Abstract
Objective and design
Roxithromycin, a macrolide antibiotic, exhibits anti-inflammatory property. The present study was designed to evaluate its protective effect in a rat model of colitis.
Methods
The anti-inflammatory property of roxithromycin was first validated in rat paw edema model at 5 and 20 mg/kg doses where it produced 19 and 51% inhibition of paw swelling induced by carrageenan. The efficacy of roxithromycin was evaluated at these doses in a rat model where colitis was induced by intra-colonic instillation of acetic acid. Rats were divided into six groups viz. normal control, experimental control and drug-treated groups: roxithromycin 5 and 20 mg/kg, diclofenac 10 mg/kg and mesalazine 300 mg/kg. All drugs were given orally 1 h before induction of colitis. The macro and microscopic changes, mean ulcer score, mucus content and markers of oxidative stress and inflammation were evaluated in all the groups after 24 h.
Results
Pretreatment with roxithromycin markedly decreased hyperemia, ulceration, edema and restored histological architecture. The protection afforded by roxithromycin was substantiated by dose-dependent increase in mucus content, normalization of markers of oxidative stress (GSH and TBARS) and levels of TNF-α, PGE2 and nitrite along with marked decrease in expression of NFκB (p65), IL-1β and COX-2. The protective effect of roxithromycin was found to be comparable to mesalazine while diclofenac was found ineffective.
Conclusion
Our study demonstrates that roxithromycin ameliorates experimental colitis by maintaining redox homeostasis, preserving mucosal integrity and downregulating NFκB-mediated pro-inflammatory signaling and suggests that it has a therapeutic potential in inflammatory conditions of the colon.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ulcerative colitis (UC), a subtype of inflammatory bowel disease, is an inflammatory condition of the large intestine associated with abdominal pain, bloody diarrhea and other systemic manifestations with alternating episodes of exacerbations and remission [1, 2]. There is rising trend in its incidence in developing countries due to change in lifestyle [3, 4]. Along with genetic, environmental and immunological factors, both inflammation and oxidative stress play an important role in its pathogenesis. The loss of mucosal integrity in this condition is brought about by the release of various pro-inflammatory mediators accompanied by recruitment of cells such as activated neutrophils, macrophages and mast cells [5]. Biopsy samples obtained from the patients with active disease show elevated levels of prostaglandins (PGE2, PGI2 and PGF2α), interleukin (IL-1β), tumor necrosis factor-alpha (TNF-α) and interferon gamma (IFN-γ) [6,7,8]. Inflammation and overproduction of free radicals such as reactive oxygen and nitrogen species (ROS and RNS) together contribute to the macromolecular damage and loss of mucosal integrity. Mucosal damage as seen in colitis can be induced by several agents in experimental models. Acetic acid-induced colitis in rat resembles human UC with respect to histological changes, mediator release and responsiveness to drugs and this model is widely used for the evaluation of drugs effective in colitis [9, 10]. The currently used drugs in UC produce beneficial effect through their anti-inflammatory, immunomodulatory and/or anti-adhesion properties where the aim is to induce and maintain remission, reduce the frequency and severity of exacerbations, preserve mucosal integrity, avoid surgical intervention and to prevent transformation into cancer [1, 11,12,13]. It is also well known that the drugs initially effective may lose efficacy over a period of time or their use is discontinued due to side effects.
Macrolides, the antibacterial drugs, have been reported to produce non-antibiotic effects in various disease conditions primarily through their anti-inflammatory and immunomodulatory properties [14, 15]. Besides respiratory tract infections, they have been found effective in reducing airway inflammation and hyper-responsiveness by inhibiting neutrophil migration, generation of reactive oxygen species, activation of transcription factor and production of pro-inflammatory cytokines [15, 16]. Roxithromycin is one such macrolide that has been found effective in inhibiting an acute inflammatory response induced in a rodent model. It has been shown to inhibit NFκB signaling and endoplasmic reticulum stress in intestinal epithelial cells in a mouse model of colitis [14, 17]. The present study was designed to evaluate the efficacy of roxithromycin in an acetic acid-induced colitis model where parameters such as ulcer score, histoarchitecture, adherent mucus content, markers of inflammation, oxidative stress and expression of NFκB (p65), IL-1β and COX-2 were included and a comparison was made with standard anti-colitic drug mesalazine and anti-inflammatory drug diclofenac.
