Abstract
The synapsins, the first identified synaptic vesicle-specific proteins, are phosphorylated on multiple sites by a number of protein kinases and are involved in neurite outgrowth and synapse formation as well as in synaptic transmission. In mammals, the synapsin family consists of at least 10 isoforms encoded by 3 distinct genes and composed by a mosaic of conserved and variable domains. The synapsins are highly conserved evolutionarily, and orthologues have been found in invertebrates and lower vertebrates. Within nerve terminals, synapsins are implicated in multiple interactions with presynaptic proteins and the actin cytoskeleton. Via these interactions, synapsins control several mechanisms important for neuronal homeostasis. In this review, we describe the main functional features of the synapsins, in relation to the complex role played by these phosphoproteins in neuronal development.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The remarkable spatial and temporal precision of the process of neurotransmitter release is generated and maintained by the activity of a number of proteins that are localized within the presynaptic terminal, interact with each other in a complex network, and participate in synapse formation, maintenance, and function. Among several presynaptic players identified so far, the synapsins, the most abundant family of neuron-specific, synaptic vesicle (SV)-associated phosphoproteins, control multiple steps of the SV life cycle and participate in developmental processes that precede the formation of mature nerve terminals.
Synapsins are present in all organisms endowed with a nervous system and, in mammals, are encoded by three distinct genes (synI, synII, and synIII) located on chromosomes X, 3, and 22, respectively. Alternative splicing generates distinct isoforms (Fig. 1), termed synapsins a, b, and b-like. These transcripts are composed of a mosaic of individual and shared domains highly conserved during evolution [1–3]. Synapsins I and II are selectively expressed at nerve terminals in mature neurons [4], where they associate with the cytoplasmic surface of small SVs [5, 6], by binding to both phospholipid and protein components of the vesicle membrane in a manner modulated by site-specific phosphorylation [7, 8]. In contrast, the expression of synapsin III is downregulated in mature neurons (Fig. 2a), and the protein is not strictly confined to synaptic terminals [9, 10]. Synapsins are the substrate of several protein kinases, including cyclic AMP-dependent protein kinase (PKA), Ca2+/calmodulin-dependent protein kinases (CaMK) -I, -II, and -IV, mitogen-activated protein kinase (MAPK) Erk1/2, and cyclin-dependent kinase 5 (Cdk5), that phosphorylate them on distinct serine residues, as well as for the tyrosine kinase Src. These phosphorylation events modulate the interactions of synapsins with lipid and protein components of SVs [7], as well as with various cytoskeletal proteins including actin [11–13], and are key determinants of synapsin function in developing neurons and mature terminals [3, 14].
Schematic protein domain model of the mammalian synapsin family with functional properties and protein kinase phosphorylation sites. Within domain A, site 1 (Ser9 in synapsin I) is phosphorylated by PKA and CaMKI/IV [33–35]. Within domain B, sites 4 and 5 (Ser62 and Ser67, respectively) are phosphorylated by MAPKs [36]. All synapsin isoforms have a conserved tyrosine in domain C (site 8) which has been shown to be phosphorylated by the tyrosine kinase Src in synapsins I and II [29]. Domain C also contains putative ATP binding residues [26]. The D domain includes the proline-rich strand binding SH3 domains [29, 89] and the phosphorylation sites 2 and 3 (Ser566 and Ser603, respectively) that are phosphorylated by CaMKII [35]. Within domain D, site 6 (Ser549) is phosphorylated by MAPKs, Cdk1/5 [36, 37]. The latter kinase also phosphorylates an adjacent site (site 7, Ser551 in synapsin I) [37, 38]. Only one isoform is represented for Syn III, although multiple synIII products have been described in the adult brain [90]
Temporal expression pattern of synapsin isoforms. a Expression level during in vitro development of cultured hippocampal neurons of synapsins I, II, and III. Neuronal extracts were prepared from E16 mouse dissociated hippocampal neurons kept in culture for up to 30 days in vitro (DIV). The proteins were separated by electrophoresis and immunoblots were reacted with specific antibodies and densitometrically analyzed. Results are plotted as percentage of the highest level detected. While synapsin III has the highest peak of expression at 7 DIV, when synaptogenesis is still ongoing, synapsins I and II levels increase with the onset of synaptic activity. Modified with permission from [91]; based on data from [9]. b Expression of synapsin III and lineage markers during development of neuronal precursor cells. The mitotic markers PCNA and Ki67 are expressed during proliferation; nestin is expressed before the cells become committed to either the neuronal or the glial lineage; GFAP is expressed in undifferentiated precursors and mature glial cells; PSA-NCAM is expressed in immature neurons, whereas NeuN is expressed in mature neurons. Tuj1 begins its expression in immature neurons and persists in mature neurons. The temporal expression profile for synapsin III is based on its colocalization with the other established markers [10]. Note the precocious expression of synapsin III, which is already present in nestin-positive cells prior to neuronal commitment. Reprinted with permission of John Wiley and Sons from [10]
Since synapsins are able to interact in vitro with various cytoskeletal proteins, such as actin, and oligomerize, they have been proposed to contribute to the clustering of SVs and to their attachment to the actin-based cytoskeleton [12, 13, 15, 16]. Perturbation of synapsin function in a variety of preparations leads to the disruption of the organization of SVs in the presynaptic compartment and to an increase in synaptic depression, underlining the role of synapsins in sustaining neurotransmitter release in response to high levels of neuronal activity [17–20]. Recent studies indicate that, in addition to affecting the SV pools, perturbation of synapsin activity leads to an inhibition and slowing of neurotransmitter release kinetics [14, 21–23] and to an imbalance between the activities of excitatory and inhibitory neurons [19, 24], suggesting additional roles for synapsins at the active zone.
Besides the well-documented role at mature synapses, a plethora of data implicates synapsins in neuronal development, from the early stages of neurite sprouting to the regulation of synapse formation and refinement. However, a clear picture of synapsin function in neuronal development has not yet emerged and a unitary model has not been formulated, likely because of the large and heterogeneous body of experimental evidence derived from distinct approaches and model systems. Here, we review this vast literature in the attempt to provide a conceptual framework that facilitates further investigations of the role of synapsins in the developing nervous system.
Structural organization of the synapsins
Three regions are shared by vertebrate and invertebrate synapsins. At the amino-terminus (domain A), all synapsins share consensus phosphorylation sites for PKA and CaMKI/IV. The high conservation of the site across isoforms and orthologues points to the functional relevance of its phosphorylation [25].
The most extensive homology is found within domain C, a large central region of about 300 amino acids. The elucidation of the domain C crystal structure suggests the possibility that synapsins exhibit enzymatic properties. Indeed, more than 80% of the amino acid backbone of synapsin I domain C is superimposable with the structure of a group of ATP-dependent synthetases [26]. In addition, recombinant domain C regions from all the three synapsin isoforms are able to bind ATP in vitro, although with a distinct Ca2+-dependent regulation. The C domain of all synapsin isoforms is able to induce the formation of homo- and hetero-dimers, even if with variable efficiency, thus giving rise to different combinations of synapsin dimers on the synaptic vesicle surface [27, 28]. Moreover, the domain C of all synapsins displays a highly conserved phosphorylation site for the tyrosine kinase Src [29].
Although derived from three distinct genes by alternative splicing mechanisms, the vertebrate a-type synapsin isoforms share a carboxy-terminal region termed domain E. This domain is conserved in selected invertebrate synapsins and appears to be involved in regulating the interactions with actin and the clustering of SVs [21, 22, 30]. In addition, the involvement of domain E in the formation of synapsin dimers may explain the ability of a-isoforms to bring to synaptic terminals synapsin isoforms with weak targeting potential, such as Syn Ib [31, 32]. Although the region between domains A and C (domain B) and between domain C and the carboxy-terminus domain (domains D, G, H, J in the various isoforms) are poorly conserved among the synapsins, certain features within these domains are maintained. Of particular relevance is the presence of consensus sites for MAPKs, Cdk5, and CaMKII, as well as proline-rich sequences binding SH3 domains (Fig. 1). The structural similarity among vertebrate and invertebrate synapsins suggests that the basic mechanisms of synapsin function are evolutionarily conserved [25] (see below).
