Other vertebrates
Although the genetics of colour determination may seem to be complicated in mammals, they represent the simplest example among vertebrates. As is obvious from simple observation, mammals are less colourful than any other class of vertebrates, i.e. fishes, amphibians, reptiles and birds. Not surprisingly, in fishes, amphibians and reptiles, colour is not determined by a single cell type as in mammals, but by three cell layers, the melanophore (containing melanin), the xanthophore (containing carotenoid and pteridine colours) and the iridophore (“structural” colours due to reflection). Together, these cell layers can produce almost any combination of colours, with xantophores being responsible for yellow, red and orange colours, melanophores for brown, black and gray, and iridophores for short wave length colours (blue, violet and green) as well as for silvery colours. However, it should be noted that blue pigments, called cyanophores, do exist in fishes [43]. Many of these pigments (or their precursors) cannot be synthesised by vertebrates and must therefore be taken up with the diet.
While much has still to be learned about the genetics of colour variation and distribution, some genes stand out as key players and have been identified time and again as the cause of colour variation within or between species. Before discussing examples for this, we will first outline which evolutionary forces may be responsible for colour variation.
Evolutionary forces behind colour variation
Generally, there are two classes of individuals that can be the target of colour signals: conspecifics and individuals belonging to different species. In the latter case, there is only one major function for colouration, namely avoiding predation. However, this aim can be reached via two very different pathways, namely crypsis and aposematics, although in the latter case, the signal may not always be honest.
Crypsis
Cryptic colouration is ubiquitous among vertebrates, be it the vast majority of fishes, most (female) birds or almost all small mammals. However, as cryptic colouration depends heavily on the habitat of the species—or population—in question, numerous examples for the power of predation risk as an evolutionary force exist [44–47]. For example, pocket mice of the genera Chaetodipus and Perognathus occur on different coloured substrates in North America that range from almost black (basaltic lava) to almost white (gypsum dunes). As already noted by naturalists in the first half of the 20th century, mice living on different substrates also differ in coat colour, matching their specific environment [48]. Interestingly, the same pattern of dark colouration on dark substrate and light colouration on light substrate has also been observed in Western fence lizards (Sceloporus undulates) [47] that live in the same habitat as the pocket mice. Experimental studies on another genus of mice, Peromyscus, which also shows variation in coat colour matching the respective substrate, have moreover shown that this has indeed a strong influence on predation rates by visually orientating avian predators [49]. Such adaptations to the colour of the environment can happen very fast. In populations of the deer mouse (Peromyscus maniculatus) living on the Nebraska sand hills (Fig. 2), the adaptation to light-coloured ground could be linked to a single amino acid deletion in the agouti gene and an increased agouti expression probably due to cis-acting mutation(s) [28]. Further evidence suggests that this change happened very rapidly during the last 8,000 years. Similarly, in New Mexico, three lizard species have, during the last 6,000 years, rapidly evolved so-called blanched phenotypes as concealing colouration on the gypsum dunes of White Sands in the Chihuahuan Desert, New Mexico, [50].
In general, crypsis is likely to be one of the most potent forces influencing colour as many species match with the background colour in their habitat. This may even be the fact for the—to the human eye—unusually colourful coral reef fishes [51]. In contrast to humans, many coral reef fishes are dichromats and therefore, as noted by Marshall and Vorobyev [52], “the reef is probably less colourful to many fishes than it appears to us” and “colours of reef fishes are almost always for camouflage”.
However, crypsis is not only a strategy of prey species in an attempt to avoid predation, it may be equally important for predators to avoid being spotted by prey too early. There are fewer examples for variation in colour among different populations of predator species, possibly due to the on average larger size and therefore habitat range, but recently a very interesting example has been published. In the Yellowstone National Park wolf population, which goes back to a limited number of founder animals, a black colour morph is present in very high frequency. This phenotype has been traced back to the K locus, which encodes a beta-defensin protein, CBD103 [53]. Interestingly, it seems to be derived from domestic dogs, having entered the wolf gene pool via introgression, possibly already shortly after introduction of domestic dogs to the New World some 10,000–15,000 years ago [32]. In contrast to wolf populations from tundra environments where this variant is rare, it is generally common in wolf populations from forest habitats. Thus, if the tundra habitat declines due to human impact and/or climate change, the allele derived by introgression from domestic dogs may actually prove beneficial for adaptation of wolves to new environmental conditions.
Aposematism
Given that cryptic colouration should be adaptive for both predators and prey, why is it then that so many animals are extremely colourful? Obviously, there are occasions when an animal has an evolutionary advantage of being seen. This can have a number of reasons. First, poisonous animals can signal to a potential predator that they do not represent suitable prey, by various means such as odour or, very often, bright colours. There are numerous examples for such so-called aposematic signals, for example, in poisonous frogs, snakes or lionfish. Although a naive predator may try—and succeed—to kill a member of such an aposematic species, the experience will result in it avoiding such prey in the future. Although individuals of the aposematic species may thus still be killed occasionally by predators, the overall risk will be low enough for the system to evolutionary survive. Interestingly, similar colouration may be displayed by both two poisonous species (Mullerian mimicry) as well as by a poisonous and a non-poisonous species (Batesian mimicry). In the first case, the two (or more) species both profit from each other, as the more common the signal, the more likely it is that predators will have encountered it before and consequently avoid it. In contrast, in Batesian mimicry, the non-poisonous species exploits the warning signal of the poisonous one, as predators avoid them without the mimicking species having to bear the cost of producing venom. One example in vertebrates is provided by some species of king snakes (genus Lampropeltis) that mimic the highly venomous coral snakes (genus Micrucurus). However, if the non-poisonous species becomes too frequent, predators will learn that the signal is not honest. This is one of several examples of frequency effects when it comes to evolution and maintenance of colour.