Materials and methods
Drugs, chemicals and reagents
Roxithromycin, diclofenac and mesalazine were provided by Next Wave (India), Himachal Pradesh, India, Arbo Pharmaceuticals, New Delhi, India and Win-Medicare, New Delhi, India, respectively. The Prostaglandin E2 EIA kit was purchased from Cayman Chemicals, USA, and TNF-α ELISA kit was procured from Boster Biological Technology Ltd, USA. Immunohistochemistry (IHC) was performed with UltraVision Quanto Detection System HRP DAB purchased from Thermo Fisher Scientific, MA, USA. The antibodies for COX-2 and NFκB (p65) were procured from Thermo Fisher Scientific, MA, USA and IL-1β from Santa Cruz, Texas, USA. All other reagents and the chemicals used were of analytical grade and were purchased from Sisco Research Laboratories, India, Qualigens Fine Chemicals, Mumbai, India and Sigma–Aldrich, St. Louis, MO, USA.
Animals
The study was conducted on adult Wistar albino rats of either sex (110–200 g) procured from Central Animal Facility of All India Institute of Medical Sciences, New Delhi, India. The animals were maintained in the animal house, acclimatized before the experiments for a week and were provided food and water ad libitum. The study protocol was approved by Institutional Animal Ethics Committee (773/IAEC/13).
Paw edema model
The validation of anti-inflammatory activity and determination of dose of roxithromycin was carried out in rat paw edema model where inflammation was induced by sub-plantar injection of 0.1 ml of 0.5% carrageenan as described by Winter et al. [18]. Rats were divided into five groups (n = 6), where Group I served as normal control (NC) and normal saline (NS) was injected in paw whereas inflammation was induced in groups II–V. Group II was given NS orally and served as experimental control (EC), Group III and IV were given roxithromycin at 5 and 20 mg/kg doses (RXM 5 and RXM 20) and Group V was given diclofenac at 10 mg/kg (D 10) orally (in 1 ml volume) 1 h before injecting carrageenan. The doses of roxithromycin and diclofenac were selected based on the previous studies [14, 19]. The paw volume was measured up to a fixed mark on the lateral malleolus before inducing inflammation and at 3 h when the inflammation is at its peak. The difference in two readings gave the edema volume and the anti-inflammatory effect of drugs was expressed as % inhibition of edema with respect to the EC group [20].
Colitis model
Colitis was induced in 24 h fasted rats (water provided ad libitum) by intra-colonic instillation of 1 ml of 4% acetic acid followed by 2 ml of air bolus through a soft pediatric catheter inserted per rectum and reaching up to 8 cm from anal orifice [21]. Rats were divided into six groups (n = 6), where Group I served as NC (1 ml water instilled in colon) and colitis was induced in groups II–VI. Group II served as experimental control (EC) and Group III and IV were administered roxithromycin at doses 5 and 20 mg/kg (RXM 5 and RXM 20), Group V and VI were administered diclofenac (10 mg/kg; D 10) and mesalazine (300 mg/kg; MSZ 300) [19, 22]. The test and reference drugs were administered orally in 1 ml volume 1 h before the induction of colitis, and thereafter, the animals were provided water ad libitum but deprived of food. The rats were euthanized after 24 h of induction of colitis, the distal colon was dissected out and the mucosal injury was scored as described earlier; 0: normal mucosa; 1: localized hyperemia; 2: diffused hyperemia; 3: single linear ulcer (< 1 cm) with inflammation; 4: two or more small linear ulcers (all < 1 cm) with inflammation; 5: two or more linear ulcers (at least one ≥ 1 cm) with inflammation; 6: multiple ulcers with intense hyperemia, hemorrhage and edema [23]. After scoring, a portion of tissue was fixed in 10% buffered formalin for histological examination and immunohistochemical analysis and remaining was stored at − 70 °C for biochemical analysis. In a separate set of experiment, the inflamed colonic tissue was dissected out for the estimation of adherent mucus content.