Regulation of synapsin function by phosphorylation
During neuronal transmission, synapsin properties are modulated in a complex manner through phosphorylation. All synapsins contain a consensus site for phosphorylation by PKA and by CaMKI/IV (site 1) within domain A (Fig. 1) [33–35]. Synapsin I contains three phosphorylation sites for MAPK/Erk named sites 4, 5, and 6 [36]. Sites 4 and 5 are located within domain B, while site 6 is located within domain D and is also subjected to phosphorylation by Cdk5, together with an adjacent site (site 7) [37]. Putative phosphorylation sites (sites 4 and 5) for MAPK/Erk are also present in domain B of synapsins II and III, although their phosphorylation has not been experimentally demonstrated [38]. In the C domain, all synapsin isoforms contain a highly conserved site (site 8) for the tyrosine kinase Src, whose phosphorylation has been experimentally demonstrated for synapsins I and II [29]. In addition, synapsin I, but not synapsin II, is a physiological substrate for a vesicle-associated form of CaMKII at two sites located within domain D (sites 2 and 3) [35]. Similar consensus sites are present in synapsin III, although their accessibility for phosphorylation remains to be determined.
Phosphorylation at site 1 (PKA/CaMKI) induces only subtle changes in the overall conformation of the synapsin molecule [39], but appears to modulate the binding of synapsins to actin and SVs [11, 40]. On the other hand, phosphorylation at sites 2 and 3 by CaMKII leads to major conformational changes [39], as well as to a drastic decrease in the ability of synapsin I to interact with actin [13] and SVs [7, 41]. Similar to the PKA/CaMKI site, phosphorylation at sites 4, 5, and 6 results in a moderate decrease in the ability of synapsins to interact with actin, but has no marked effects on the binding of synapsins to SVs [36]. Through the modulation of synapsin interactions, these phosphorylation events affect SV mobility and exo-endocytosis in both mature nerve terminals and growth cones.
In addition, the various phosphorylation sites are subjected to dephosphorylation by specific protein phosphatases. While sites 1, 2, and 3 are dephosphorylated by protein phosphatase 1, the MAPK sites 4, 5, and 6 are specific substrates for the Ca2+-calmodulin-dependent protein phosphatase 3/calcineurin, formerly known as protein phosphatase 2B [38]. As a consequence, the temporal pattern of phosphorylation in response to electrical activity is rather complex: phosphorylation of synapsin I at sites 1, 2, and 3 occurs rapidly after the Ca2+ influx that follows the arrival of the depolarizing stimulus and results in dissociation from SVs, which therefore become available for exocytosis [42, 43], while phosphorylation of sites 4, 5, and 6 (MAPK/Cdk5) dramatically decreases soon after the Ca2+ influx due to calcineurin activation, and slowly recovers afterwards. These two opposing, rapid, Ca2+-dependent processes play a crucial role in the modulation of SV trafficking within the presynaptic terminal, contributing to maintaining the equilibrium among the various pools of synaptic vesicles.
Role of synapsins in the control of neurotransmitter release
Various experimental approaches, from injection of peptides and antibodies to genetic ablation of the synapsin genes, have helped in defining the role of synapsins in neuroexocytosis at mature nerve terminals. Intracellular injection of synapsin I into the squid giant axon or into goldfish mauthner neurons reduces synaptic transmission, whereas introduction of an active form of CaMKII produces opposite effects, i.e., it increases the rate of rise and the amplitude of postsynaptic potentials and decreases their latency [44–46]. Similar results were obtained by introducing synapsin I or CaMKII into rat brain synaptosomes and measuring the depolarization-induced release of glutamate and noradrenaline [47, 48]. Altogether, these results support a model whereby dephosphorylated synapsin I provides an inhibitory constraint on neurosecretion that is relieved upon phosphorylation by CaMKII [2].
In the frog nerve muscle preparation, synapsin I partially dissociates from SVs during exocytosis and reassociates following endocytosis in response to high frequency electrical stimulation [42, 49]. In agreement with these results, in rat brain synaptosomes treated with depolarizing agents phosphorylation of synapsin I is associated with a rapid translocation of the protein from the membrane fraction to the synaptosol [43]. These data have been confirmed in live hippocampal neurons, where synapsin was observed to disperse within the presynaptic terminal and preterminal axon during depolarization and to recluster at SV sites following recovery [50]. Interestingly, the rates of synapsin dispersion and reclustering are respectively controlled by site-specific phosphorylation and dephosphorylation events. CaMK-mediated phosphorylation on sites 2 and 3 controls SV mobilization at low frequency of stimulation, whereas MAP kinase phosphorylation on sites 4 and 5 is triggered at both low and high frequencies of stimulation [50, 51].
Although single and multiple knock-out mice for either synapsin isoforms do not display overt alterations in neuroanatomy and behavior [17–19, 52–57], all mutants except single synapsin III knock-out, exhibit early onset (2 months of age) spontaneous and sensory stimuli-evoked epileptic seizures with severity positively correlated with the number of inactivated synapsin genes [17, 18]. SynII −/− and synI,II −/− mice have a mild behavioral phenotype characterized by impaired contextual conditioning and spatial memory loss [58, 59], while triple knock-out mice exhibit impaired motor coordination and defective spatial learning [19]. However, during aging, the behavioral phenotype of synapsin II knock-out mice becomes more severe and behavioral defects also appear in synI −/− mice [59]. Ultrastructural examination of mouse synI −/−, synI,II,−/−, and synI,II,III −/− presynaptic terminals reveals that the SV density is greatly decreased in the region comprised between 150 and 500 nm from the active zone, suggesting a role for synapsins in the organization of the presynaptic cytoarchitecture and of the distal SV pool [17, 18, 20, 54].
Both the number of SVs exocytosed during brief action potential trains and the total recycling SV pool are significantly reduced in hippocampal neurons from synapsin I knock-out mice, while the kinetics of endocytosis and SV repriming appear normal [55]. The selective loss of a major pool of SVs, distal to the active zone, in synI −/− mice is consistent with the results obtained with injection of anti-synapsin antibodies in lamprey reticulo-spinal axons [30] and with the enhanced synaptic depression during prolonged high frequency stimulations observed in synI −/−, synII −/− synI,II −/−, and synI,II,III −/− mice [14, 17, 19]. In addition, quantal analysis of excitatory postsynaptic potentials combined with the ultrastructural study of neuromuscular junctions in synII −/− mice shows that the lack of synapsin II results in a 40% decrease in the density of SVs in the reserve pool, while the number of docked vesicles remains unchanged. Interestingly, at reduced Ca2+ concentrations, quantal release and facilitation of synchronous release are significantly increased in synII −/− synapses [60].
The causal link between synapsin deficiency and the epileptic phenotype starts to be elucidated. It has been hypothesized that synaptic depression during repetitive stimulation may contribute to seizure development by causing an imbalance between excitatory and inhibitory systems. Indeed, gamma-aminobutyric acid (GABA) release is particularly sensitive to the depletion of the SV reserve pool observed with synapsin deletion, since inhibitory GABAergic interneurons often experience high frequency firing. Recent works have demonstrated that synI −/− mice display an impaired function of GABAergic neurons, with decreased size of both reserve and readily-releasable pools and high susceptibility to synaptic depression [14, 24, 56]. Such impairment in the inhibitory systems is also accompanied by an increased size of the readily releasable pool in glutamatergic terminals that further enhances the imbalance between excitatory and inhibitory activity, leading to network hyperexcitability and eventually to the generation of seizures [24]. Interestingly, a nonsense mutation in the synapsin I gene has been found in a family whose males are affected by epilepsy and mental retardation [61], and synapsin II has been identified as a major susceptibility gene for human epilepsy [62].