Frequency-dependent selection can also occur within species with regard to predators. One well-studied example comes from guppies, in which males show bright nuptial colours. Interestingly, rare colour morphs show higher survival rates possibly due to predators developing a search image that targets the most common colour pattern [54]. This effect favours the persistence of multiple colour morphs within a population.
Sexual selection
Such a frequency dependent effect, favouring the rarer colour morphs, is also known from sexual selection, when females preferentially mate with rare colour morph males, a phenomenon also known from guppies [55, 56]. In contrast, in Lake Victoria cichlids, it has been observed that males from populations that lack a certain colour polymorphism show mating preference against females of the colour morph that is lacking in the test population [57]. However, it should be noted that this is a special case, as the colour-determining locus is associated with both male and female mating preference. This observation may explain the rapid sympatric evolution of numerous species in Lake Victoria. Polymorphic mating preference is not restricted to males. In swordtail fishes (Xiphophorus cortezi), females prefer either males with bars or such without bars [58]. Moreover, assortative mating with regard to colour polymorphisms has been observed in numerous bird species [59]. It has even be suggested for the white colour morph (Kermode bears) of the American black bear (Ursus americanus), although the sample size in the study was too small to determine this with certainty [60].
Thus, sexual selection on colour variants can have opposing outcomes. As preference for rare males will result in the maintenance of many different colour variants, it should promote gene flow among populations and impair speciation. In contrast, assortative mating or female polymorphism with regard to preference for male colour variants will support speciation. Although there may be a bias with regard to the studies conducted and/or reported, it seems that the latter mechanism is more common in nature. It is now also well established that sexual selection on colour is in many cases environment-dependent. Thus, it was recently shown [61] that speciation in Lake Victoria cichlids takes place according to the sensory-drive hypothesis [62, 63]. This hypothesis predicts that adaptation of both the sensory and signalling system in populations living in different environment can lead to speciation. In Lake Victoria, species pairs that show red and blue nuptial colouration (Fig. 3), respectively, are quite common. In their study, the authors found for several species pairs that the absorption maximum of opsins is shifted to a longer wave length for species that live at greater water depth. This results in the populations living at greater depths showing a red-shift in their light sensitivity maximum. In addition, females from populations living at greater depths prefer red- over blue-coloured males [61]. Thus, in accord with the sensory-drive hypothesis, perception evolved according to the environment, and both female preference and male nuptial colouration followed the perceptual restrictions. In this example, speciation occurred even in symaptry along gradients in light condition. Sticklebacks are another example for speciation via sensory-drive sexual selection. In several Canadian Lakes, species pairs of a benthic and a limnetic species are found, and again perceptual sensitivity, male nuptial colour and female preference differ between the limnetic and benthic form as expected from the wavelength shift in the ambient light of the habitat [63]. Interestingly, in cichlids, different light regimes or even bright colours are no prerequisite for sexual selection. For deep-water cichlids of the genus Diplotaxodon, living in Lake Malawi, it was found that males differ in colour patterns, which are detectable despite the narrow wave length spectrum in their habitat [64]. However, it is unknown whether these species evolved in sympatry or in allopatry.
Different colour morphs of Lake Victoria cichlids. a Chessboard pattern associated with non-territorial life in structured littoral habitats; here on blue background (Paralabidochromis sp. “rockkribensis”); b midlateral stripe pattern: associated with life in the open water (Enterochromis cf. paropius); c chessboard pattern on red background (Paralabidochromis sp. “rockkribensis”); d vertical bars pattern: associated with highly territorial life in structured littoral habitats (Pundamilia nyererei). Pictures courtesy of Ole Seehausen
Conditions for sensory perception may not be the only cause for driving evolution of a certain mate preference via “sensory bias”. An interesting example is provided again by guppies, in which females show a preference for males with orange spots. Moreover, the intensity with which females prefer orange males varies across populations. Interestingly, both males and females react strongly to orange objects also outside a mating context, and the variability in this reaction explains a striking 94% of the variability in female preference against orange males among populations. The authors of this study therefore suggest that male guppies actually “are mimicking fruit” [65]. This is an interesting example for a “sensory bias” that may have developed due to foraging triggering sexual preference (but see below). A similar observation has been reported for sticklebacks, despite the fact that red nuptial colouration correlates with numerous fitness traits such as male condition, male mating success and resistance of offspring to parasites [66–68]. Although the extent of red colouration therefore obviously represents an honest male signal, both male and female sticklebacks also react to red objects outside a mating context, and this is even true for species that do not have red nuptial colours. Thus, red nuptial colour again most likely evolved because of a sensory bias of sticklebacks towards red colour, which in itself may have evolved in a foraging context.
While sensory bias seems to be a strong force with regard to the development of female colour preferences, as noted above, nuptial colouration may still serve as an important signal for females when assessing male quality. In fact, it was shown for guppies that females even show a plastic phenotypic response to nuptial colour. Thus, females that grow up with a high-carotenoid diet show a weaker response to bright orange nuptial colour in males than females that grow up with a low-carotenoid diet [69]. Due to the combination of several factors, this reaction makes evolutionary sense. Fish cannot produce carotenoids, the chemical components for orange colour, but have to take them up with their diet. However, carotenoids cannot only be used as pigment but are also important effectors in the immune response. Therefore, in low-carotenoid environments, only high-quality males can afford allocating the limited amounts of carotenoids available to orange coloured pigment patterns. In contrast, with increasing availability of carotenoids, low quality males will also be able to express orange pigment spots, making this type of signal increasingly useless for females to discriminate between high and low quality males. Thus, the negative correlation between carotenoid availability and female response to the orange coloured males observed is exactly what would be expected evolutionarily.