Preparation of tissue homogenate and post-mitochondrial supernatant (PMS)
The tissue was homogenized in 0.1 M phosphate buffer (pH 7.4) to prepare a 10% homogenate (w/v) for the estimation of reduced glutathione (GSH) and thiobarbituric acid-reactive substances (TBARS). An aliquot of homogenate was centrifuged at 3000 rpm for 10 min to remove nuclear debris and the supernatant was again centrifuged at 12,000 rpm for 20 min at 4 °C to yield PMS that was used for the determination of levels of nitrite and TNF-α [24]. The homogenate for the estimation of PGE2 was prepared as per the protocol provided in ELISA kit.
Determination of tissue GSH levels
Equal volumes of homogenate and 5% trichloroacetic acid were mixed and centrifuged at 3000 rpm for 10 min at 4 °C to get the supernatant. Briefly, to 0.1 ml of supernatant, 4 ml of phosphate buffer and 0.5 ml of 0.04% of 5,5′-dithiobis-2-nitrobenzoic acid were added. The mixture obtained was vortexed and kept in dark for 10 min at room temperature. The absorbance of yellow-colored solution was read at 412 nm. Tissue GSH levels were calculated from the graph plotted using various concentrations of GSH (0.1–200 µg) and expressed as μg/g tissue [25].
Determination of tissue TBARS levels
The levels of TBARS are measure of lipid peroxidation and are quantitative equivalents of malonyldialdehyde (MDA). Briefly, 0.1 ml of homogenate, 0.2 ml of 8.1% sodium dodecylsulphate, 1.5 ml of 20% acetic acid and 1.5 ml of 0.8% thiobarbituric acid were mixed and heated at 95 °C for 1 h. It was allowed to cool and 5 ml of n-butanol/pyridine (15:1) was added and then centrifuged at 4000×g for 10 min. A pink-colored chromogen was obtained and its absorbance was read at 532 nm. TBARS levels were calculated from the standard curve plotted using different concentrations of 1,1,3,3-tetraethoxy propane and expressed as nmol/g tissue [26].
Determination of tissue nitrite levels
Tissue nitrite levels were determined by Griess reaction which involves a simple colorimetric reaction between nitrite, sulphanilamide and N-(1-Naphthyl) ethylenediamine dihydrochloride. Equal volumes of PMS and Griess reagent were mixed and incubated at room temperature for 10 min to obtain a pink-colored product that was read at 550 nm. The nitrite level was determined from the standard curve prepared using different concentrations of sodium nitrite and expressed as µg/g tissue [27].
Determination of tissue TNF-α and PGE2 levels
The TNF-α and PGE2 levels were measured by ELISA Kits as per the protocol provided by the manufacturers.
Estimation of colonic adherent mucus content
Freshly excised colon specimens were weighed and immersed in 0.1% alcian blue prepared in sucrose solution buffered with sodium acetate (pH 5.0) for 2 h at room temperature and the specimens were washed twice with sucrose solution for 15 and 45 min. The mucus-bound dye was extracted with 10 ml of MgCl2 for 2 h and an aliquot of 5 ml was shaken with an equal volume of diethyl ether and centrifuged at 4000 rpm for 10 min. Absorbance of supernatant was read at 580 nm and the amount of alcian blue extracted was calculated from the standard curve and expressed as μg/g tissue [28].
Tissue histology
Formalin fixed tissue specimens were embedded in paraffin and thin sections (4 µm) were cut, stained with hematoxylin and eosin. The sections were examined microscopically for histological changes at 10 × magnification.
Immunohistochemical analysis for NFκB (p65), IL-1β and COX-2
The immunohistochemical analysis was performed on colon tissue with UltraVision Quanto Detection System HRP DAB. Briefly, thin sections of tissue mounted on poly-l-lysine coated slides were deparaffinized in xylene and then rehydrated with ethanol. The slides were washed and blocked for non-specific background staining and were incubated overnight with primary antibodies, anti-rat NFκB (p65) (1:1000), anti-rat IL-1β (1:100) and anti-rat COX-2 (1:100) at 4 °C and washed. The slides were incubated with primary antibody amplifier for 10 min and then incubated with HRP polymer. After washing, a mixture of DAB chromogen and DAB substrate was applied to the specimens and incubated for 5 min. The slides were washed and counterstained with hematoxylin and visualized under light microscope at 20 × magnification. Based on the percent positive cells and DAB staining, the immunoreactivity score (IR score) was determined as described by Fedchenko and Reifenrath [29]. The percent positive cells were scored 0: no positive cells; 1: < 10% of positive cells; 2: 10–50% positive cells; 3: 51–80% positive cells; 4: > 80% positive cells and DAB staining was scored 0: no color reaction; 1: mild reaction; 2: moderate reaction; 3: strong reaction. The product of scoring for percent positive cells and DAB staining gave the final IR score ranging from 0 to 12. The results were expressed as mean ± SEM.
