Abstract
Background
Fibroblast-like synoviocytes (FLS) play an essential role in the pathogenesis of chronic inflammatory diseases, such as rheumatoid arthritis. Paeonol (Pae) is a phenolic compound found in many traditional Chinese medicine remedies. However, the effects of Pae on TNF-α-stimulated FLS and the underlying molecular mechanism are unknown. In this study, we aimed to investigate the anti-proliferative and anti-inflammatory effect of Pae against activated FLS.
Materials and methods
Rheumatoid arthritis FLS (RA-FLS) were pre-treated with different doses (25, 50, and 100 µM) of Pae or miR-155 inhibitor for 30 min or transfected with miR-155 mimic, and then treated with 50 ng/mL of tumor necrosis factor alpha (TNF-α) for 1 h. Cells that were untreated served as control. At 24 h after drug pretreatment, the proliferation of FLS was detected using the MTT assay. The concentrations of interleukin IL-6 and IL-1β in cell culture supernatant were examined by enzyme-linked immunosorbent assay (ELISA), and mRNA levels of Foxo3 and miR-155 expression in FLS were quantified by reverse transcription-polymerase chain reaction (RT-PCR). Protein expressions of forkhead box O3 (FOXO3), cyclin D1, and c-Myc were detected by Western Blot.
Results
TNF-α induced the proliferation of FLS, whereas Pae inhibited this proliferation in a dose-dependent manner. Pae attenuated TNF-α-induced production of IL-6 and IL-1β, and inhibited the expression of miR-155 in a dose-dependent manner. In addition, miR-155 inhibitor decreased TNF-α-induced proliferation of FLS, and attenuated TNF-α-induced production of IL-6 and IL-1β. In addition, pretreatment with different doses of Pae or miR-155 inhibitor markedly attenuated TNF-α-induced decrease in protein expression of FOXO3 in FLS. Mechanistic studies revealed FOXO3 as miR-155-5p direct target and inhibition of FOXO3 led to the abolishment of Pae protective effects.
Conclusions
Paeonol protected against TNF-α-induced proliferation and cytokine release of FLS by decreasing the expression of miR-155 and upregulating its target FOXO3.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Fibroblast-like synoviocytes (FLS) play a crucial role in the pathogenesis of chronic inflammatory diseases, such as rheumatoid arthritis. During the progression of rheumatoid arthritis, constant inflammatory processes take place in the synovial membrane, which results in cartilage damage, joint destruction, and deformation. The proliferative and apoptotic processes significantly increase the number of FLS. FLS, together with other immune cells such as macrophages, lymphocytes, and neutrophils, create an inflammatory environment in the synovium which recruits more immune cells and contributes to the joint destruction [1–3]. Stimulated by inflammatory cytokines such as tumor necrosis factor (TNF-α), FLS produce a number of pro-inflammatory signaling molecules, including interleukin (IL)-6 and IL-1β, the release of which affect other cells and further amplification of the inflammatory processes [3].
Paeonol (Pae), a phenolic compound found in peony such as Paeonia suffruticosa [4] and Arisaema erubescens [4], is a component found in many traditional Chinese medicine remedies [5] that is known for its therapeutic effects against a multitude of diseases. For instance, Pae has been shown to increase cortical cytochrome oxidase and improve behavior in a rat model of Alzheimer’s disease [6]. It was equally reported that Pae can reduce cerebral infarction and microglia activation in ischemia–reperfusion injured rats [6]. More importantly, Pae has been proven to exert anti-inflammatory and analgesic effects in carrageenan-induced thermal hyperalgesia [7]. Additional studies have equally demonstrated the inhibitory effects of Pae on anaphylactic reaction through regulating histamine and TNF-α [8] and on the activity of monoamine oxidase [9]. It was also demonstrated that Pae can exert some anti-inflammatory and immune-regulatory effects and has been proven effective in the treatment of rheumatoid arthritis (RA). However, the molecular mechanisms underlying the therapeutic effects of Pae, especially regarding the proliferation of FLS and its associated inflammatory responses, are still largely unknown.