The above result also shows that male signalling depends on the environment, an observation not restricted to guppies. Studies on a number of fish species have shown that especially eutrophication, mostly due to human activity, can dramatically reduce the strength of selection on traditional mating traits. In sticklebacks, increased algal growth was found to reduce the strength of selection on both male nuptial colouration and courtship display [70]. A similar effect has been observed in the sand goby, where male mating success was more evenly distributed under turbid water conditions, thus relaxing both opportunity for and strength of sexual selection [71]. Finally, a breakdown of sexual selection and consequently reproductive isolation among differently coloured species have been found in parts of Lake Victoria, where visual conditions changed dramatically because of eutrophication caused by human activity [61]. This example of a breakdown in species barriers shows how dramatic the evolutionary effect of environmental changes influencing sexual selection can be.
Finally, female preference and/or sensory bias may not be the only intra-specific evolutionary force influencing colour evolution. For several cichlid species from lakes Malawi and Victoria, it has been described that males show more aggressive behaviour against similarly coloured opponents than against those that show different colour [72, 73]. Therefore, differently coloured males may potentially have a selective advantage, an effect that could eventually lead to speciation via colour morph separation [73, 74].
The genetic basis of coat colour variation: the MC1R case
As noted above, more than 150 genes have been described to date that play a role in colour determination. However, for a variety of reasons, only a much more limited number are generally studied as candidate genes in non-model organisms that show colour variation. One of the best examples for this is MC1R. With the advent of large, publicly available genomic data sets and the completion of numerous vertebrate genome sequences, there has been much effort to identify the origin and to follow the evolutionary history of the endocrine melanocortin system. Although its importance for pigmentation was demonstrated at the molecular level more than 15 years ago [75], the interest of the scientific community in its relevance in adaptation processes and diseases not only in humans but also vertebrates from fish to mammals is still growing. Therefore, in the following section we will discuss examples for colour variation that have been studied specifically with regard to variation in MC1R sequence and for which the molecular basis could, or could not, be determined.
During the last few years, the number of studies that discuss coat colour variation in non-domesticated species as well as the genetics underlying variation has increased almost exponentially [76]. A major reason for this has been the concentration on the MC1R gene, which seems to be involved in colour variation in a disproportional fraction of the studies. In 2005, Garcia-Borron and colleagues listed already 60 natural variants of the MC1R gene [77], and this number has increased considerably since then. Moreover, MC1R is a short gene, consisting of only about 350 amino acids, contains a single protein-encoding exon, and functional tests are established, making it an ideal target for molecular studies. It becomes increasingly popular to screen for variants in MC1R and in its antagonist/inverse agonist, the vertebrate ASIP [28, 29], which consists of 4 exons with a total protein size of about 130 amino acids [78–80]. Finally, MC1R plays a role in pigmentation in a large group of species ranging from fishes, amphibians, reptiles across birds to mammals [81], and in contrast to some other genes involved in colour determination such as the KIT gene, where mutations may be lethal at least in the homozygote state [82], generally neither activating nor inactivating mutations in MC1R have major pleiotropic effects. In fact, researchers have become so accustomed to MC1R being responsible for colour differences that research articles have started to state already in the abstract when MC1R is NOT responsible for an observed colour difference (e.g., [29]).
The skin colouration effect of α-MSH is well established for fish, amphibians and reptiles since decades [83]. Consistently, MC1R, the other four fish orthologs of the mammalian melanocortin receptor family (MC2R-MC5R), ASIP and POMC have been cloned and sequenced in multiple fish species [79, 84, 85]. This indicates that the different components of the melanocortin system were already present before the radiation of gnathostomes, but melanocortin receptors were not yet found in non-chordate species. The action of the melanocortin system has been implicated in fish dorsal ventral pigmentation and background adaptation in several teleost fishes [79, 86]. The pharmacological properties of fish MC1R and its peptide agonists appear to be very similar when compared to the mammalian system [79, 85]. MC1R is a single copy gene in all fishes investigated so far [81]. Until recently, there was no report on the variability of MC1R or other melanocortin system components in fishes. Using a QTL approach combined with sequence and functional analyses, two distinct genetic alterations in the coding sequence of the gene Mc1r were found to cause reduced pigmentation in Mexican cave fishes [87]. Interestingly, the depigmented phenotype has arisen independently in geographically separate caves, mediated through different mutations of the same gene and probably due to loss-of-constraint—a perfect example for parallel evolution targeting one gene.
Although Xenopus melanophores are frequently used to bioassay MC1R function [88, 89], there is no report to our best knowledge on variants in the melanocortin receptor or signalling pathways in amphibians. There are several albino phenotypes in for example axolotl and blind cave salamander, but the contribution of the melanocortin system to depigmented phenotypes, as shown in cave fishes [87], is not analysed yet.
In reptiles and birds several intraspecies colouration differences were traced to MC1R variants. For example, melanic or blanched forms of lizard species living in the Chihuahuan Desert [50] of New Mexico were associated with partially inactive MC1R variants. There are numerous examples in birds that suggest that intraspecies variations in colour are associated with MC1R variants. For example, melanic plumage in swans is related to amino acid changes at important functional sites in MC1R that are consistent with increased MC1R activity and melanism. Since the putative melanizing mutations were independently derived in the two melanic swan lineages, this is another example of convergent evolution at MC1R [90]. Similarly, red-footed booby [91], quails [92, 93], fairy-wrens [94], bananaquit [95] and chicken [17] carry MC1R variants that correlate with melanic plumage. Melanism and MC1R variants are strongly associated in geese and skuas, with melanism being the derived trait that seems to have evolved during the Pleistocene [96]. For both species, positive assortative mating of the colour morphs has been described [97, 98]. Evidence for sexual selection affecting the MC1R locus was also found for some galliform birds [99].