Statistical analysis
Data were analyzed by one-way ANOVA followed by Bonferroni multiple comparison test (post hoc) using GraphPad InStat version 9 (GraphPad Software Inc., San Diego, USA). The results are expressed as mean ± SEM. The differences among the groups were considered statistically significant at p < 0.05.
Results
Effect of roxithromycin on edema formation in paw model
Sub-planter injection of carrageenan produced a significant increase in paw volume as compared to NC group. Treatment with roxithromycin at 5 and 20 mg/kg produced a dose-dependent reduction in paw inflammation (19 and 51% inhibition, respectively). The standard anti-inflammatory drug diclofenac (10 mg/kg) produced 58% reduction in edema formation (Fig. 1).
Effect of roxithromycin on macroscopic and histological changes, ulcer score and adherent mucus content in colitis model
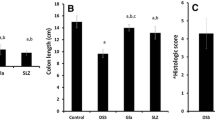
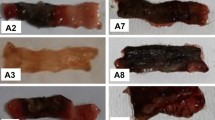
Macroscopically, intra-colonic instillation of acetic acid caused substantial injury to the colonic mucosa and produced hyperemia, ulceration and edema, while histological examination of these specimens revealed damaged mucosa, epithelial destruction, crypt distortion, inflammatory cell influx and loss of architecture. These changes were accompanied by ulceration of colonic mucosa with a mean ulcer score of 5.50 ± 0.22 as compared to NC group where no ulceration was observed. Treatment with roxithromycin produced a dose-dependent reduction in mucosal injury, while treatment with anti-inflammatory drug diclofenac did not afford protection. The effect of RXM 20 was comparable to that of standard drug mesalazine (Fig. 2a–c).
As the mucus coating over the colonic epithelium serves as a protective barrier, the adherent mucus content was measured in the colon tissue. Instillation of acetic acid in the colon produced a significant reduction in mucus content as compared to the normal rats. Treatment of colitic rats with roxithromycin (RXM 20) and mesalazine restored the mucus content to near normal values. The anti-inflammatory drug diclofenac, however, did not produce any change in mucus content as compared to the EC group (Fig. 2d).
Effect of roxithromycin on tissue levels of GSH and TBARS in colitis model
Instillation of acetic acid in colon significantly decreased GSH levels and increased TBARS levels in EC group as compared to NC group (p < 0.001). Treatment of colitic rats with roxithromycin restored the levels of these markers of oxidative stress in a dose-dependent manner. The effect of RXM 20 was comparable to that of MSZ 300, while diclofenac was found to be ineffective (Fig. 3a, b).
Effect of roxithromycin on tissue levels of TNF-α, PGE2 and nitrite in colitis model
As ulcerative colitis is an inflammatory condition, the levels of inflammatory markers such as TNF-α, PGE2 and nitrite were estimated in the colonic tissue. Induction of colitis significantly increased tissue levels of all these markers as compared to NC group. Treatment with roxithromycin produced a dose-dependent reduction in the levels of TNF-α, PGE2 and nitrite (p < 0.001). The effect of RXM 20 was comparable to that of MSZ 300. It was interesting to note that diclofenac did not affect TNF-α and nitrite level in colonic tissue but significantly decreased PGE2 level (Fig. 4a, b).
Effect of roxithromycin on expression of NFκB (p65), IL-1β and COX-2 in colitis model
Intra-colonic instillation of acetic acid produced an increase in tissue expression of NFκB (p65), IL-1β and COX-2 as revealed by the IR score in respective EC group as compared to NC group. Treatment with both roxithromycin and mesalazine suppressed the expression of all these markers. Diclofenac on the other hand produced an effect such as mesalazine in suppressing IL-1β and COX-2, while it was found to be weak in suppressing NFκB (p65) expression (Fig. 5).