In recent years, the small non-coding RNAs (miRNAs) have been the focus of a plethora of scientific investigations due to their involvement in the regulation of many physiological and pathological processes, including cell metabolism, invasion, migration, proliferation, and inflammation. Previous studies have conveyed that mir-155 works as a pro-inflammatory regulator in experimental and clinical arthritis. In another in vivo study, miR-155 was identified as a possible therapeutic target for autoimmune arthritis. However, whether there is a connection between mir-155 and the anti-inflammatory properties of Pae during RA treatment and whether this potential link is involved in the proliferation of FLS is still unknown.
Therefore, the present study was designed to investigate the effects of Pae on TNF-α-induced proliferation and cytokine release of FLS. We similarly aimed to elucidate the functional role of mir-155-5p in these potential effects.
Methods
Cells and reagents
Cells
Human rheumatoid arthritis fibroblast-like synoviocytes (RA-FLS) (Cell application, Inc.). Reagents: Pae (Sigma–Aldrich Inc., St. Louis, MO, USA); ELISA kit for TNF-α, IL-6, and IL-1β (Cusabio Biotech Inc., Wuhan, China); miR155-5p inhibitor (GenePharma Inc., Shanghai, China); miR-155-5p mimic (RiboBio, Guangzhou, China); FOXO3 siRNA (abm); RIPA tissue lysis buffer (Beyotime Biotechnology Inc., Shanghai, China); BCA protein quantification kit (Thermo Fisher Scientific Inc., Rockford, IL, USA); 30% acrylamide; Tris–HCl; sodium dodecyl sulfate (SDS); ammonium persulfate; tetramethylethylenediamine (TEMED); polyvinylidene fluoride (PVDF) membrane; phosphate buffering solution (PBS); Tween-20; developing powder; fixing powder; X-ray films; primary antibody against FOXO3A (FOXO3; Cusabio Biotech Inc., Wuhan, China); Anti-Cyclin D1 Antibody (abm, Catalogue #: Y050095), Anti-c-myc Antibody (abm, Catalogue #: Y054036), primary antibody against β-actin (ZSGB-Bio Inc., Beijing, China); and HRP-labeled goat anti-rabbit secondary antibody (Invitrogen, Carlsbad, CA, USA).
Cell transfection
Cells were transiently transfected with miR-155-5p mimic (50 nmol/L), miR-155-5p inhibitor (100 nmol/L), or their respective negative controls using Lipofectamine® 2000 Reagent (Thermo Fisher Scientific) for 24 h in line with the vendor’s guidelines. For silencing of FOXO3, cells were equally transfected with Foxo3 siRNA (abm) or negative control using Lipofectamine 2000 reagent protocol as indicated above.
MTT assay
FLS were pre-treated with Pae at different doses (25, 50, and 100 µM), 10 pg/mL of miR-155 inhibitor for 30 min or transfected with miR-155 mimic, and then treated with 50 ng/mL of TNF-α for 1 h (n = 5 wells/group). Cells that were untreated served as control. At 24 h after Pae or miR-155 treatment, the proliferation of FLS was detected using the MTT assay. Following culture, 20 µL of MTT solution (5 mg/mL) was added to each well, and the cells were cultured at 37 °C for an additional 4 h. The cell supernatants were removed, and 150 µL of DMSO was added to each well. The plates were then shaken for 15 min to dissolve crystals, and the absorbance of each sample was detected at 490 nm (A490) using an ELISA microplate reader.
Enzyme-linked immunosorbent assay (ELISA)
FLS were pre-treated with Pae at different doses (25, 50, and 100 µM), 10 pg/mL of miR-155 inhibitor for 30 min or transfected with miR-155 mimic, and then treated with 50 ng/mL of TNF-α for 1 h (n = 5/group). At 24 h after treatment with Pae or miR-155 inhibitor, the concentrations of IL-6 and IL-1β in cell culture supernatant were detected by ELISA according to the instructions of kits.