There are also numerous examples for MC1R mutations being causal for colour variations in mammals, ranging from humans over mice to wolves, bears and various cat species. For example, melanistic coat colouration occurs as a common polymorphism in 11 of 37 felid species and reaches high population frequency in some cases, but never achieves complete fixation [100]. Association and transmission analyses showed that a 2-bp deletion in the ASIP gene specifies black colouration in domestic cats, and two different in-frame deletions in the MC1R gene are implicated in melanism in jaguars and jaguarondis [100]. Because melanistic individuals from other felid species did not carry any of these mutations, there must be additional independent genetic origins for melanism in the cat family.
Coat-colour polymorphisms based on MC1R variants were identified also in the extinct Pleistocene mammoth (Mammuthus primigenius) [101]. One of these, Arg67Cys, is carried at the homologous sequence position by light-coloured populations of the beach mouse, which have lighter coloured coats than their inland counterparts, driven by natural selection for camouflage against the pale sand dunes (Fig. 4). Functional tests and crossing experiments revealed both a reduction in basal and induced activity highly similar to that observed for the mammoth MC1R protein and a strong association between this amino acid polymorphism and adaptive coat colour phenotype [46].
Humans are probably the best studied species for pigment variations. Thus, a number of genome-wide association studies have been conducted in humans to identify candidate genes involved in pigmentation. SNP polymorphisms in a number of genes such as TYR, SLC24A4, SLC45A2, SLC24A5, ASIP, OCA2, KITLG and HERC2 present in human populations can account for differences between those of darkest and lightest skin [9, 102–104]. Blue-brown eye colour can be explained by a single SNP in the HERC2 locus proposed to regulate OCA2 expression [105, 106]. However, the best correlations between the biochemical signalling properties of the encoded receptor and the red-hair fair skin pigmentation phenotype are again shown by variant MC1R alleles. Over 150 sequence variations at the human MC1R locus, including about 90 missense, non-sense and frame shifting mutations, have been reported so far [22, 23]. Interestingly, these studies also show that there is no ‘hot spot’ for mutations in MC1R. Missense mutations often lead to altered receptor trafficking and loss of high affinity binding of MSH [107]. Many of the variant MC1R found to be functionally impaired have been associated with red hair and pale skin in humans. The majority of red-haired individuals are compound heterozygotes or homozygotes for loss-of-function mutations [108]. In contrast to other species, activating mutations in MC1R, such as those identified in dark-coat coloured mice [18], have not yet been described in humans. The worldwide pattern of MC1R diversity indicates functional constraint and consequently purifying selection in African populations, whereas the greater allelic diversity seen in non-African populations seems to be consistent with neutrality rather than with positive selection [109]. Its key role in regulating skin pigmentation makes MC1R a major determinant of sun sensitivity and, therefore, a genetic risk factor for melanoma and non-melanoma type skin cancer [110–112]. This function may be an important selective force that confers the functional constraint of MC1R in regions with high UV irradiation.
Differences in skin and hair pigmentation due to functional MC1R variants are not only a phenomenon in humans of European origin during recent times. Lalueza-Fox and co-workers [113] amplified and sequenced a fragment of the MC1R gene from two Neanderthal remains. Both specimens had a mutation (Arg307Gly) that was not found in approximately 3,700 modern humans analysed. Functional analyses revealed that this variant reduces MC1R activity to a level that alters hair and/or skin pigmentation in modern humans. The impaired activity of this variant suggested that Neanderthals also varied in pigmentation levels and inactive MC1R variants evolved independently in both modern humans and Neanderthals.
Conclusion
The wide variety of colours and colour patterns found in nature is realised by concerted action of more than 150 genes that realise and influence pigmentation. Nucleotide changes in these genes are responsible for different pigmentation with such diverse effects as avoidance of predation, conspicuous attraction or optical warning. Ultimately, deviating pigmentation may allow adaptation to different environments and even result in speciation. Changes in a single coat colour-determining gene and sometimes even a single nucleotide can be responsible for both divergent and convergent evolution of different species. Vice versa, similar or almost identical phenotypes even in the same species may be due to mutations in different genes. We have progressed quite far in understanding some of the components involved in pigmentation such as the melanocortin system, but future studies of Mother Nature’s paint box will surely reveal further fascinating causalities of vertebrate evolution.