Similar findings were observed in synI,II,III −/− mice, in which excitatory synapses exhibited normal basal transmission, but decreased size of the reserve pool and marked synaptic depression, whereas inhibitory synapses exhibited impaired basal transmission, mild changes in the number of SVs and no changes in synaptic depression [19]. Thus, it is possible that the critical role of synapsins in the balance between excitatory and inhibitory synapses in brain networks underlies the seizure propensity observed in synapsin mutant mice.
Notwithstanding the absence of an overt epileptic phenotype, knock-out mice for the most recently identified member of the synapsin family, synapsin III, also showed an impairment of GABAergic transmission, while excitatory transmission was unaffected. These results raise the possibility that the function of synapsin III in inhibitory terminals may differ from that at excitatory synapses [57]. Since synapsin III is down-regulated at the time of synapse maturation and refinement and its expression levels in mature neurons are quite low [9], it is possible that these effects are the consequence of an impairment in the early steps of synapse assembly, which might lead to the establishment of altered networks.
Role of synapsins in neuronal development
Neurons are postmitotic cells generated from neuroepithelial precursors. The term neuronal development designates the processes by which neurons become phenotypically and morphologically specialized to integrate, conduct, and deliver electrochemical signals in a highly regulated spatiotemporal pattern. The finely orchestrated developmental program that leads to the establishment of a functional neuronal network proceeds through key steps, including cell migration, establishment of cell polarity, neurite outgrowth, axonal navigation, and synapse formation/maturation. The expression of synapsin genes during development is differentially regulated. The expression of synapsins I and II correlates with neuropil development and synapse formation: it rises quickly after birth and reaches the adult levels at 1–2 months of age (for review, see [1]). In contrast, synapsin III is already expressed by nestin-positive neuronal progenitors and later becomes restricted to cells of the neuronal linage (Fig. 2b) [10]. In primary mammalian neurons, the levels of synapsin III peak at 7 days in vitro (DIV) and decrease thereafter, while the expression of synapsin I and synapsin II increases over time during the formation of neurites, the specification of axons, and the establishment of synaptic contacts (Fig. 2a) [4, 9, 63]. These observations, strengthened by the highly neuro-specific localization of synapsins, prompted researchers to investigate the contribution of these proteins to neuronal development.
Synapsin I or synapsin IIa loaded into early blastomeres of Xenopus embryos increase both the amplitude and the frequency of spontaneous synaptic currents in the derived nerve-muscle co-cultures [64, 65]. Synapsin loading also increases the amplitude of evoked synaptic currents, suggesting that the elevated synaptic activity in these cells is due to an accelerated development of neurons rather than to a general increase in their excitability (Fig. 3a). It is important to underline that, even if synapsins I and IIa have overlapping effects (as expected by the high degree of conservation of multiple synapsin genes in higher vertebrates; see [25]), their function is not completely redundant. As an example, in the case of synapsin I-injected neurons, impulse-evoked synaptic currents also trigger a strong synaptic depression [64] that is usually correlated with the selection of mature synapses competing for their targets [66, 67].
Effects of synapsin I or II overexpression on the maturation of Xenopus neuromuscular synapses in culture. a Spontaneous acetylcholine release from Xenopus neurons after 1 DIV. Shown are spontaneous synaptic currents recorded from whole-cell voltage clamped myocytes that were innervated by either a control neuron or by a neuron injected with either exogenous synapsin I [64] or recombinant synapsin IIa [65]. Inward currents are shown as downward deflections at low (white background) and high (black background) time resolution. Note the higher frequency and amplitude of the spontaneous currents recorded in synapsin-injected neurons (scale bars 250 pA, 40 s, and 100 pA, 10 ms for the slow and fast traces, respectively). Reprinted with permission from [64] (Copyright 1992, Cell Press, USA.) and from [65] (Copyright 1994, National Academy of Sciences, USA.). b Fine structure of two neuromuscular synapses formed by either an uninjected neuron (SynI (−), left) or a neuron injected with purified rat synapsin I (SynI (+), right). Magnification: ×40,000 SynI (−) and ×45,000 SynI (+). Note the cluster of SVs (V) in the injected axoplasm in correspondence with basal lamina deposition (arrowheads). Modified with permission from [78]
The accelerated maturation of the quantal secretion properties in synapsin-injected neurons prompted the idea that synapsins have a role in synapse formation. Such a role might be achieved either indirectly, through the ability of the proteins to modulate neurotransmitter release, or by acting directly on the phenomena regulating the process of synapse formation and refinement.
Cytoskeletal regulation, neurite elongation and branching
Several cellular mechanisms may account for the ability of synapsins to control aspects of neuronal development. The evidence that in vitro synapsins exhibit actin-binding and bundling activity [11, 13] led to hypothesizing the involvement of the synapsins in the cytoskeletal changes underlying neurodevelopmental processes. Consistently, ectopic expression of synapsins Ia, Ib, IIa, or IIb in non-neuronal cells induces a dramatic reorganization of the actin cytoskeleton, leading to the extension of neurite-like processes. Synapsins expression also promotes the formation of synaptic-like varicosities. Notably, the rate of growth in these cells is reduced, suggesting a cell-cycle switch toward differentiation [68].
Subsequent studies carried out in hippocampal neurons were inspired by the “cytoskeleton reorganization hypothesis”. Depletion of synapsin II mRNA through an oligonucleotide antisense (OAS) approach or through gene targeting in mouse interferes with neurite outgrowth and triggers the formation of enlarged F-actin lamellipodium veils (Fig. 4) [69, 70]. Later in development, when neurite outgrowth in culture is virtually completed, synapsin II OASs cause cell body clustering and axonal fasciculation [71]. SynI −/− hippocampal neurons show an overall delay in neurite outgrowth and a decreased branching of the primary neurite, which are autonomously caught up at later stages of development [53, 72]. Since synapsin III has an expression peak immediately after birth and is enriched in axonal growth cones, it was thought to be involved in axon elongation. Synapsin III OASs impair both major and total neurite elongation at early developmental stages [9]. Surprisingly, defective neurite outgrowth observed at DIV 1 is spontaneously rescued 1 day later in synIII −/− mouse hippocampal neurons [57].
Effects of the absence of various synapsin isoforms on early stages of rodent neuronal development in vitro. Blue color designates normal development, while red color indicates developmental defects. Wild-type (WT) rodent hippocampal neurons in culture undergo a series of well-characterized developmental stages [92]. Between 1 and 3 days DIV, stage III neurons show a longer neuritic process that has the highest probability to become the axon. Mouse synI −/− hippocampal neurons at 1 and 3 DIV show an overall growth delay accompanied by decreased branching of the primary neurite [53, 72]. Mouse synII −/− neurons have a stronger phenotype, being devoid of a major neurite at 1 DIV and showing a slower growth phenotype [70]. In addition synII −/− neurons have enlarged lamellipodia veils. Mouse hippocampal synIII −/− neurons display a milder phenotype at 1 DIV and are completely normal at 2 DIV [57]. Neurons knocked out for multiple synapsin genes are normal [19, 70]. However, a comprehensive comparative work with all these genotypes is still missing
Counterintuitively, the neurite outgrowth delay observed in single mutants is rescued in neurons bearing multiple synapsin mutations. As an example, synI,II −/− and triple synI,II,III −/− mouse neurons in culture have a normal growth rate and do not show overt differences with respect to the controls [19, 70]. These unexpected compensatory changes might explain why early studies failed to detect neurite outgrowth defects in synapsin mutants [17]; the delayed outgrowth might be visible only in a very narrow temporal window and be completely rescued in the course of development.