Quantitative real-time PCR analysis
To measure the expression level of miR-155-5p, the total RNA extracted from the FLS with TRIZOL reagent was reverse-transcribed using the miScript II RT Kit (QIAGEN). For the determination of Foxo3 mRNA expression, iScript™ Advanced cDNA Synthesis Kit for RT-qPCR was used with the Prime PCR™ Assay Validation Report primer reagent. The qRT-PCR experiment was achieved using the CFX96 Touch™ Real-Time PCR Detection System (Bio-Rad). The amplification conditions were as follows: 30 s at 95 °C, followed by 45 cycles at 95 °C for 5 s and 58 °C for 34 s. The relative expression of miR-155-5p was evaluated using the ∆∆Ct method. U6 small nuclear RNA (snRNA) was used as a normalizing control.
Luciferase assay
A fragment of FOXO3 including its 3′-UTR (WT) was PCR-amplified and cloned into psiCHECK-2 vectors. Site-directed mutagenesis of miR-155-5p seed sequence in FOXO 3′-UTR (MUT) was performed with the QuikChange II Site-Directed Mutagenesis Kit from Agilent Technologies (CA, USA). Cells (5 × 104/well) were seeded in 24-well plates and cultured for 24 h, cotransfected with hsa-mir-155-5p mimic or negative control (NC) and FOXO-3′-UTR-MUT or WT FOXO3 3′-UTR vectors using Lipofectamine 2000 Reagent (Invitrogen, Carlsbad, CA). After incubation, cells were lysed and the relative dual-luciferase activity was detected 48 h following transfection with the Dual-Luciferase Reporter Assay kit (Promega) following the manufacturer’s guidelines. The transfection efficiency was checked by normalization with the Renilla luciferase activity obtained from phRG-TK.
Western blot
Protein expressions of FOXO3, Cyclin D1, and c-Myc in FLS were detected by Western Blot. Total proteins in FLS were extracted and separated by sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE) at 120 V, and then transferred to polyvinylidene fluoride (PVDF) membranes at 100 V for 120 min. Membranes were blocked with 5% non-fat milk powder for 1 h at room temperature, and then incubated with primary antibodies against FOXO3, Cyclin D1, and c-Myc at 4 °C overnight. The membranes were then incubated with goat anti-rabbit secondary antibody labeled with HRP at room temperature for 1 h. Membranes were treated with electro-chemi-luminescence (ECL) solution, and films were exposed in a dark room. Densitometric analysis was done using the Image J software.
Statistical analysis
Results were expressed as mean ± SEM. One-way or two-way analysis of variance (ANOVA) was utilized to compare differences among three or more groups, followed by Bonferroni post hoc testing for multiple comparisons. Student’s t test was utilized to compare differences between two groups. P values lesser than 0.05 were regarded statistical significant. Figures and statistical analysis were made by the GraphPad Prism 6.0 software (GraphPad Software Inc., La Jolla, CA, USA).
Results
Pae inhibited TNF-α-induced proliferation and inflammation of FLS
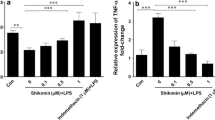
To investigate the effect of Pae on cell proliferation, FLS were pre-treated with Pae at different doses (25, 50, and 100 µM) for 30 min, and then treated with 50 ng/mL of TNF-α for 1 h. At 24 h after treatment with Pae, the proliferation of FLS was measured using the MTT assay. The results (Fig. 1a) showed that comparatively to the control group, TNF-α induced the proliferation of FLS. Compared to the TNF-α treatment group, pretreatment with Pae inhibited the proliferation of FLS in a dose-dependent manner. The most significant (P < 0.001) inhibitory effect was obtained with 100 µM Pae treatment.