References
Otaki JM (2008) Physiologically induced color-pattern changes in butterfly wings: mechanistic and evolutionary implications. J Insect Physiol 54:1099–1112
Parchem RJ, Perry MW, Patel NH (2007) Patterns on the insect wing. Curr Opin Genet Dev 17:300–308
Papa R, Martin A, Reed RD (2008) Genomic hotspots of adaptation in butterfly wing pattern evolution. Curr Opin Genet Dev 18:559–564
O’Grady PM, DeSalle R (2000) How the fruit fly changed (some of) its spots. Curr Biol 10:R75–R77
Oetting WS, Austin LM, Bennett DC (2009) Color genes: European Society for Pigment Cell Research. http://www.espcr.org/micemut/
Simon JD, Peles D, Wakamatsu K, Ito S (2009) Current challenges in understanding melanogenesis: bridging chemistry, biological control, morphology, and function. Pigment Cell Melanoma Res 22:563–579
Barsh G, Cotsarelis G (2007) How hair gets its pigment. Cell 130:779–781
Weiner L, Han R, Scicchitano BM, Li J, Hasegawa K, Grossi M, Lee D, Brissette JL (2007) Dedicated epithelial recipient cells determine pigmentation patterns. Cell 130:932–942
Sturm RA (2009) Molecular genetics of human pigmentation diversity. Hum Mol Genet 18:R9–R17
Ito S, Wakamatsu K (2008) Chemistry of mixed melanogenesis: pivotal roles of dopaquinone. Photochem Photobiol 84:582–592
Slominski A, Tobin DJ, Shibahara S, Wortsman J (2004) Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol Rev 84:1155–1228
Schallreuter KU (2007) Advances in melanocyte basic science research. Dermatol Clin 25:283–291 vii
Cone RD, Lu D, Koppula S, Vage DI, Klungland H, Boston B, Chen W, Orth DN, Pouton C, Kesterson RA (1996) The melanocortin receptors: agonists, antagonists, and the hormonal control of pigmentation. Recent Prog Horm Res 51:287–317 discussion 318
Fredriksson R, Schioth HB (2005) The repertoire of G-protein-coupled receptors in fully sequenced genomes. Mol Pharmacol 67:1414–1425
Rees JL (2003) Genetics of hair and skin color. Annu Rev Genet 37:67–90
Ha T, Naysmith L, Waterston K, Oh C, Weller R, Rees JL (2003) Defining the quantitative contribution of the melanocortin 1 receptor (MC1R) to variation in pigmentary phenotype. Ann N Y Acad Sci 994:339–347
Kerje S, Lind J, Schutz K, Jensen P, Andersson L (2003) Melanocortin 1-receptor (MC1R) mutations are associated with plumage colour in chicken. Anim Genet 34:241–248
Robbins LS, Nadeau JH, Johnson KR, Kelly MA, Roselli-Rehfuss L, Baack E, Mountjoy KG, Cone RD (1993) Pigmentation phenotypes of variant extension locus alleles result from point mutations that alter MSH receptor function. Cell 72:827–834
Kijas JM, Wales R, Tornsten A, Chardon P, Moller M, Andersson L (1998) Melanocortin receptor 1 (MC1R) mutations and coat color in pigs. Genetics 150:1177–1185
Vage DI, Klungland H, Lu D, Cone RD (1999) Molecular and pharmacological characterization of dominant black coat color in sheep. Mamm Genome 10:39–43
Vage DI, Lu D, Klungland H, Lien S, Adalsteinsson S, Cone RD (1997) A non-epistatic interaction of agouti and extension in the fox, Vulpes vulpes. Nat Genet 15:311–315
Kazius J, Wurdinger K, van Iterson M, Kok J, Back T, Ijzerman AP (2008) GPCR NaVa database: natural variants in human G protein-coupled receptors. Hum Mutat 29:39–44
Kanetsky PA, Rebbeck TR, Hummer AJ, Panossian S, Armstrong BK, Kricker A, Marrett LD, Millikan RC, Gruber SB, Culver HA, Zanetti R, Gallagher RP, Dwyer T, Busam K, From L, Mujumdar U, Wilcox H, Begg CB, Berwick M (2006) Population-based study of natural variation in the melanocortin-1 receptor gene and melanoma. Cancer Res 66:9330–9337
Lefkowitz RJ, Cotecchia S, Samama P, Costa T (1993) Constitutive activity of receptors coupled to guanine nucleotide regulatory proteins. Trends Pharmacol Sci 14:303–307
Haitina T, Ringholm A, Kelly J, Mundy NI, Schioth HB (2007) High diversity in functional properties of melanocortin 1 receptor (MC1R) in divergent primate species is more strongly associated with phylogeny than coat color. Mol Biol Evol 24:2001–2008
Schaffer JV, Bolognia JL (2001) The melanocortin-1 receptor: red hair and beyond. Arch Dermatol 137:1477–1485
Norris BJ, Whan VA (2008) A gene duplication affecting expression of the ovine ASIP gene is responsible for white and black sheep. Genome Res 18:1282–1293
Linnen CR, Kingsley EP, Jensen JD, Hoekstra HE (2009) On the origin and spread of an adaptive allele in deer mice. Science 325:1095–1098
Kingsley EP, Manceau M, Wiley CD, Hoekstra HE (2009) Melanism in peromyscus is caused by independent mutations in agouti. PLoS One 4:e6435
Slominski A, Plonka PM, Pisarchik A, Smart JL, Tolle V, Wortsman J, Low MJ (2005) Preservation of eumelanin hair pigmentation in proopiomelanocortin-deficient mice on a nonagouti (a/a) genetic background. Endocrinology 146:1245–1253
Candille SI, Kaelin CB, Cattanach BM, Yu B, Thompson DA, Nix MA, Kerns JA, Schmutz SM, Millhauser GL, Barsh GS (2007) A -defensin mutation causes black coat color in domestic dogs. Science 318:1418–1423
Anderson TM, vonHoldt BM, Candille SI, Musiani M, Greco C, Stahler,DR, Smith DW, Padhukasahasram B, Randi E, Leonard JA, Bustamante CD, Ostrander EA, Tang H, Wayne RK, Barsh GS (2009) Molecular and evolutionary history of melanism in North American gray wolves. Science 323:1339–1343