The possible impact of the delay in neurite formation observed in vitro on brain development has been tested in synapsin KO mice. Puzzlingly, the overall cytoarchitecture and wiring of the brain is normal in synapsin mutants [17, 19, 53, 54, 70], although brain weight is lower in synII −/− embryos [70] and synIII −/− embryos show a defect in the proliferation of neuronal progenitors that leads to a decreased neurogenesis [10].
An alternative clue for the interpretation of synapsin-dependent cytoskeletal regulation has recently emerged. Since the ablation of synapsins does not completely block neurite outgrowth, synapsins are likely to be modulators rather than determinants of cytoskeletal rearrangements. Thus, synapsins could be present in two forms, acting as either positive or negative modulators of cytoskeletal changes. At steady state, the positive and negative components are counterbalanced and the outcome of the system is indistinguishable from that of a system completely devoid of the modulator in question. When positive and negative fractions of the modulator are unbalanced, via, e.g., posttranslational modifications or mutations, changes are detectable. From a biological point of view, such “push–pull mechanism” is a very efficient way to regulate a process bidirectionally with a single protein. Data from Kao et al. provide experimental support for this model: expression of the site 1 non-phosphorylatable mutant S9A synapsin II in Xenopus spinal neurons in culture decreases initiation and growing rate of neurites, while the S9E phospho-mimetic mutant has an opposite effect. Xenopus embryos injected with the DNA encoding for either the S9A or the S9E phospho-mutant synapsin II recapitulate the results: the spinal neurons expressing S9A are shorter than the contralateral wild-type (WT) controls, while the S9E injected neurons have longer neurites than the controls (Fig. 5a) [73]. A work on synapsin I fucosylation also suggests that the synapsin-mediated cytoskeletal changes are mainly due to unbalanced levels of synapsin conformers. Synapsin I is the major neuronal target of fucosylation, a post-translational modification that regulates protein activity and turnover. Hippocampal neurons treated with a fucosylation inhibitor have a reduced mean neurite length, while synI −/− neurons are resistant to this treatment [74]. Altogether these data support the idea that the pattern of active synapsin conformers, and therefore the synapsin activation state, rather than the absolute expression levels of the protein could be the key factor that induces the cytoskeletal rearrangements required for neurite growth.
Effects of synapsin phosphorylation on neuronal development. a Images of spinal nerves from the thoracic and abdominal areas derived from Xenopus embryos injected unilaterally at the two-cell stage with WT or mutated Xenopus synapsin IIa (SynII) mRNA. The serine to alanine mutation (SynII S9A, upper panel) mimics unphosphorylated synapsin, while the serine to glutamic acid mutation (SynII S9E, middle panel) mimics phosphorylated synapsin. Each image is the summation of a Z-stack of optical slices spaced 4 μm apart from each side of the embryo. Green, spinal nerves from injected side; red, nerves from uninjected side. WT synapsin II injection does not change the growth rate of Xenopus spinal nerves. Following injection of the non-phosphorylatable S9A mutant nerves are significantly shorter than the contralateral controls. Conversely, the pseudophosphorylated S9E mutant increases the outgrowth of injected nerves. These data indicate that the pattern of synapsin activation is more important than the increase of its total level in the determination of cytoskeletal rearrangements. Scale bar 100 μm. Reproduced with permission from [70]. b Images of growth cones of hippocampal neurons derived from synI −/− mice coexpressing synaptophysin I-EYFP (SypI-EYFP) and either WT synapsin I (SynI, upper two rows) or its non-phosphorylatable S9A mutant (SynI S9A, bottom two rows). The white traces in the left row outline the distal edges of the peripheral domain, as determined based on differential interference contrast images. Growth cones were imaged before and after a 5-min incubation with forskolin, an adenylate cyclase activator that increases intracellular cAMP levels and activates PKA. In the right column, SypI–EYFP image gray scales were transposed into a pseudocolor spectrum surface plot, with warmer hues corresponding to pixels of higher fluorescence intensity. Forskolin induces dispersion of SypI–EYFP-positive SVs in growth cones expressing WT, but not S9A, SynI, confirming that phosphorylation of synapsin I at site 1 by PKA is required for mobilization of SVs from the central domain of the growth cone. Scale bar 10 μm. Modified with permission from [87]
The molecular roles of synapsins in the determination of neurite growth are unknown. The possibility that this effect is linked to the modulation of spontaneous activity is unlikely, since some of the reported experiments were performed at stages in which neuronal activity in culture is undetectable. The evidence that cultured hippocampal neurons from synII −/− mice [70] or depleted for synapsin III [9] exhibit abnormal growth cone morphology associated with aberrant actin organization supports the idea that the actin-binding activity of synapsins is important for neuritogenesis. An attractive hypothesis is that synapsin-dependent cytoskeletal rearrangements are influenced by c-Src phosphorylation, which is a key signaling event in several aspects of neuronal development, including neurite outgrowth [75, 76].
Role of synapsins in synapse formation and maturation
Several reports indirectly indicate that synapsins modulate neuronal development through the promotion of synaptogenesis. A pioneering work in neuroblastoma/glioma cells showed that the stable overexpression of synapsin IIb increases the number of varicosities under differentiating conditions [77]. Consistently, rat hippocampal neurons chronically depleted of synapsin II mRNA have a decreased number of synapses [71]. Similar effects on the modulation of synaptogenesis have also been described in the case of synapsin I. For instance, developing neuromuscular synapses of nerve-muscle co-cultures prepared from Xenopus embryos loaded with synapsin I exhibit a precocious development of active zone-like structures. Ultrastructural analysis reveals that synapsin I favors the deposition of basal lamina components in the synaptic cleft and triggers the development of thickenings at the level of the postsynaptic plasma membrane (Fig. 3b) [78]. Consistent with these findings, hippocampal mossy fiber terminals and cerebellar granule cell terminals from synI −/− mouse show presynaptic defects that could be linked to impaired synapse formation. In particular, these terminals have a reduced area projection and do not show the varicosity-like expansions present in control neurons [54]. In contrast, no defects in synapse structure, connectivity, and SV distribution are observed in synIII −/− mice [57]. Together with the evidence that mutations in synapsin genes in human are linked to neurological phenotypes, these results from animal models point to the importance of the synapsins for the establishment and functions of neuronal networks.
Changes in the density of synaptic contacts are expected to produce corresponding variations in the levels of synaptic proteins. Hence, it is noteworthy that the expression of various presynaptic proteins, particularly SV components, is reduced in synI −/− and synII −/− mice [17, 54]. Consistent with the limited effects of synapsin III on synaptic maturation, no further reduction in SV levels are observed in triple synI,II,III −/− mice [19]. Notably, the expression of SV proteins, but not of pre- and postsynaptic scaffolding components, is specifically affected by ablation of synapsin genes [19]. Acute changes in synapsin II levels by overexpression or depletion approaches are sufficient to induce matching changes in SV protein levels [69, 71, 77].
Interestingly, in single and double synI −/− and synII −/−, the levels of the small GTPase Rab3a are diminished, whereas Rab5 levels increase [17, 79]. Since the interaction with synapsin has been implicated in the regulation of the late (i.e., post-docking) steps of SV exocytosis by Rab3a [79–81], it is possible that a Rab5-dependent compensatory endocytic process is active in synapsin mutants. Although the effects of synapsins on SV protein levels constitute a logical correlate to synaptogenesis, these observations are not sufficient to understand whether SV protein loss is a cause or an effect of the modulation of synaptogenesis.