Pae inhibited TNF-α-induced proliferation of FLS. a FLS were pre-treated with Pae at different doses (25, 50, and 100 µM) for 30 min, and then treated with 50 ng/mL of TNF-α for 1 h. Cells that were untreated served as control. At 24 h after treatment with Pae, the proliferation of FLS was detected using the MTT assay. TNF-α induced the proliferation of FLS, whereas Pae inhibited this proliferation in a dose-dependent manner (mean ± SEM, n = 5/group). b Concentrations of IL-6 in cell culture supernatant were detected by ELISA at 24 h after Pae treatment. Pae markedly attenuated TNF-α-induced production of IL-6 in a dose-dependent manner (mean ± SEM, n = 5/group). b Concentrations of IL-1β in cell culture supernatant were detected by ELISA at 24 h after Pae treatment. Pae markedly attenuated TNF-α-induced production of IL-1β in a dose-dependent manner (mean ± SEM, n = 5/group). *p < 0.05; **p < 0.01 and ***p < 0.001 when compared to the control group. ## p < 0.0001; # p < 0.01 as compared to the cells treated uniquely with TNF-α. Ns non-significant, TNF tumor necrosis factor, Pae paeonol
To assess the effect of Pae on inflammation, FLS were pre-treated with Pae at different doses (25, 50, and 100 µM) for 30 min, and then treated with 50 ng/mL of TNF-α for 1 h. The concentrations of IL-6 and IL-1β in cell culture supernatant were subsequently measured using the ELISA assay at 24 h after Pae treatment. Pae pretreatment markedly attenuated TNF-α-induced production of IL-6 (p < 0.01; Fig. 1b) and IL-1β (p < 0.01; Fig. 1c) dose-dependently.
Pae inhibited TNF-α-induced miR-155 expression
The expression level of miR-155 in FLS was determined using RT-PCR at 24 h after Pae pretreatment. The results (Fig. 2) showed that in cells treated uniquely with TNF-α, the miR-155 expression was significantly increased when compared with the control group. On the contrary, we found that Pae pretreatment inhibited the expression of miR-155 in a dose-dependent manner. In particular, pretreatment with 100 µM of Pae decreased the relative expression of miR-155 markedly in FLS (p < 0.0001; Fig. 2).
Pae inhibited TNF-α-induced mir-155 expression in FLS. FLS were pre-treated with Pae at different doses (25, 50, and 100 µM) for 30 min, and then treated with 50 ng/mL of TNF-α for 1 h. Pae inhibited the expression of miR-155 in a dose-dependent manner (mean ± SEM, n = 5/group). *p < 0.05; **p < 0.01 when compared to the control group. # p < 0.05 and ## p < 0.01 when compared to the TNF-α group. TNF: tumor necrosis factor. Pae paeonol, ns $ non-significant when compared to TNF-α
Pae inhibited TNF-α-induced proliferation of FLS and cytokine release by downregulating miR-155
To confirm whether the inhibitory effect of Pae on cell proliferation and cytokine release was due to the decreased miR-155 expression, FLS were pre-treated with 10 pg/mL of miR-155 inhibitor for 30 min, and then treated with 50 ng/mL of TNF-α for 1 h. At 24 h after treatment with miR-155 inhibitor, the proliferation of FLS was examined by MTT assay. The results showed that TNF-α induced the proliferation of FLS, whereas miR-155 inhibitor decreased the proliferation compared to cells treated uniquely with TNF-α (p < 0.01; Fig. 3a). Similarly, pretreatment with miR-155 inhibitor markedly attenuated TNF-α-induced production of IL-6 (Fig. 3b) and IL-1β (p < 0.05; Fig. 3c). Western Blot analysis showed that pretreatment with different doses of Pae as well as miR-155 inhibitor markedly reversed TNF-α-induced decrease in protein expression of FOXO3 in FLS (p < 0.001; Fig. 3c, d). In the inverse, transfection with mir-155 mimic led to the abolishment of Pae on the TNF-α-induced FLS proliferation, cytokine release, and FOXO3 expression. Pae induced FOXO3A than miR155 inhibitor did, indicating Pae may upregulate FOXO3A, not only by miR155 but also by other pathways. These results indicated that Pae-mediated inhibition of mir-155 and increase of FOXO3 is involved in its inhibitory effects on TNF-α-induced FLS proliferation and inflammation of FLS.