Bassi MT, Schiaffino MV, Renieri A, De Nigris F, Galli L, Bruttini M, Gebbia M, Bergen AA, Lewis RA, Ballabio A (1995) Cloning of the gene for ocular albinism type 1 from the distal short arm of the X chromosome. Nat Genet 10:13–19
Lopez VM, Decatur CL, Stamer WD, Lynch RM, McKay BS (2008) l-DOPA is an endogenous ligand for OA1. PLoS Biol 6:e236
Schiaffino MV, Bassi MT, Galli L, Renieri A, Bruttini M, De Nigris F, Bergen AA, Charles SJ, Yates JR, Meindl A et al (1995) Analysis of the OA1 gene reveals mutations in only one-third of patients with X-linked ocular albinism. Hum Mol Genet 4:2319–2325
Incerti B, Cortese K, Pizzigoni A, Surace EM, Varani S, Coppola M, Jeffery G, Seeliger M, Jaissle G, Bennett DC, Marigo V, Schiaffino MV, Tacchetti C, Ballabio A (2000) Oa1 knock-out: new insights on the pathogenesis of ocular albinism type 1. Hum Mol Genet 9:2781–2788
Protas ME, Hersey C, Kochanek D, Zhou Y, Wilkens H, Jeffery WR, Zon LI, Borowsky R, Tabin CJ (2006) Genetic analysis of cavefish reveals molecular convergence in the evolution of albinism. Nat Genet 38:107–111
Fukamachi S, Shimada A, Shima A (2001) Mutations in the gene encoding B, a novel transporter protein, reduce melanin content in medaka. Nat Genet 28:381–385
Haase B, Brooks SA, Schlumbaum A, Azor PJ, Bailey E, Alaeddine F, Mevissen M, Burger D, Poncet PA, Rieder S, Leeb T (2007) Allelic heterogeneity at the equine KIT locus in dominant white (W) horses. PLoS Genet 3:e195
Geissler EN, Ryan MA, Housman DE (1988) The dominant-white spotting (W) locus of the mouse encodes the c-kit proto-oncogene. Cell 55:185–192
Albert FW, Carlborg O, Plyusnina I, Besnier F, Hedwig D, Lautenschläger S, Lorenz D, McIntosh J, Neumann C, Richter H, Zeising C, Kozhemyakina R, Shchepina O, Kratzsch J, Trut L, Teupser D, Thiery J, Schöneberg T, Andersson L, Päbo S (2009) Genetic architecture of tameness in a rat model of animal domestication. Genetics 182:541–554
Johansson Moller M, Chaudhary R, Hellmen E, Hoyheim B, Chowdhary B, Andersson L (1996) Pigs with the dominant white coat color phenotype carry a duplication of the KIT gene encoding the mast/stem cell growth factor receptor. Mamm Genome 7:822–830
Goda M, Fujii R (1998) The blue coloration of the common surgeonfish, Paracanthurus hepatus-II: color revelation and color changes. Zool Sci 15:323–333
Nachman MW, Hoekstra HE, D’Agostino SL (2003) The genetic basis of adaptive melanism in pocket mice. Proc Natl Acad Sci USA 100:5268–5273
Hoekstra HE, Nachman MW (2003) Different genes underlie adaptive melanism in different populations of rock pocket mice. Mol Ecol 12:1185–1194
Hoekstra HE, Hirschmann RJ, Bundey RA, Insel PA, Crossland JP (2006) A single amino acid mutation contributes to adaptive beach mouse color pattern. Science 313:101–104
Rosenblum EB, Hoekstra HE, Nachman MW (2004) Adaptive reptile color variation and the evolution of the Mc1r gene. Evolution 58:1794–1808
Dice L, Blossom PM (1937) Studies of mammalian ecology in southwestern North America, with special attention to the colors of desert mammals. Publ Carnegie Inst Washington 485:1–25
Kaufmann DW (1974) Adaptive coloration in Peromyscus polionotus: experimental selection by owls. J Mammal 55:271–283
Rosenblum EB, Römpler H, Schöneberg T, Hoekstra HE (2010) Molecular and functional basis of phenotypic convergence in white lizards at White Sands. Proc Natl Acad Sci USA 107:2113–2117
Price AC, Weadick CJ, Shim J, Rodd FH (2008) Pigments, patterns, and fish behavior. Zebrafish 5:297–307
Marshall NJ, Vorobyev M (2003) The design of color signals and color vision in fishes. In: Collin SP, Marshall NJ (eds) Sensory Processing in Aquatic Environments. Springer, New York, pp 194–222
Leonard JA, Wayne RK, Wheeler J, Valadez R, Guillen S, Vila C (2002) Ancient DNA evidence for old world origin of new world dogs. Science 298:1613–1616
Olendorf R, Rodd FH, Punzalan D, Houde AE, Hurt C, Reznick DN, Hughes KA (2006) Frequency-dependent survival in natural guppy populations. Nature 441:633–636
Eakley AL, Houde AE (2004) Possible role of female discrimination against ‘redundant’ males in the evolution of colour pattern polymorphism in guppies. Proc Biol Sci 271(Suppl 5):S299–S301
Zajitschek SR, Brooks RC (2008) Distinguishing the effects of familiarity, relatedness, and color pattern rarity on attractiveness and measuring their effects on sexual selection in guppies (Poecilia reticulata). Am Nat 172:843–854
Pierotti MER, Seehausen O (2006) Male mating preferences pre-date the origin of a female trait polymorphism in an incipient species complex of Lake Victoria cichlids. J Evol Biol 20:240–248
Morris MR, Rios-Cardenas O, Scarlett Tudor M (2006) Larger swordtail females prefer asymmetrical males. Biol Lett 2:8–11
Roulin A (2004) The evolution, maintenance and adaptive function of genetic colour polymorphism in birds. Biol Rev Camb Philos Soc 79:815–848
Marshall HD, Ritland K (2002) Genetic diversity and differentiation of Kermode bear populations. Mol Ecol 11:685–697