Changes in SV protein levels are likely to result in changes in the number of SVs present at nerve terminals. The correlation between SV protein level and SV number is presented in several reports showing that synapsin overexpression increases, and synapsin depletion reduces SV number. As an example, SVs are increased in both neuroblastoma cells overexpressing synapsin IIb and developing neuromuscular cultures from synapsin I-injected Xenopus embryos [77, 78]. Synaptic vesicle density is decreased in several brain regions of adult synI −/− and synII −/− mice [17, 54], while no changes are observed in synIII −/− mice [57]. Electron tomography of the hippocampal CA1 area on triple synI,II,III −/− mice shows that SV density is drastically decreased, while the surface area of the bouton is only slightly reduced [20]. The SV reduction is not restricted to the central nervous system, since motor terminals devoid of synapsins have a similar phenotype, although in the absence of detectable transmission deficits [82]. In synI,II,III −/−, hippocampal neurons in culture SVs are reduced in both excitatory and inhibitory terminals, while docked SVs are decreased only at inhibitory terminals [19]. A recent work proposes that the overexpression of synapsin IIa is able to functionally rescue the SV loss found in synI,II,III −/− mice [83]. A study on Drosophila synapsin null mutant lines implies that in invertebrates the situation is different, since in larval type I terminals synapsin deletion does not affect either synapse formation or the number of SVs [84].
The data concerning SV protein levels and SV density at synapses suggest that synapsins I and II have relevant roles in SV maintenance, but this evidence does not fully clarify their function during synapse formation. A relevant piece of data that needs to be added to this picture is the localization of SVs at the presynaptic terminals when synapsin levels are altered. Some experiments confirm that synapsin I regulates the intervesicular distance, increasing the tendency of SVs to occur in clusters and favoring their precocious compartmentalization [78]. In the spinal cord asymmetrical synapses and in the granule cells of synI −/−, mice SVs have a normal distribution within 150 nm from the active zone, but their density is reduced by 50% in the peripheral region of the synapse [18, 54]. Taken together, these data indicate that the synapsins could regulate SV compartmentalization at synapses.
A set of experiments carried out in cultured neurons derived from synapsin mutant mice has been instrumental in understanding the contribution of synapsins to synaptogenesis. A limitation of these studies stems from the fact that in most instances the density of SV protein fluorescent puncta was taken as a morphological correlate of synapse formation. However, “SV positive puncta” may represent either SV protein transport vesicles required to assemble new synapses or mature functional terminals (for review, see [85]). In synI −/− neurons, the number of SV positive puncta is reduced by 90% at 7 DIV as compared to the control and is progressively rescued at 10 DIV. At 14 DIV, the number of SV positive puncta does not show significant differences from the controls [53, 72]. In hippocampal neurons depleted of synapsin II by OAS, the number of SV puncta is also reduced by 90% at 8 DIV and rescued later on. Interestingly, the effect is reversible upon OAS wash [71]. These data are substantially confirmed by a subsequent comparative report on synI −/−, synII −/−, and synI,II −/− mice. The neurons from these three KO mouse lines have a decreased number of SV-positive puncta at 4 DIV in culture. The delay persists until 10 DIV in the case of synI −/− and synII −/− neurons, while in the synI,II −/− neurons the phenotype seems to be milder [70]. As expected, the absence of synapsin III did not affect the density of SV positive puncta [9]. With a heterochronic primary cell culture approach combining synI −/− and WT neurons at different ages, Ferreira and collaborators [72] showed that, if synI −/− neurons are plated on 4 DIV WT neurons, the delay in the appearance of SV-positive puncta is partially rescued. Conversely, when WT neurons were plated on 4 DIV synI −/− neurons, the WT cells also showed a delayed appearance of SV-positive puncta. These data suggest that the delay in the formation of SV-positive puncta may require the crosstalk between the pre- and the postsynaptic cell. Although the contribution of synapsins to the formation of SV puncta in developing neurons is evident, additional studies are needed to determine whether these structures correspond to novel bona fide synapses or rather to clusters of SV precursors in transit to their final destination.
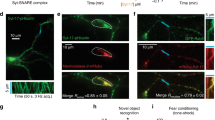
The effect of synapsins on the dynamics of SV precursors has been investigated in recent years. A work from Bonanomi and collaborators [86] shows that synapsins are important for the regulation of SV precursor dynamics in developing neurons in culture. It has been known for a long time that SV precursors vesicles are concentrated in the central domain of axonal growth cones of neurons developing in culture. PKA activation causes dispersion of SV precursors throughout the axonal growth cone, and this effect requires the presence of the PKA phosphorylation site in the synapsin I molecule (Fig. 5b). Interestingly, in synI −/− neurons (as well as in synI,II,III −/− neurons), SV precursors are already dispersed throughout the whole area of the growth cone under basal conditions (Fig. 6). Re-introduction of synapsin I is sufficient to relocalize SV precursors in the central domain. These data demonstrate that synapsin is necessary for the spatial arrangement of SV precursors in the growth cone and that synapsin I phosphorylation state regulates the dynamics of SV precursors during axonal outgrowth [87]. In addition, a report from Sabo and collaborators indicates that SV precursors in the synI,II,III −/− neurons have a decreased pause probability, a shorter pause duration, and an increased pause frequency, confirming the impaired dynamic behavior of SV precursors in the absence of synapsins [88]. Under this perspective, the reduction of SV-positive puncta in KO neurons could also be contributed by the altered transport and re-organization of SV precursors at early stages of development, and this could in turn regulate synapse formation.
Differential role of synapsin isoforms in SV clustering in growth cones. Hippocampal neurons from either WT, synI −/−, synII −/−, or synI,II,III −/− were fixed and stained with an anti-synaptotagmin I antibody (green in the merged images) and with fluorescently-tagged phalloidin that specifically labels F-actin (red in the merged images). In culture, neuronal growth cones show two main cytoskeleton defined regions: a central domain that is enriched in organelles and microtubules, and a peripheral domain that is characterized by the absence of organelles an the deposition of filamentous actin [86]. Synaptotagmin I-positive SV precursors are confined to the central domain in WT and synII −/− neurons, but they disperse throughout the growth cone in synI −/− and synI,II,III −/− neurons. The lack of phenotype in synII −/− neurons reinforces the idea that synapsin I is the major determinant for the localization of SV precursors in neuronal growth cones [87]. The white traces in the left row outline the distal edges of the peripheral domain, as determined based on differential interference contrast images. Scale bar 5 μm. Original from Bonanomi and Valtorta, unpublished
Conclusions
In invertebrates, synapsins are present as a single gene, while in higher organisms gene duplication, together with differential splicing, have given rise to multiple isoforms. In spite of their high degree of conservation throughout evolution, synapsins are not essential for neuronal development and synaptic transmission, and deletion of their genes is compatible with life. However, they appear to be critical for synapse rearrangement, refinement, and plasticity. Indeed, synapsins interfere with several developmental processes, being involved in neurite outgrowth, SV spatial localization at growth cones, SV targeting at synapses, synapse formation and maturation. Synapsins are the most abundant family of phosphoproteins in the presynaptic compartment. Most importantly, they are at the crossroads of many fundamental signal transduction pathways including those triggered by G protein-coupled receptors, voltage-gated and ligand-gated Ca2+ channels, and neurotrophins.
In spite of the detailed structural and functional characterization of the synapsins in a variety of experimental models, several questions still remain open, particularly concerning the molecular mechanisms that underlie the participation of these phosphoproteins in such a diverse range of developmental processes. The regulation of actin dynamics and the reversible interaction of synapsins with SVs are the two most likely events at the basis of synapsin role on neuronal development. The presence of multiple conserved domains suggests a substantial functional redundancy among synapsin isoforms. However, distinct domain composition, differential temporal and spatial expression patterns, as well as distinct substrate properties for post-translational modifications, namely phosphorylation, suggest that the various synapsin isoforms may also have specific and non-overlapping functions. The situation is further complicated by the observation that, on the SV membrane, synapsins are present as homo- or hetero-dimers, thus giving rise to a high number of possible combinations, which might behave as slightly different functional units.
Since the ablation of synapsins does not completely block neurite outgrowth and synapse formation, we propose that synapsins should be considered as modulators rather than direct determinants of developmental processes. In this respect, thanks to their high number of phosphorylation sites and their dynamic localization, synapsins could function as integrators of the many signals which impinge on a given presynaptic compartment or growth cone at any given moment, thus enabling the neuron or its single terminals to respond in an appropriate manner to complex environmental changes.