MiR-155 inhibitor inhibited TNF-α-induced proliferation of FLS and cytokines production. FLS were pre-treated with 10 pg/mL of miR-155 inhibitor for 30 min, and then treated with 50 ng/mL of TNF-α for 1 h. a At 24 h after treatment with miR-155 inhibitor, the proliferation of FLS was examined by MTT assay. MiR-155 inhibitor decreased the proliferation compared to TNF-α alone group (mean ± SEM, n = 5/group). b Concentrations of IL-6 in cell culture supernatant were detected by ELISA at 24 h after treatment with miR-155 inhibitor. Pretreatment with miR-155 inhibitor markedly attenuated TNF-α-induced production of IL-6 (mean ± SEM, n = 5/group). c Concentrations of IL-1β in cell culture supernatant were detected by ELISA at 24 h after treatment with miR-155 inhibitor. Pretreatment with miR-155 inhibitor markedly attenuated TNF-α-induced production of IL-1β (mean ± SEM, n = 5/group). d Western blot results indicated that Pae and miR-155 inhibitor attenuated TNF-α-induced decrease in protein expression of FOXO3 in FLS. Protein expression of FOXO3 was detected by western blot at 24 h after drug pretreatment. Western blot results were then quantified and relative protein expression of FOXO3 in experimental groups and the control group was presented. Pretreatment with Pae and miR-155 inhibitor attenuated TNF-α-induced decrease in protein expression of FOXO3 in FLS markedly (mean ± SEM, n = 5/group). *p < 0.05; **p < 0.01; ***p < 0.001 and ****p < 0.0001 when compared to the negative control (NC) group. # p < 0.05, ## p < 0.01, ### p < 0.001 as compared to the TNF-α group. TNF tumor necrosis factor, Pae paeonol, IL interleukin
miR-155-5p directly binds to FOXO3 3′-UTR
We next performed bioinformatics analyses using the online Targetscan bioinformatics tool to find whether miR-155-5p directly binds to FOXO3 3′ UTR. The result indicated that the 3′-untranslated region (3′-UTR) of FOXO3 possesses one binding site for miR-155 (Fig. 4a). To further confirm whether FOXO3 3′-UTR is a direct target for miR-155, FOXO3 3′-UTR reporter luciferase assay was achieved. The results revealed that miR-155-5p mimic significantly altered FOXO3 3′-UTR reporter luciferase activity (Fig. 4b). mRNA expression of FOXO3 did not change following the delivery of miR-155-5p mimic (Fig. 4c). Western blot analyses indicated that miR-155-5p mimic negatively regulated the expression of FOXO3 protein, while mir-155-5p inhibitor led to increased expression of FOXO3 (Fig. 4d, e). These data suggest that miR-155-5p negatively regulates the expression of FOXO3 protein by acting on FOXO3 mRNA 3′-UTR post-transcriptionally.
