Abstract
Background
Calprotectin is calcium-binding protein which can be found in the cytosol of neutrophils. Several studies have studied its levels in preeclamptic women; however, to date there is no consensus regarding its effectiveness in the field.
Purpose
To investigate whether serum calprotectin levels are elevated among preeclamptic women compared to healthy controls.
Materials and methods
We used Medline (1966–2015), Scopus (2004–2015), ClinicalTrials.gov (2008–2015), Cochrane Central Register of Controlled Trials CENTRAL (1999–2015) and Google Scholar (2004–2015) search engines in our primary search, together with reference lists from included studies.
Results
Seven studies were finally included in our systematic review which recruited 439 women (245 with preeclampsia and 194 healthy controls). Their methodological quality was relatively high as they reached a score that ranged between 6 and 7 according to the Ottawa–Newcastle classification. All included studies reported that the serum calprotectin levels were significantly elevated among preeclamptic patients (p < 0.05). One study suggested that patients with severe preeclampsia have significantly higher levels of calprotectin than patients with mild preeclampsia (p = 0.01). However, to date there is no evidence regarding specific cut-off values which would help screen women for preeclampsia, or even follow the course of the disease.
Conclusion
Current evidence suggests that serum calprotectin is significantly raised among women with preeclampsia during the third trimester. Future research is needed to reach firm conclusions regarding its use as a potential screening and surveillance marker during the pregnancy course of women at risk of developing preeclampsia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Preeclampsia is a pregnancy-specific hypertensive disorder which is accompanied by increased urinary protein excretion. It affects 2–5 % of pregnant women and is associated with adverse maternal and neonatal outcomes [1–5]. It usually presents during the third trimester, however, its severe form (early onset preeclampsia) can become apparent even during the late second trimester [6]. The pathogenesis of preeclampsia takes place earlier than the actual clinical symptoms. Several pathways seem to interplay during this process including abnormal decidualization, abnormal placental vascularization, endothelial dysfunction and an abnormal inflammatory response [7, 8]. Various factors contribute to the establishment of the disease including anti-angiogenic factors such as soluble fms-like tyrosine kinase 1 (sFlt-1), soluble endoglin (sEng), placental growth factor (PlGF) and the transforming growth factors TGF-beta 1 and TGF-beta 3 [9]. These factors suppress the normal trophoblast invasion and the development of a vascular network which is essential for the normal activity of the placenta [10–12]. The accompanying oxidative stress seems to be related to local placental production of metalloproteinase (MMPs), growth factors and proinflammatory cytokines [13, 14]. Besides, preeclampsia is also associated with leucocyte activation and circulating leukocyte-derived microparticles and mRNA expression [15].
To date, several markers of inflammation have been extensively investigated in the field of preeclampsia. In their meta-analysis, Lau et al. reported that the maternal circulating TNF-α, IL-6, and IL-10 levels are significantly elevated in preeclamptic patients [16]. These cytokines seem to generate a widespread dysfunction of the maternal endothelium [16]. Recently, Yang et al. reported that increased levels of IFN-γ might be associated with preeclampsia [16]. The last decade novel markers of inflammation have been studied extensively in the literature. Among them, calprotectin gained increased attention. This protein is a complex of two mammalian calcium binding proteins named S100A8 and S100A9. Other synonyms of calprotectin are S100A8/A9, MRP8/14, calgranulin A/B, L1 protein, 27E10 antigen, cystic fibrosis antigen, myeloid–histiocyte antigen and CP-10. It constitutes up to 60 % of soluble protein content in the cytosol of neutrophil granulocytes and it can also be found in lower concentration in monocytes, macrophages and squamous epithelial cells [17]. Its expression is linked with elevated immunological activity and chronic inflammation [17, 18]. Several researchers suggested that calprotectin seems to be a marker of preeclampsia. However, to date there is a lack of consensus in this field. The aim of the present systematic review is to accumulate the current evidence and investigate the expression of calprotectin in the plasma of preeclamptic patients.