Given the complexity of the phenomena at play, the roles of the synapsins in neuronal development have been studied in cultured neurons. This approach, however, does not allow the full evaluation of the possible consequences of the observed phenomena on the establishment of the extremely complex neuronal networks of the brain. Indeed, in vivo, apparently minor phenomena, such as a delay in neurite outgrowth, might also have important consequences, since the establishment of appropriate connections critically depends on the timing and location of occurrence of the various developmental steps.
In conclusion, synapsins contribute to the formation of neuronal contacts starting from the elongation of neurites and the regulation of SV precursors at growth cones up to the housekeeping, remodeling and plasticity of synapses. In their absence, neuronal networks and synapses can still form and perform basic activities, but with a much lower degree of freedom. In mammals, the importance of synapsins for proper brain function is undeniable and is reflected by the pathologies of the central nervous system that develop in both rodents and human upon their deletion or mutation.
References
De Camilli P, Benfenati F, Valtorta F, Greengard P (1990) The synapsins. Annu Rev Cell Biol 6:433–460
Greengard P, Valtorta F, Czernik AJ, Benfenati F (1993) Synaptic vesicle phosphoproteins and regulation of synaptic function. Science 259:780–785
Fdez E, Hilfiker S (2006) Vesicle pools and synapsins: new insights into old enigmas. Brain Cell Biol 35:107–115
De Camilli P, Cameron R, Greengard P (1983) Synapsin I (protein I), a nerve terminal-specific phosphoprotein. I. Its general distribution in synapses of the central and peripheral nervous system demonstrated by immunofluorescence in frozen and plastic sections. J Cell Biol 96:1337–1354
De Camilli P, Harris SM Jr, Huttner WB, Greengard P (1983) Synapsin I (protein I), a nerve terminal-specific phosphoprotein. II. Its specific association with synaptic vesicles demonstrated by immunocytochemistry in agarose-embedded synaptosomes. J Cell Biol 96:1355–1373
Huttner WB, Schiebler W, Greengard P, De Camilli P (1983) Synapsin I (protein I), a nerve terminal-specific phosphoprotein. III. Its association with synaptic vesicles studied in a highly purified synaptic vesicle preparation. J Cell Biol 96:1374–1388
Benfenati F, Bahler M, Jahn R, Greengard P (1989) Interactions of synapsin I with small synaptic vesicles: distinct sites in synapsin I bind to vesicle phospholipids and vesicle proteins. J Cell Biol 108:1863–1872
Benfenati F, Valtorta F, Rubenstein JL, Gorelick FS, Greengard P, Czernik AJ (1992) Synaptic vesicle-associated Ca2+/calmodulin-dependent protein kinase II is a binding protein for synapsin I. Nature 359:417–420
Ferreira A, Kao HT, Feng J, Rapoport M, Greengard P (2000) Synapsin III: developmental expression, subcellular localization, and role in axon formation. J Neurosci 20:3736–3744
Kao HT, Li P, Chao HM, Janoschka S, Pham K, Feng J, McEwen BS, Greengard P, Pieribone VA, Porton B (2008) Early involvement of synapsin III in neural progenitor cell development in the adult hippocampus. J Comp Neurol 507:1860–1870
Bahler M, Greengard P (1987) Synapsin I bundles F-actin in a phosphorylation-dependent manner. Nature 326:704–707
Benfenati F, Valtorta F, Chieregatti E, Greengard P (1992) Interaction of free and synaptic vesicle-bound synapsin I with F-actin. Neuron 8:377–386
Valtorta F, Greengard P, Fesce R, Chieregatti E, Benfenati F (1992) Effects of the neuronal phosphoprotein synapsin I on actin polymerization. I. Evidence for a phosphorylation-dependent nucleating effect. J Biol Chem 267:11281–11288
Baldelli P, Fassio A, Valtorta F, Benfenati F (2007) Lack of synapsin I reduces the readily releasable pool of synaptic vesicles at central inhibitory synapses. J Neurosci 27:13520–13531
Valtorta F, Benfenati F, Greengard P (1992) Structure and function of the synapsins. J Biol Chem 267:7195–7198
Ceccaldi PE, Grohovaz F, Benfenati F, Chieregatti E, Greengard P, Valtorta F (1995) Dephosphorylated synapsin I anchors synaptic vesicles to actin cytoskeleton: an analysis by videomicroscopy. J Cell Biol 128:905–912
Rosahl TW, Spillane D, Missler M, Herz J, Selig DK, Wolff JR, Hammer RE, Malenka RC, Sudhof TC (1995) Essential functions of synapsins I and II in synaptic vesicle regulation. Nature 375:488–493
Li L, Chin LS, Shupliakov O, Brodin L, Sihra TS, Hvalby O, Jensen V, Zheng D, McNamara JO, Greengard P, Andersen P (1995) Impairment of synaptic vesicle clustering and of synaptic transmission, and increased seizure propensity, in synapsin I-deficient mice. Proc Natl Acad Sci USA 92:9235–9239
Gitler D, Takagishi Y, Feng J, Ren Y, Rodriguiz RM, Wetsel WC, Greengard P, Augustine GJ (2004) Different presynaptic roles of synapsins at excitatory and inhibitory synapses. J Neurosci 24:11368–11380
Siksou L, Rostaing P, Lechaire JP, Boudier T, Ohtsuka T, Fejtova A, Kao HT, Greengard P, Gundelfinger ED, Triller A, Marty S (2007) Three-dimensional architecture of presynaptic terminal cytomatrix. J Neurosci 27:6868–6877
Hilfiker S, Schweizer FE, Kao HT, Czernik AJ, Greengard P, Augustine GJ (1998) Two sites of action for synapsin domain E in regulating neurotransmitter release. Nat Neurosci 1:29–35
Hilfiker S, Benfenati F, Doussau F, Nairn AC, Czernik AJ, Augustine GJ, Greengard P (2005) Structural domains involved in the regulation of transmitter release by synapsins. J Neurosci 25:2658–2669
Fassio A, Merlo D, Mapelli J, Menegon A, Corradi A, Mete M, Zappettini S, Bonanno G, Valtorta F, D’Angelo E, Benfenati F (2006) The synapsin domain E accelerates the exo-endocytotic cycle of synaptic vesicles in cerebellar Purkinje cells. J Cell Sci 119:4257–4268
Chiappalone M, Casagrande S, Tedesco M, Valtorta F, Baldelli P, Martinoia S, Benfenati F (2009) Opposite changes in glutamatergic and GABAergic transmission underlie the diffuse hyperexcitability of synapsin I-deficient cortical networks. Cereb Cortex 19:1422–1439
Kao HT, Porton B, Hilfiker S, Stefani G, Pieribone VA, DeSalle R, Greengard P (1999) Molecular evolution of the synapsin gene family. J Exp Zool 285:360–377
Esser L, Wang CR, Hosaka M, Smagula CS, Sudhof TC, Deisenhofer J (1998) Synapsin I is structurally similar to ATP-utilizing enzymes. EMBO J 17:977–984
Hosaka M, Sudhof TC (1998) Synapsins I and II are ATP-binding proteins with differential Ca2+ regulation. J Biol Chem 273:1425–1429
Hosaka M, Sudhof TC (1999) Homo- and heterodimerization of synapsins. J Biol Chem 274:16747–16753
Onofri F, Messa M, Matafora V, Bonanno G, Corradi A, Bachi A, Valtorta F, Benfenati F (2007) Synapsin phosphorylation by SRC tyrosine kinase enhances SRC activity in synaptic vesicles. J Biol Chem 282:15754–15767