Discussion
Roxithromycin, a well-established semi-synthetic macrolide antibiotic possessing a vast antimicrobial spectrum is commonly used for respiratory, urinary and soft tissue infections [30]. In various in vitro and in vivo studies, it has also been shown to exhibit anti-inflammatory, immunomodulatory, anti-arthritic, cardioprotective, and anti-cancer properties [14,15,16, 31,32,33]. In a recent study, it has been shown to afford protection against dextran sulfate sodium-induced colitis in a murine model by inhibiting NFκB signaling and endoplasmic reticulum stress [17]. However, its effect on tissue redox status and inflammation in colitis remains to be explored. In present study, the anti-inflammatory dose of roxithromycin was determined in the carrageenan-induced paw edema model where a dose-dependent inhibitory effect was observed at 5 and 20 mg/kg doses as also reported by Scaglione and Rossoni [14]. These doses were selected for further evaluation of its anti-colitic effect in a rat model where colitis was induced by intra-colonic instillation of acetic acid, a widely used model that mimics human ulcerative colitis. Acetic acid alters the redox status of the intestinal epithelial cells, increases cellular influx and produces inflammatory changes, mucosal and sub-mucosal layer damage and epithelial cell necrosis [34, 35]. In colitic rats, mucosal ulceration was associated with hemorrhage and edema as evident from a significantly high ulcer score which on microscopic examination was revealed as crypt distortion, epithelial denudation, edema and inflammatory cell influx. These changes were associated with a significant reduction in mucus content that provides a protective layer and safeguards the mucosal immune system [36]. Pretreatment with roxithromycin produced a dose-dependent reduction in ulcer score with restoration of mucus content and mucosal integrity. The protection afforded by roxithromycin at 20 mg/kg dose was comparable to that of mesalazine, a drug effective in the treatment of ulcerative colitis and well known to bring about restoration of mucosal barrier by inhibiting TNF-α-mediated goblet cell exfoliation, while the anti-inflammatory drug diclofenac was found ineffective [37]. Diclofenac, though not effective in colitis, was included as a reference drug in view of its comparable anti-inflammatory efficacy with that of roxithromycin in paw edema model and to compare its protective effect, if any, on various oxidative stress and inflammation-related parameters in colitis. Like other NSAIDs, diclofenac has been reported to exacerbate and even induce colitis [38].
Oxidative stress is one of the major contributors to the pathogenesis of UC. Inflammatory cell accumulation by acetic acid, as observed in the present study, is associated with generation of free radicals. Enhanced production of ROS, RNS and unstable byproducts of electron transfer reaction bring about lipid peroxidation and macromolecular damage in the colonic mucosa [39]. Increased level of free radicals has also been reported in the colonic tissue of ulcerative colitis patients [40]. Besides generation of highly reactive species, altered redox modulation as a result of insufficient antioxidant mechanisms also play a critical role in the pathophysiology of UC. Glutathione is a non-enzymatic antioxidant that inhibits oxidative stress via its sulfhydryl group and is considered a first line of defense against free radicals. In the present study, instillation of acetic acid in colon produced oxidative damage that was evident from reduced tissue level of GSH and elevated levels of TBARS as also reported earlier [22]. Nitric oxide produced by iNOS under pathological conditions produces peroxynitrite by interacting with superoxide anions and leads to free radical generation and brings about nitrosative and oxidative stress and initiates a cascade of inflammatory reactions. It also plays an important role in maintenance of inflammation in UC [41]. In a previous study, it has been suggested that direct or indirect inhibition of NOS and resultant reduction in NO generation could be an important targeted approach for the treatment of intestinal inflammatory changes [42]. It is interesting to note that treatment with roxithromycin and mesalazine maintained oxidative homeostasis as evident from the normalized levels of these markers while diclofenac failed to do so. Drug therapies effective in UC have been shown to interfere with free radical generation and cytokine production. Further, compounds possessing antioxidant activity have also been reported to ameliorate colonic inflammation in this condition [39, 43].