FOXO3 3′-UTR is a target for miR-155-5p. a Diagram of FOXO3 3′-UTR-containing reporter constructs. Mut contains 7-base-mutation at the miR-155 target region, abolishing its binding. b Reporter assay, FLS, with cotransfection of 500 ng Wt- or mut-reporter and 50 nM control-miR, or miR-155-5p mimic as indicated. c mRNA expression of FOXO3 was not affected by miR-155-5p. d Representative images of protein expression of FOXO3 as determined by western blot analysis. e Densitometry analysis of protein expression of FOXO3 as determined by western blot analysis. Each bar represents values from three independent experiments performed in quadruplicates. ***p < 0.001 when compared to negative control, ****p < 0.0001 when compared to control inhibitor (ctrl inhibitor), and #### p < 0.0001 when compared to control mimic (ctrl mimic)
Pae inhibits TNF-α-induced synoviocyte proliferation by upregulation of FOXO3 via inhibition of mir-155-5p
Due to the effect of Pae on miR-155 and FOXO3 and due to FOXO3 targeting by mir-155-5p, we planned to evaluate the role of FOXO3 in the anti-proliferative and anti-inflammatory effects of Pae. The results showed that the silencing of FOXO3 reversed the anti-proliferative effect of Pae (Fig. 5a). Especially, the expression of cell proliferation markers Cyclin D1 and c-Myc was significantly and dose-dependently inhibited in TNF-α activated FLS following Pae pretreatment (Fig. 5b, c). However, these effects were reversed in FLS transfected with FOXO3 siRNA (Fig. 5b, c). Similarly, silencing of FOXO3 resulted in the annihilation of the anti-inflammatory effects of Pae as demonstrated by the production of IL-6 and IL-β in different treatment groups. These results suggests that Pae inhibits TNF-α-induced synoviocyte proliferation by upregulation of FOXO3 via inhibition of mir-155-5p.
Silencing of FOXO3 annihilated the anti-proliferative effect of Pae. a After FOXO3 silencing with FOXO3 siRNA, MTT assay showed that the inhibitory effect of Pae was abolished. b Representative images of protein expression of cell cycle markers as determined by western blot analysis. c Densitometry analysis of protein expression of Cyclin D1 and c-Myc as determined by western blot analysis. d Concentrations of IL-6 in cell culture supernatant were detected by ELISA at 24 h after Pae treatment. FOXO3 siRNA abolished the TNF-α-induced production of IL-6 in FLS. e Concentrations of IL-1β in cell culture supernatant were detected by ELISA at 24 h after Pae treatment. FOXO3 siRNA abolished the TNF-α-induced production of IL-1β in FLS. Each bar represents values from three independent experiments performed in triplicates. *p < 0.05; **p < 0.01, ***p < 0.001, and ***p < 0.001 when compared to the control siRNA (ctrl siRNA) group. # p < 0.05, ## p < 0.01, AND ### p < 0.001 as compared to the TNF-α group. TNF tumor necrosis factor, Pae paeonol, IL interleukin, ns $ non-significant when compared to TNF-α group
Discussion
Fibroblast-like synoviocytes (FLS) play an essential role in the pathogenesis of chronic inflammatory diseases, such as rheumatoid arthritis (RA), and are thought to be involved in both inflammation and joint destruction in RA [10–12]. At present, effective therapies for RA are generally directed against the immune system, and the development of new drugs is highly encouraged [13]. Targeting mesenchymal FLS may be effective against both inflammation and tissue destruction in the joint [13, 14].
Paeonol (Pae) is a phenolic compound found in many traditional Chinese medicine medications. Preclinical studies show that Pae is able to diminish pain, joint swelling, synovial hypertrophy, and the severity of bone erosion and cartilage degradation in experimental arthritis [15]. However, the effects of Pae on activated FLS and the underlying molecular mechanism are ill-defined.
In this study, we investigated the effects of Pae on TNF-α-induced FLS proliferation and the associated release of pro-inflammatory cytokines and found that Pae protected against TNF-α-induced FLS proliferation, decreased the expression of cell proliferation markers Cyclin D1 and c-Myc, and decreased the levels of inflammatory factors IL-6 and IL-1β. The present results indicated the anti-proliferative and anti-inflammatory properties of Pae against activated FLS.