Seehausen O, Terai Y, Magalhaes IS, Carleton KL, Mrosso HD, Miyagi R, van der Sluijs I, Schneider MV, Maan ME, Tachida H, Imai H, Okada N (2008) Speciation through sensory drive in cichlid fish. Nature 455:620–626
Schluter D, Price T (1993) Honesty, perception and population divergence in sexually selected traits. Proc Biol Sci 253:117–122
Boughman JW (2001) Divergent sexual selection enhances reproductive isolation in sticklebacks. Nature 411:944–948
Genner MJ, Nichols P, Carvalho GR, Robinson RL, Shaw PW, Turner GF (2007) Reproductive isolation among deep-water cichlid fishes of Lake Malawi differing in monochromatic male breeding dress. Mol Ecol 16:651–662
Rodd FH, Hughes KA, Grether GF, Baril CT (2002) A possible non-sexual origin of mate preference: are male guppies mimicking fruit? Proc Biol Sci 269:475–481
Barber I, Arnott SA, Braithwaite VA, Andrew J, Huntingford FA (2001) Indirect fitness consequences of mate choice in sticklebacks: offspring of brighter males grow slowly but resist parasitic infections. Proc Biol Sci 268:71–76
Milinski M, Bakker TCM (1990) Female sticklebacks use male coloration in mate choice and hence avoid parasitized males. Nature 344:330–333
Bakker TCM and Mundwiler B (1994). Female mate choice and male red colouration in a natural three-spined stickleback (Gasterosteus aculeatus) population. Behav Ecol 5:74–80
Grether GF, Kolluru GR, Rodd FH, de la Cerda J, Shimazaki K (2005) Carotenoid availability affects the development of a colour-based mate preference and the sensory bias to which it is genetically linked. Proc Biol Sci 272:2181–2188
Candolin U, Salesto T, Evers M (2007) Changed environmental conditions weaken sexual selection in sticklebacks. J Evol Biol 20:233–239
Jarvenpaa M, Lindstrom K (2004) Water turbidity by algal blooms causes mating system breakdown in a shallow-water fish, the sand goby Pomatoschistus minutus. Proc Biol Sci 271:2361–2365
Pauers MJ, Kapfer JM, Fendos CE, Berg CS (2008) Aggressive biases towards similarly coloured males in Lake Malawi cichlid fishes. Biol Lett 4:156–159
Seehausen O, Schluter D (2004) Male-male competition and nuptial-colour displacement as a diversifying force in Lake Victoria cichlid fishes. Proc Biol Sci 271:1345–1353
Turner GF, Burrows MT (1995) A model of sympatric speciation by sexual selection. Proc R Soc Lond B 260:287–292
Mountjoy KG, Robbins LS, Mortrud MT, Cone RD (1992) The cloning of a family of genes that encode the melanocortin receptors. Science 257:1248–1251
Hoekstra HE (2006) Genetics, development and evolution of adaptive pigmentation in vertebrates. Heredity 97:222–234
Garcia-Borron JC, Sanchez-Laorden BL, Jimenez-Cervantes C (2005) Melanocortin-1 receptor structure and functional regulation. Pigment Cell Res 18:393–410
Bultman SJ, Michaud EJ, Woychik RP (1992) Molecular characterization of the mouse agouti locus. Cell 71:1195–1204
Cerda-Reverter JM, Haitina T, Schioth HB, Peter RE (2005) Gene structure of the goldfish agouti-signaling protein: a putative role in the dorsal-ventral pigment pattern of fish. Endocrinology 146:1597–1610
Klovins J, Schioth HB (2005) Agouti-related proteins (AGRPs) and agouti-signaling peptide (ASIP) in fish and chicken. Ann N Y Acad Sci 1040:363–367
Selz Y, Braasch I, Hoffmann C, Schmidt C, Schultheis C, Schartl M, Volff JN (2007) Evolution of melanocortin receptors in teleost fish: the melanocortin type 1 receptor. Gene 401:114–122
Nocka K, Majumder S, Chabot B, Ray P, Cervone M, Bernstein A, Besmer P (1989) Expression of c-kit gene products in known cellular targets of W mutations in normal and W mutant mice–evidence for an impaired c-kit kinase in mutant mice. Genes Dev 3:816–826
Bagnara JT, Hadley ME (1973) Chromatophores and colour change: the comparative physiology of animal pigmentation. Prentice Hall, New Jersey
Metz JR, Peters JJ, Flik G (2006) Molecular biology and physiology of the melanocortin system in fish: a review. Gen Comp Endocrinol 148:150–162
Klovins J, Haitina T, Fridmanis D, Kilianova Z, Kapa I, Fredriksson R, Gallo-Payet N, Schioth HB (2004) The melanocortin system in Fugu: determination of POMC/AGRP/MCR gene repertoire and synteny, as well as pharmacology and anatomical distribution of the MCRs. Mol Biol Evol 21:563–579
van der Salm AL, Metz JR, Bonga SE, Flik G (2005) Alpha-MSH, the melanocortin-1 receptor and background adaptation in the Mozambique tilapia, Oreochromis mossambicus. Gen Comp Endocrinol 144:140–149
Gross JB, Borowsky R, Tabin CJ (2009) A novel role for Mc1r in the parallel evolution of depigmentation in independent populations of the cavefish Astyanax mexicanus. PLoS Genet 5:e1000326
Ollmann MM, Lamoreux ML, Wilson BD, Barsh GS (1998) Interaction of Agouti protein with the melanocortin 1 receptor in vitro and in vivo. Genes Dev 12:316–330
Barsh G, Gunn T, He L, Schlossman S, Duke-Cohan J (2000) Biochemical and genetic studies of pigment-type switching. Pigment Cell Res 13(Suppl 8):48–53
Pointer MA, Mundy NI (2008) Testing whether macroevolution follows microevolution: are colour differences among swans (Cygnus) attributable to variation at the MCIR locus? BMC Evol Biol 8:249