Pieribone VA, Shupliakov O, Brodin L, Hilfiker-Rothenfluh S, Czernik AJ, Greengard P (1995) Distinct pools of synaptic vesicles in neurotransmitter release. Nature 375:493–497
Gitler D, Xu Y, Kao HT, Lin D, Lim S, Feng J, Greengard P, Augustine GJ (2004) Molecular determinants of synapsin targeting to presynaptic terminals. J Neurosci 24:3711–3720
Monaldi I, Vassalli M, Bachi A, Millo E, Valtorta F, Raiteri R, Benfenati F, Fassio A (2009) The highly conserved synapsin domain E mediates synapsin dimerization and phospholipid vesicle clustering. Biochem J (in press)
Johnson EM, Ueda T, Maeno H, Greengard P (1972) Adenosine 3′, 5-monophosphate-dependent phosphorylation of a specific protein in synaptic membrane fractions from rat cerebrum. J Biol Chem 247:5650–5652
Huttner WB, Greengard P (1979) Multiple phosphorylation sites in protein I and their differential regulation by cyclic AMP and calcium. Proc Natl Acad Sci USA 76:5402–5406
Czernik AJ, Pang DT, Greengard P (1987) Amino acid sequences surrounding the cAMP-dependent and calcium/calmodulin-dependent phosphorylation sites in rat and bovine synapsin I. Proc Natl Acad Sci USA 84:7518–7522
Jovanovic JN, Benfenati F, Siow YL, Sihra TS, Sanghera JS, Pelech SL, Greengard P, Czernik AJ (1996) Neurotrophins stimulate phosphorylation of synapsin I by MAP kinase and regulate synapsin I-actin interactions. Proc Natl Acad Sci USA 93:3679–3683
Matsubara M, Kusubata M, Ishiguro K, Uchida T, Titani K, Taniguchi H (1996) Site-specific phosphorylation of synapsin I by mitogen-activated protein kinase and Cdk5 and its effects on physiological functions. J Biol Chem 271:21108–21113
Jovanovic JN, Sihra TS, Nairn AC, Hemmings HC Jr, Greengard P, Czernik AJ (2001) Opposing changes in phosphorylation of specific sites in synapsin I during Ca2+-dependent glutamate release in isolated nerve terminals. J Neurosci 21:7944–7953
Benfenati F, Neyroz P, Bahler M, Masotti L, Greengard P (1990) Time-resolved fluorescence study of the neuron-specific phosphoprotein synapsin I. Evidence for phosphorylation-dependent conformational changes. J Biol Chem 265:12584–12595
Hosaka M, Hammer RE, Sudhof TC (1999) A phospho-switch controls the dynamic association of synapsins with synaptic vesicles. Neuron 24:377–387
Schiebler W, Jahn R, Doucet JP, Rothlein J, Greengard P (1986) Characterization of synapsin I binding to small synaptic vesicles. J Biol Chem 261:8383–8390
Torri Tarelli F, Bossi M, Fesce R, Greengard P, Valtorta F (1992) Synapsin I partially dissociates from synaptic vesicles during exocytosis induced by electrical stimulation. Neuron 9:1143–1153
Sihra TS, Wang JK, Gorelick FS, Greengard P (1989) Translocation of synapsin I in response to depolarization of isolated nerve terminals. Proc Natl Acad Sci USA 86:8108–8112
Llinas R, McGuinness TL, Leonard CS, Sugimori M, Greengard P (1985) Intraterminal injection of synapsin I or calcium/calmodulin-dependent protein kinase II alters neurotransmitter release at the squid giant synapse. Proc Natl Acad Sci USA 82:3035–3039
Llinas R, Gruner JA, Sugimori M, McGuinness TL, Greengard P (1991) Regulation by synapsin I and Ca(2+)-calmodulin-dependent protein kinase II of the transmitter release in squid giant synapse. J Physiol 436:257–282
Hackett JT, Cochran SL, Greenfield LJ Jr, Brosius DC, Ueda T (1990) Synapsin I injected presynaptically into goldfish mauthner axons reduces quantal synaptic transmission. J Neurophysiol 63:701–706
Nichols RA, Sihra TS, Czernik AJ, Nairn AC, Greengard P (1990) Calcium/calmodulin-dependent protein kinase II increases glutamate and noradrenaline release from synaptosomes. Nature 343:647–651
Nichols RA, Chilcote TJ, Czernik AJ, Greengard P (1992) Synapsin I regulates glutamate release from rat brain synaptosomes. J Neurochem 58:783–785
Torri-Tarelli F, Villa A, Valtorta F, De Camilli P, Greengard P, Ceccarelli B (1990) Redistribution of synaptophysin and synapsin I during alpha-latrotoxin-induced release of neurotransmitter at the neuromuscular junction. J Cell Biol 110:449–459
Chi P, Greengard P, Ryan TA (2001) Synapsin dispersion and reclustering during synaptic activity. Nat Neurosci 4:1187–1193
Chi P, Greengard P, Ryan TA (2003) Synaptic vesicle mobilization is regulated by distinct synapsin I phosphorylation pathways at different frequencies. Neuron 38:69–78
Rosahl TW, Geppert M, Spillane D, Herz J, Hammer RE, Malenka RC, Sudhof TC (1993) Short-term synaptic plasticity is altered in mice lacking synapsin I. Cell 75:661–670
Chin LS, Li L, Ferreira A, Kosik KS, Greengard P (1995) Impairment of axonal development and of synaptogenesis in hippocampal neurons of synapsin I-deficient mice. Proc Natl Acad Sci USA 92:9230–9234
Takei Y, Harada A, Takeda S, Kobayashi K, Terada S, Noda T, Takahashi T, Hirokawa N (1995) Synapsin I deficiency results in the structural change in the presynaptic terminals in the murine nervous system. J Cell Biol 131:1789–1800
Ryan TA, Li L, Chin LS, Greengard P, Smith SJ (1996) Synaptic vesicle recycling in synapsin I knock-out mice. J Cell Biol 134:1219–1227
Terada S, Tsujimoto T, Takei Y, Takahashi T, Hirokawa N (1999) Impairment of inhibitory synaptic transmission in mice lacking synapsin I. J Cell Biol 145:1039–1048
Feng J, Chi P, Blanpied TA, Xu Y, Magarinos AM, Ferreira A, Takahashi RH, Kao HT, McEwen BS, Ryan TA, Augustine GJ, Greengard P (2002) Regulation of neurotransmitter release by synapsin III. J Neurosci 22:4372–4380
Silva AJ, Rosahl TW, Chapman PF, Marowitz Z, Friedman E, Frankland PW, Cestari V, Cioffi D, Sudhof TC, Bourtchuladze R (1996) Impaired learning in mice with abnormal short-lived plasticity. Curr Biol 6:1509–1518
Corradi A, Zanardi A, Giacomini C, Onofri F, Valtorta F, Zoli M, Benfenati F (2008) Synapsin-I- and synapsin-II-null mice display an increased age-dependent cognitive impairment. J Cell Sci 121:3042–3051
Samigullin D, Bill CA, Coleman WL, Bykhovskaia M (2004) Regulation of transmitter release by synapsin II in mouse motor terminals. J Physiol 561:149–158
Garcia CC, Blair HJ, Seager M, Coulthard A, Tennant S, Buddles M, Curtis A, Goodship JA (2004) Identification of a mutation in synapsin I, a synaptic vesicle protein, in a family with epilepsy. J Med Genet 41:183–186
Cavalleri GL, Weale ME, Shianna KV, Singh R, Lynch JM, Grinton B, Szoeke C, Murphy K, Kinirons P, O’Rourke D, Ge D, Depondt C, Claeys KG, Pandolfo M, Gumbs C, Walley N, McNamara J, Mulley JC, Linney KN, Sheffield LJ, Radtke RA, Tate SK, Chissoe SL, Gibson RA, Hosford D, Stanton A, Graves TD, Hanna MG, Eriksson K, Kantanen AM, Kalviainen R, O’Brien TJ, Sander JW, Duncan JS, Scheffer IE, Berkovic SF, Wood NW, Doherty CP, Delanty N, Sisodiya SM, Goldstein DB (2007) Multicentre search for genetic susceptibility loci in sporadic epilepsy syndrome and seizure types: a case-control study. Lancet Neurol 6:970–980