Over-expression of miR-155 has been reported to cause chronic inflammatory state in human [16], and there was higher expression of miR-155 in tissues and synovial fibroblasts of patients with autoimmune disorders such as rheumatoid arthritis [17]. MiR-155 was also overexpressed in patients with atopic dermatitis and it modulates proliferative responses of T lymphocytes by targeting cytotoxic T-lymphocyte-associated antigen 4 [18]. Meanwhile, the expression of miR-155 in patients with multiple sclerosis increased in peripheral and CNS-resident myeloid cells, such as circulating monocytes and activated microglia [19]. To verify whether Pae may interfere with the expression of mir-155-5p, we measured the effect of mir-155-5p in different treatment groups. We found that Pae decreased the expression of miR-155, and both Pae and miR-155 inhibitor inhibited TNF-α-induced proliferation and cytokine release of FLS. Further investigations indicated that Pae exerts its anti-proliferative and anti-inflammatory effects by downregulating miR-155. Pae may be useful in treating chronic inflammatory disease such as rheumatoid arthritis.
In addition, we have shown that the protein expression of FOXO3 increased in Pae or MiR-155 inhibitor pretreatment groups, compared to cells treated uniquely with TNF-α. FOXO3 belongs to the O subclass of the forkhead family of transcription factors, and has distinct fork head DNA-binding domain. FOXO3 is translocated out of the nucleus after it is phosphorylation by proteins such as Akt and protein kinase B in the PI3K signaling pathway [20]. FOXO3 was reported to induce apoptosis by upregulating genes necessary for cell death including PUMA and Bim [21] or downregulating anti-apoptotic proteins such as FLIP [22]. In addition, FOXO3 protected from oxidative stress by upregulating antioxidants including catalase and MnSOD. It was shown that female exhibited a dramatic age-dependent infertility due to premature ovarian failure in FOXO3-knockout mice [23]. Thus, the increase in FOXO3 after Pae treatment and inhibition of MiR-155 may induce the apoptosis of FLS. This explains the decreased proliferation rate of FLS after treatment with Pae and miR-155 inhibitor. The decreased number of FLS may also result in decreased release of inflammatory cytokines.
In conclusion, we revealed that Pae protected against TNF-α-induced proliferation and cytokine release of FLS by upregulating, partly, FOXO3 expression via inhibition of miR-155-5p expression. Although research investigations are needed to unveil more underlying molecular mechanisms, Pae may represent a novel therapeutic approach for chronic inflammatory joint diseases.
References
Chang SK, Gu Z, Brenner MB. Fibroblast-like synoviocytes in inflammatory arthritis pathology: the emerging role of cadherin-11. Immunol Rev. 2010;233:256–66.
Dasuri K, Antonovici M, Chen K, Wong K, Standing K, Ens W, El-Gabalawy H, Wilkins JA. The synovial proteome: analysis of fibroblast-like synoviocytes. Arthr Res Ther. 2004;6:R161–R168.
Bartok B, Firestein GS. Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol Rev. 2010;233:233–55.
Ducki S, Hadfield JA, Lawrence NJ, Zhang X, McGown AT. Isolation of paeonol from Arisaema erubescens. Planta Med. 1995;61:586–7.
Deng C, Yao N, Wang B, Zhang X. Development of microwave-assisted extraction followed by headspace single-drop microextraction for fast determination of paeonol in traditional Chinese medicines. J Chromatogr A. 2006;1103:15–21.
Zhou J, Zhou L, Hou D, Tang J, Sun J, Bondy SC. Paeonol increases levels of cortical cytochrome oxidase and vascular actin and improves behavior in a rat model of Alzheimer’s disease. Brain Res. 2011;1388:141–7.
Chou TC. Anti-inflammatory and analgesic effects of paeonol in carrageenan-evoked thermal hyperalgesia. Br J Pharmacol. 2003;139:1146–52.
Kim SH, Kim SA, Park MK, Kim SH, Park YD, Na HJ, Kim HM, Shin MK, Ahn KS. Paeonol inhibits anaphylactic reaction by regulating histamine and TNF-alpha. Int Immunopharmacol. 2004;4:279–87.