Baiao PC, Schreiber E, Parker PG (2007) The genetic basis of the plumage polymorphism in red-footed boobies (Sula sula): a melanocortin-1 receptor (MC1R) analysis. J Hered 98:287–292
Nadeau NJ, Mundy NI, Gourichon D, Minvielle F (2007) Association of a single-nucleotide substitution in TYRP1 with roux in Japanese quail (Coturnix japonica). Anim Genet 38:609–613
Nadeau NJ, Minvielle F, Mundy NI (2006) Association of a Glu92Lys substitution in MC1R with extended brown in Japanese quail (Coturnix japonica). Anim Genet 37:287–289
Doucet SM, Shawkey MD, Rathburn MK, Mays HL Jr, Montgomerie R (2004) Concordant evolution of plumage colour, feather microstructure and a melanocortin receptor gene between mainland and island populations of a fairy-wren. Proc Biol Sci 271:1663–1670
Theron E, Hawkins K, Bermingham E, Ricklefs RE, Mundy NI (2001) The molecular basis of an avian plumage polymorphism in the wild: a melanocortin-1-receptor point mutation is perfectly associated with the melanic plumage morph of the bananaquit, Coereba flaveola. Curr Biol 11:550–557
Mundy NI, Badcock NS, Hart T, Scribner K, Janssen K, Nadeau NJ (2004) Conserved genetic basis of a quantitative plumage trait involved in mate choice. Science 303:1870–1873
Cooke F, Finney GH, Rockwell RF (1976) Assortative mating in lesser snow geese (Anser caerulescens). Behav Genet 6:127–140
Phillips RA, Furness RW (1998) Polymorphism, mating preferences and sexual selection in the Arctic skua. J Zool 245:245–252
Nadeau NJ, Burke T, Mundy NI (2007) Evolution of an avian pigmentation gene correlates with a measure of sexual selection. Proc Biol Sci 274:1807–1813
Eizirik E, Yuhki N, Johnson WE, Menotti-Raymond M, Hannah SS, O’Brien SJ (2003) Molecular genetics and evolution of melanism in the cat family. Curr Biol 13:448–453
Römpler H, Rohland N, Lalueza-Fox C, Willerslev E, Kuznetsova T, Rabeder G, Bertranpetit J, Schöneberg T, Hofreiter M (2006) Nuclear gene indicates coat-color polymorphism in mammoths. Science 313:62
Duffy DL, Zhao ZZ, Sturm RA, Hayward NK, Martin NG, Montgomery GW (2010) Multiple pigmentation gene polymorphisms account for a substantial proportion of risk of cutaneous malignant melanoma. J Invest Dermatol 130:520–528
Han J, Kraft P, Nan H, Guo Q, Chen C, Qureshi A, Hankinson SE, Hu FB, Duffy DL, Zhao ZZ, Martin NG, Montgomery GW, Hayward NK, Thomas G, Hoover RN, Chanock S, Hunter DJ (2008) A genome-wide association study identifies novel alleles associated with hair color and skin pigmentation. PLoS Genet 4:e1000074
Sulem P, Gudbjartsson DF, Stacey SN, Helgason A, Rafnar T, Magnusson KP, Manolescu A, Karason A, Palsson A, Thorleifsson G, Jakobsdottir M, Steinberg S, Palsson S, Jonasson F, Sigurgeirsson B, Thorisdottir K, Ragnarsson R, Benediktsdottir KR, Aben KK, Kiemeney LA, Olafsson JH, Gulcher J, Kong A, Thorsteinsdottir U, Stefansson K (2007) Genetic determinants of hair, eye and skin pigmentation in Europeans. Nat Genet 39:1443–1452
Sturm RA, Frudakis TN (2004) Eye colour: portals into pigmentation genes and ancestry. Trends Genet 20:327–332
Eiberg H, Troelsen J, Nielsen M, Mikkelsen A, Mengel-From J, Kjaer KW, Hansen L (2008) Blue eye color in humans may be caused by a perfectly associated founder mutation in a regulatory element located within the HERC2 gene inhibiting OCA2 expression. Hum Genet 123:177–187
Schöneberg T, Schulz A, Biebermann H, Hermsdorf T, Rompler H, Sangkuhl K (2004) Mutant G-protein-coupled receptors as a cause of human diseases. Pharmacol Ther 104:173–206
Flanagan N, Healy E, Ray A, Philips S, Todd C, Jackson IJ, Birch-Machin MA, Rees JL (2000) Pleiotropic effects of the melanocortin 1 receptor (MC1R) gene on human pigmentation. Hum Mol Genet 9:2531–2537
Harding RM, Healy E, Ray AJ, Ellis NS, Flanagan N, Todd C, Dixon C, Sajantila A, Jackson IJ, Birch-Machin MA, Rees JL (2000) Evidence for variable selective pressures at MC1R. Am J Hum Genet 66:1351–1361
Meyle KD, Guldberg P (2009) Genetic risk factors for melanoma. Hum Genet 126:499–510
Ibrahim N, Haluska FG (2009) Molecular pathogenesis of cutaneous melanocytic neoplasms. Annu Rev Pathol 4:551–579
Landi MT, Bauer J, Pfeiffer RM, Elder DE, Hulley B, Minghetti P, Calista D, Kanetsky PA, Pinkel D, Bastian BC (2006) MC1R germline variants confer risk for BRAF-mutant melanoma. Science 313:521–522
Lalueza-Fox C, Römpler H, Caramelli D, Stäubert C, Catalano G, Hughes D, Rohland N, Pilli E, Longo L, Condemi S, de la Rasilla M, Fortea J, Rosas A, Stoneking M, Schöneberg T, Bertranpetit J, Hofreiter M (2007) A melanocortin 1 receptor allele suggests varying pigmentation among Neanderthals. Science 318:1453–1455
Acknowledgments
We thank Brenda Bradley, Hopi Hoekstra and Ole Seehausen for providing pictures and Regina Querner for help with the figure design. This work was funded by the DFG, the Max Planck Society and the University of York.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hofreiter, M., Schöneberg, T. The genetic and evolutionary basis of colour variation in vertebrates. Cell. Mol. Life Sci. 67, 2591–2603 (2010). https://doi.org/10.1007/s00018-010-0333-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-010-0333-7