Fletcher TL, Cameron P, De Camilli P, Banker G (1991) The distribution of synapsin I and synaptophysin in hippocampal neurons developing in culture. J Neurosci 11:1617–1626
Lu B, Greengard P, Poo MM (1992) Exogenous synapsin I promotes functional maturation of developing neuromuscular synapses. Neuron 8:521–529
Schaeffer E, Alder J, Greengard P, Poo MM (1994) Synapsin IIa accelerates functional development of neuromuscular synapses. Proc Natl Acad Sci USA 91:3882–3886
Dunia R, Herrera AA (1993) Synapse formation and elimination during growth of the pectoral muscle in Xenopus laevis. J Physiol 469:501–509
Cash S, Zucker RS, Poo MM (1996) Spread of synaptic depression mediated by presynaptic cytoplasmic signalling. Science 272:998–1001
Han HQ, Greengard P (1994) Remodeling of cytoskeletal architecture of non-neuronal cells induced by synapsin. Proc Natl Acad Sci USA 91:8557–8561
Ferreira A, Kosik KS, Greengard P, Han HQ (1994) Aberrant neurites and synaptic vesicle protein deficiency in synapsin II-depleted neurons. Science 264:977–979
Ferreira A, Chin LS, Li L, Lanier LM, Kosik KS, Greengard P (1998) Distinct roles of synapsin I and synapsin II during neuronal development. Mol Med 4:22–28
Ferreira A, Han HQ, Greengard P, Kosik KS (1995) Suppression of synapsin II inhibits the formation and maintenance of synapses in hippocampal culture. Proc Natl Acad Sci USA 92:9225–9229
Ferreira A, Li L, Chin LS, Greengard P, Kosik KS (1996) Postsynaptic element contributes to the delay in synaptogenesis in synapsin I-deficient neurons. Mol Cell Neurosci 8:286–299
Kao HT, Song HJ, Porton B, Ming GL, Hoh J, Abraham M, Czernik AJ, Pieribone VA, Poo MM, Greengard P (2002) A protein kinase A-dependent molecular switch in synapsins regulates neurite outgrowth. Nat Neurosci 5:431–437
Murrey HE, Gama CI, Kalovidouris SA, Luo WI, Driggers EM, Porton B, Hsieh-Wilson LC (2006) Protein fucosylation regulates synapsin Ia/Ib expression and neuronal morphology in primary hippocampal neurons. Proc Natl Acad Sci USA 103:21–26
Onofri F, Giovedi S, Vaccaro P, Czernik AJ, Valtorta F, De Camilli P, Greengard P, Benfenati F (1997) Synapsin I interacts with c-Src and stimulates its tyrosine kinase activity. Proc Natl Acad Sci USA 94:12168–12173
Tucker BA, Rahimtula M, Mearow KM (2008) Src and FAK are key early signalling intermediates required for neurite growth in NGF-responsive adult DRG neurons. Cell Signal 20:241–257
Han HQ, Nichols RA, Rubin MR, Bahler M, Greengard P (1991) Induction of formation of presynaptic terminals in neuroblastoma cells by synapsin IIb. Nature 349:697–700
Valtorta F, Iezzi N, Benfenati F, Lu B, Poo MM, Greengard P (1995) Accelerated structural maturation induced by synapsin I at developing neuromuscular synapses of Xenopus laevis. Eur J Neurosci 7:261–270
Giovedì S, Darchen F, Valtorta F, Greengard P, Benfenati F (2004) Synapsin is a novel Rab3 effector protein on small synaptic vesicles. II. Functional effects of the Rab3A-synapsin I interaction. J Biol Chem 279:43769–43779
Giovedì S, Vaccaro P, Valtorta F, Darchen F, Greengard P, Cesareni G, Benfenati F (2004) Synapsin is a novel Rab3 effector protein on small synaptic vesicles. I. Identification and characterization of the synapsin I-Rab3 interactions in vitro and in intact nerve terminals. J Biol Chem 279:43760–43768
Geppert M, Ullrich B, Green DG, Takei K, Daniels L, De Camilli P, Sudhof TC, Hammer RE (1994) Synaptic targeting domains of synapsin I revealed by transgenic expression in photoreceptor cells. EMBO J 13:3720–3727
Gaffield MA, Betz WJ (2007) Synaptic vesicle mobility in mouse motor nerve terminals with and without synapsin. J Neurosci 27:13691–13700
Gitler D, Cheng Q, Greengard P, Augustine GJ (2008) Synapsin IIa controls the reserve pool of glutamatergic synaptic vesicles. J Neurosci 28:10835–10843
Godenschwege TA, Reisch D, Diegelmann S, Eberle K, Funk N, Heisenberg M, Hoppe V, Hoppe J, Klagges BR, Martin JR, Nikitina EA, Putz G, Reifegerste R, Reisch N, Rister J, Schaupp M, Scholz H, Schwarzel M, Werner U, Zars TD, Buchner S, Buchner E (2004) Flies lacking all synapsins are unexpectedly healthy but are impaired in complex behaviour. Eur J Neurosci 20:611–622
Ziv NE, Garner CC (2004) Cellular and molecular mechanisms of presynaptic assembly. Nat Rev Neurosci 5:385–399
Forscher P, Kaczmarek LK, Buchanan JA, Smith SJ (1987) Cyclic AMP induces changes in distribution and transport of organelles within growth cones of Aplysia bag cell neurons. J Neurosci 7:3600–3611
Bonanomi D, Menegon A, Miccio A, Ferrari G, Corradi A, Kao HT, Benfenati F, Valtorta F (2005) Phosphorylation of synapsin I by cAMP-dependent protein kinase controls synaptic vesicle dynamics in developing neurons. J Neurosci 25:7299–7308
Sabo SL, Gomes RA, McAllister AK (2006) Formation of presynaptic terminals at predefined sites along axons. J Neurosci 26:10813–10825
Onofri F, Giovedi S, Kao HT, Valtorta F, Bongiorno Borbone L, De Camilli P, Greengard P, Benfenati F (2000) Specificity of the binding of synapsin I to Src homology 3 domains. J Biol Chem 275:29857–29867
Porton B, Kao HT, Greengard P (1999) Characterization of transcripts from the synapsin III gene locus. J Neurochem 73:2266–2271
Baldelli P, Fassio A, Corradi A, Cremona O, Valtorta F, Benfenati F (2005) Synapsins and neuroexocytosis: recent views from functional studies on synapsin null mutant mice. Arch Ital Biol 143:113–126
Dotti CG, Sullivan CA, Banker GA (1988) The establishment of polarity by hippocampal neurons in culture. J Neurosci 8:1454–1468
Acknowledgments
We thank Drs. Paul Greengard (The Rockefeller University, New York, NY) and Hung-Teh Kao (Brown University, Providence, RI) for the long-standing collaboration in the synapsin field and for invaluable and stimulating discussions. This study was supported by research grants from the Italian Ministry of University and Research (PRIN and FIRB grants), the Compagnia di San Paolo-Torino, the Cariplo Foundation-Milano and the Mariani Foundation-Milano to F.B. and F.V. The support of Telethon-Italy (Grant GGP09134 to F.B. and F.V.) is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Additional information
F. Benfenati and F. Valtorta contributed equally to this work.
Rights and permissions
About this article
Cite this article
Fornasiero, E.F., Bonanomi, D., Benfenati, F. et al. The role of synapsins in neuronal development. Cell. Mol. Life Sci. 67, 1383–1396 (2010). https://doi.org/10.1007/s00018-009-0227-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00018-009-0227-8