Along with inflammatory cell infiltration, free radical mediated generation of pro-inflammatory cytokines such as TNF-α, IL-6 and IL-1β and nuclear translocation of transcription factor NFκB play a pivotal role in modulating mucosal immune system and disrupting epithelial integrity in UC and its progression to cancer [40, 44]. TNF-α is a cardinal pro-inflammatory cytokine and its levels in colonic mucosa and serum correlate with disease activity in UC [45]. In addition, endogenously produced prostaglandins also exert predominant pro-inflammatory effect by promoting vasodilation, leukocyte recruitment and augmentation of action of other inflammatory cytokines [46]. They have also been reported to induce epithelial cell chloride secretion that may lead to diarrhea as observed in inflammatory bowel disease. In a clinical study, the levels of both TNF-α and PGE2 were found to be elevated in biopsy samples obtained from patients with active disease as compared to the levels in patients in remission [8]. This is also supported by enhanced expression of COX-2, an enzyme involved in PGE2 synthesis, in epithelial cells of inflamed colon [47]. The elevated tissue levels of TNF-α and PGE2 as observed in colitic rats in the present study were significantly reduced by both roxithromycin and mesalazine. Elevated levels of TNF-α and IL-1β are well known to regulate COX-2 expression through NFκB, a redox-sensitive transcription factor. In an inactive state, NFκB is bound to its inhibitor IκB and gets activated in response to binding of cytokines to their receptors and to various oxidants. The activation of NFκB, by phosphorylation, ubiquitination and subsequent degradation of IκB by proteasome pathway involving IκB kinase, is followed by its translocation into the nucleus where it binds to the promoter region of multiple pro-inflammatory genes, initiates transcription and accelerates a cascade of inflammation. One of the subunits of NFκB, namely p65 plays a significant role in inflammation and immunomodulation [48, 49]. In the present study, tissue expression of NFκB (p65), IL-1β and COX-2 was found to be elevated in colitic rats and was markedly suppressed by treatment with roxithromycin and mesalazine, while the anti-inflammatory drug diclofenac primarily suppressed IL-1β and COX-2 expression. Recently, roxithromycin has been shown to attenuate NFκB DNA binding activity, IκB degradation and endoplasmic reticulum stress in colitis model [17]. Together, these findings suggest that inhibition of oxidative stress and TNF-α generation coupled with inhibition of NFκB-mediated pro-inflammatory signaling is the mechanism by which roxithromycin affords protection in colitis. Various clinical and preclinical studies have shown that drugs inhibiting TNF-α and NFκB signaling produce beneficial effects in colitis [11, 17, 50].
In conclusion, the present study demonstrates that roxithromycin affords protection in acetic acid-induced colitis by inhibiting inflammation, maintaining oxidative homeostasis and preserving colonic mucosal integrity. Its protective effect is mediated through attenuation of NFκB-mediated pro-inflammatory signaling. In view of the findings of the present study, roxithromycin appears to be a promising therapeutic option for ulcerative colitis.
References
Kornbluth A, Sachar DB. Ulcerative colitis practice guidelines in adults: American college of gastroenterology, practice parameters committee. Am J Gastroenterol. 2010;105:501–23.
Danese S, Fiocchi C. Ulcerative colitis. N Engl J Med. 2011;365:1713–25.
Baumgart DC, Carding SR. Inflammatory bowel disease: cause and immunobiology. Lancet. 2007;369:1627–40.
Sartor RB. Mechanisms of disease: pathogenesis of Crohn’s disease and ulcerative colitis. Nat Clin Pract Gastroenterol Hepatol. 2006;3:390–407.
Xavier RJ, Podolsky DK. Unraveling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–34.
Harris DW, Smith PR, Swan CH. Determination of prostaglandin synthetase activity in rectal biopsy material and its significance in colonic disease. Gut. 1978;19:875–7.
Sharon P, Ligumsky M, Rachmilewitz D, Zor U. Role of prostaglandins in ulcerative colitis. Enhanced production during active disease and inhibition by sulfasalazine. Gastroenterology. 1978;75:638–40.
Vong L, Ferraz JGP, Panaccione R, Beck PL, Wallace JL. A pro-resolution mediator, prostaglandin D2, is specifically up-regulated in individuals in long-term remission from ulcerative colitis. PNAS. 2010;107:12023–7.
Keshavarzian A, Haydek J, Zabihi R, Doria M, D′Astice M, Sorensen JRJ. Agents capable of eliminating reactive oxygen species: catalase, WR-2721 or Cu(II)2(3,5-DIPS)4 decrease experimental colitis. Dig Dis Sci. 1992;37:1866–73.