Kong LD, Cheng CH, Tan RX. Inhibition of MAO A and B by some plant-derived alkaloids, phenols and anthraquinones. J Ethnopharmacol. 2004;91:351–5.
Brennan FM, McInnes IB. Evidence that cytokines play a role in rheumatoid arthritis. J Clin Invest. 2008;118:3537–45.
Kyung Chang S, Gu Z, Brenner MB. Fibroblast-like synoviocytes in inflammatory arthritis pathology: the emerging role of cadherin-11. Immunol Rev. 2010;233:256–66.
Yamanishi Y, Firestein GS. Pathogenesis of rheumatoid arthritis: the role of synoviocytes. Rheum Dis Clin N Am. 2001;27:355–71.
Noss EH, Brenner MB. The role and therapeutic implications of fibroblast-like synoviocytes in inflammation and cartilage erosion in rheumatoid arthritis. Immunol Rev. 2008;223:252–70.
Tak PP, Kalden JR. Advances in rheumatology: new targeted therapeutics. Arthr Res Ther. 2011;13:1.
Zhang W, Dai S-M. Mechanisms involved in the therapeutic effects of Paeonia lactiflora Pallas in rheumatoid arthritis. Int Immunopharmacol. 2012;14:27–31.
O’Connell RM, Rao DS, Baltimore D. microRNA regulation of inflammatory responses. Annu Rev Immunol. 2012;30:295–312.
Faraoni I, Antonetti FR, Cardone J, Bonmassar E. miR-155 gene: a typical multifunctional microRNA. Biochim Biophys Acta. 2009;1792:497–505.
Sonkoly E, Janson P, Majuri ML, Savinko T, Fyhrquist N, Eidsmo L, Xu N, Meisgen F, Wei T, Bradley M, Stenvang J, Kauppinen S, Alenius H, Lauerma A, Homey B, Winqvist O, Stahle M and Pivarcsi A. MiR-155 is overexpressed in patients with atopic dermatitis and modulates T-cell proliferative responses by targeting cytotoxic T lymphocyte-associated antigen 4. J Allergy Clin Immunol 2010;126:581–589, e581–520.
Moore CS, Rao VT, Durafourt BA, Bedell BJ, Ludwin SK, Bar-Or A, Antel JP. miR-155 as a multiple sclerosis-relevant regulator of myeloid cell polarization. Ann Neurol. 2013;74:709–20.
Brunet A, Bonni A, Zigmond MJ, Lin MZ, Juo P, Hu LS, Anderson MJ, Arden KC, Blenis J, Greenberg ME. Akt promotes cell survival by phosphorylating and inhibiting a Forkhead transcription factor. Cell. 1999;96:857–68.
Ekoff M, Kaufmann T, Engstrom M, Motoyama N, Villunger A, Jonsson JI, Strasser A, Nilsson G. The BH3-only protein Puma plays an essential role in cytokine deprivation induced apoptosis of mast cells. Blood. 2007;110:3209–17.
Skurk C, Maatz H, Kim HS, Yang J, Abid MR, Aird WC, Walsh K. The Akt-regulated forkhead transcription factor FOXO3 controls endothelial cell viability through modulation of the caspase-8 inhibitor FLIP. J Biol Chem. 2004;279:1513–25.
Castrillon DH, Miao L, Kollipara R, Horner JW, DePinho RA. Suppression of ovarian follicle activation in mice by the transcription factor FOXO3. Science. 2003;301:215–8.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Yoshiya Tanaka.
Ning Liu and Xue Feng contributed equally to this work.
Rights and permissions
About this article
Cite this article
Liu, N., Feng, X., Wang, W. et al. Paeonol protects against TNF-α-induced proliferation and cytokine release of rheumatoid arthritis fibroblast-like synoviocytes by upregulating FOXO3 through inhibition of miR-155 expression. Inflamm. Res. 66, 603–610 (2017). https://doi.org/10.1007/s00011-017-1041-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-017-1041-7