Keshavarzian A, Morgan G, Sedghi S, Gordon JH, Doria M. Role of reactive oxygen metabolites in experimental colitis. Gut. 1990;31:786–90.
Ordás I, Mould DR, Feagan BG, Sandborn WJ. Anti-TNF monoclonal antibodies in inflammatory bowel disease: pharmacokinetics-based dosing paradigms. Clin Pharmacol Ther. 2012;91:635–46.
Dignass A, Lindsay JO, Sturm A, Windsor A, Colombel JF, Allez M, et al. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 2: current management. J Crohns Colitis. 2012;6:991–1030.
Mowat C, Cole A, Windsor A, Ahmad T, Arnott I, Driscoll R, et al. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2011;60:571–607.
Scaglione F, Rossoni G. Comparative anti-inflammatory effects of roxithromycin, azithromycin and clarithromycin. J Antimicrob Chemother. 1998;41:47–50.
Kwiatkowska B, Maślińska M. Macrolide therapy in chronic inflammatory diseases. Mediators Inflamm. 2012;2012:636157.
Tamaoki J, Kadota J, Takizawa H. Clinical implications of the immunomodulatory effects of macrolides. Am J Med. 2004;117:5S–11S.
Choi Y, Koh SJ, Lee HS, Kim JW, Kim GB, Lee KL, et al. Roxithromycin inhibits nuclear factor kappaB signaling and endoplasmic reticulum stress in intestinal epithelial cells and ameliorates experimental colitis in mice. Exp Biol Med (Maywood). 2015;240:1664–71.
Winter CA, Risley EA, Nuss GW. Carrageenin-induced edema in hind paw of the rat as an assay for anti-inflammatory drugs. Proc Soc Exp Biol Med. 1962;111:544–7.
Pandey A, Kumar VL. Protective effect of metformin against acute inflammation and oxidative stress in rat. Drug Dev Res. 2016;77:278–84.
Kumar VL, Chaudhary P, Ramos MV, Mohan M, Matos MPV. Protective effect of proteins derived from the latex of Calotropis procera against inflammatory hyperalgesia in monoarthritic rats. Phytother Res. 2011;25:1336–41.
Myers BS, Martin JS, Dempsey DT, Parkman HP, Thomas RM, Ryan JP. Acute experimental colitis decreases colonic circular smooth muscle contractility in rats. Am J Physiol. 1997;273:G928–36.
Al-Rejaie SS, Abuohashish HM, Al-Enazi MM, Al-Assaf AH, Parmar MY, Ahmed MM. Protective effect of naringenin on acetic acid-induced ulcerative colitis in rats. World J Gastroenterol. 2013;19:5633–44.
Moriss GP, Beck PL, Herridge MS, Depew WT, Szewczuk MR, Wallace JL. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology. 1989;96:795–803.
Hamiza OO, Rehman MU, Tahir M, Khan R, Khan AQ, Lateef A, et al. Amelioration of 1,2 dimethylhydrazine (DMH) induced colon oxidative stress, inflammation and tumor promotion response by tannic acid in wistar rats. Asian Pac J Cancer Prev. 2012;13:4393–402.
Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–7.
Okhawa H, Ohishi N. Assay of lipid peroxide in animal tissue by thio-barbituric acid reaction. Anal Biochem. 1979;95:351–8.
Sasaki S, Miurat T, Nishikawa S, Yamada K, Hirasue M, Nakane A. Protective role of nitric oxide in Staphylococcus aureus infection in mice. Infect Immun. 1998;66:1017–22.
Popov SV, Markov PA, Nikitina IR, Petrishev S, Smirnov V, Ovodov YS. Preventive effect of a pectic polysaccharide of the common cranberry Vaccinium oxycoccos L. on acetic acid-induced colitis in mice. World J Gastroenterol. 2006;12:6646–51.
Fedchenko N, Reifenrath J. Different approaches for interpretation and reporting of immunohistochemistry analysis results in the bone tissue—a review. Diagn Pathol. 2014;9:221.
Bryskier A. Roxithromycin: review of its antimicrobial activity. J Antimicrob Chemother. 1998;41:1–21.
Otsuki N, Iwata S, Yamada T, Hosono O, Dang NH, Hatano R, et al. Modulation of immunological responses and amelioration of collagen-induced arthritis by the novel roxithromycin derivative 5-I. Mod Rheumatol. 2015;25:562–70.
Chernomortseva ES, Pokrovskii MV, Pokrovskaia TG, Artiushkova EB, Gureev VV. Experimental study of cardioprotective and endothelioprotective action of macrolides and azalides. Eksp Klin Farmakol. 2009;72:29–31.
Aoki D, Ueno S, Kubo F, Oyama T, Sakuta T, Matsushita K, et al. Roxithromycin inhibits angiogenesis of human hepatoma cell in vivo by suppressing VEGF production. Anticancer Res. 2005;25:133–8.
Randhawa PK, Singh K, Singh N, Jaggi AS. A review on chemical-induced inflammatory bowel disease models in rodents. Korean J Physiol Pharmacol. 2014;18:279–88.
Kawada M, Arihiro A, Mizoguchi E. Insights from advances in research of chemically induced experimental models of human inflammatory bowel disease. World J Gastroenterol. 2007;13:5581–93.
Johansson ME, Hansson GC. Mucus and the goblet cell. Dig Dis. 2013;31:305–9.
Jarry A, Muzeau F, Laboisse C. Cytokine effects in a human colonic goblet cell line. Cellular damage and its partial prevention by 5 aminosalicylic acid. Dig Dis Sci. 1992;37:1170–8.
Baert F, Hart J, Blackstone MO. A case of diclofenac-induced colitis with focal granulomatous change. Am J Gastroenterol. 1995;90:1871–3.
Piechota-Polanczyk A, Fichna J. Review article: the role of oxidative stress in pathogenesis and treatment of inflammatory bowel diseases. Naunyn-Schmiedeberg’s Arch Pharmacol. 2014;387:605–20.
Rana SV, Sharma S, Prasad KK, Sinha SK, Singh K. Role of oxidative stress and antioxidant defence in ulcerative colitis patients from north India. Indian J Med Res. 2014;139:568–71.
Kolios G, Valatas V, Ward SG. Nitric oxide in inflammatory bowel disease: a universal messenger in an unsolved puzzle. Immunology. 2004;113:427–37.
Rachmilewitz D, Karmeli F, Okon E, Bursztyn M. Experimental colitis is ameliorated by inhibition of nitric oxide synthase activity. Gut. 1995;37:247–55.
Nieto N, Torres MI, Fernández MI, Giron MD, Ríos A, Suárez MD, et al. Experimental ulcerative colitis impairs antioxidant defense system in rat intestine. Dig Dis Sci. 2000;45:1820–7.
Wang S, Liu Z, Wang L, Zhang X. NF-κB signaling pathway, inflammation and colorectal cancer. Cell Mol Immunol. 2009;6:327–34.
Sands BE, Kaplan GG. The role of TNF-alpha in ulcerative colitis. J Clin Pharmacol. 2007;47:930–41.
Ricciotti E, FitzGerald GA. Prostaglandins and inflammation. Arterioscler Thromb Vasc Biol. 2011;31:986–1000.
Singer II, Kawka DW, Schloemann S, Tessner T, Riehl T, Stenson WF. Cyclooxygenase 2 is induced in colonic epithelial cells in inflammatory bowel disease. Gastroenterology. 1998;115:297–306.
Andersen L, Jorgensen VL, Perner A, Hansen A, Eugen-Olsen J, Rask-Madsen J. Activation of nuclear factor κB in colonic mucosa from patients with collagenous and ulcerative colitis. Gut. 2005;54:503–9.
Verma S, Kumar VL. Attenuation of gastric mucosal damage by artesunate in rat: modulation of oxidative stress and NFκB mediated signaling. Chem Biol Interact. 2016;257:46–53.
Park SC, Jeen YT. Current and emerging biologics for ulcerative colitis. Gut Liver. 2015;9:18–27.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Responsible Editor: John Di Battista.
Rights and permissions
About this article
Cite this article
Ahmad, H., Verma, S. & Kumar, V.L. Effect of roxithromycin on mucosal damage, oxidative stress and pro-inflammatory markers in experimental model of colitis. Inflamm. Res. 67, 147–155 (2018). https://doi.org/10.1007/s00011-017-1103-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-017-1103-x