Methods
Study design
The present study was designed according to the PRISMA guidelines [19]. Eligibility criteria were predetermined by the authors. No language or date restrictions were applied during the literature search. All observational studies, prospective and retrospective were held eligible for inclusion. Case reports were excluded. AP and EP abstracted and tabulated predetermined data to a structured form, while the rest reviewed them independently. Discrepancies between the authors during data collection were resolved by the consensus of all authors.
Literature search and data collection
We used Medline (1966–2015), Scopus (2004–2015), ClinicalTrials.gov (2008–2015), Cochrane Central Register of Controlled Trials CENTRAL (1999–2015) and Google Scholar (2004–2015) search engines in our primary search, together with reference lists from included studies. Our search was restricted to a minimum number of keywords in order to assess an eligible number that could be hand searched, minimizing the loss of articles. All the articles that met or were presumed to meet the inclusion criteria were retrieved in full text.
We searched the literature using the words “calprotectin, S100A8/A9, MRP8/14, calgranulin A/B, L1 protein, 27E10 antigen, cystic fibrosis antigen, myeloid–histiocyte antigen and preeclampsia”. We specifically searched Medline using Pubmed with the terms “calprotectin AND preeclampsia”, “S100A8/A9 AND preeclampsia”, “MRP8/14 AND preeclampsia”, “calgranulin A/B AND preeclampsia”, “L1 protein AND preeclampsia”, “27E10 antigen AND preeclampsia”, “cystic fibrosis antigen AND preeclampsia” and the terms “myeloid–histiocyte antigen and preeclampsia AND preeclampsia”.
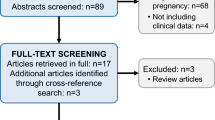
The PRISMA flow diagram summarizes the process of article retrieval (Fig. 1).
Definitions
Preeclampsia was defined as a rise of blood pressure after 20 weeks’ of gestation to >140/90 mmHg on two or more consecutive occasions (6 h apart) and proteinuria in previously normotensive women (≥+1 in urine dipstick or ≥300 mg/24 h urine excretion) on two or more consecutive occasions (6 h apart). Women with a blood pressure exceeding 160/110 mmHg and severe proteinuria (≥+2 in urine dipstick or ≥5 g/24 h urine excretion) were separately stratified as severe preeclampsia.
Quality assessment
The methodological quality of included studies was assessed with the Ottawa–Newcastle scale using a star-based system which evaluates the selection of the study groups, the comparability of the groups and the ascertainment of the outcome of interest [20].
Results
Excluded studies
Two studies were excluded from the present systematic review. The first one evaluated the cord blood levels of calprotectin [21]. The second was unrelated to calprotectin [22]. We also managed to retrieve the abstract of a study from an electronic page; however, we could not find the full text of the present study despite the fact that we tried to contact the authors; hence, this study was also excluded from the present systematic review [23].
Included studies
Seven studies were finally included in our systematic review which recruited 439 women [24–29]. Among them 245 women (55.8 %) suffered from preeclampsia while the remaining 194 (44.2 %) had uncomplicated pregnancies and served as controls. In Table 1, we present the methodological characteristics of included studies. In Table 2 we present certain maternal antenatal characteristics along with the gestational age at delivery and the birth-weight of infants. In Table 3 we present the reported levels of calprotectin from the studies included in our systematic review.
The methodology of calprotectin assays was not always comparable among studies which presented it. Braekke et al. reported that they diluted EDTA plasma samples in assay buffer and then added them to microtiter plate wells previously coated with an IgG fraction of rabbit anti-calprotectin. The diluted samples were then incubated with stirring at room temperature for 45 min, and washed three times with washing buffer. Following that they added alkaline phosphatase conjugated affinity-purified rabbit anti-calprotectin, and then the plate was incubated for further 45 min. After washing for three times they added the substrate and optical densities were recorded after 15–25 min [24]. Borekci et al. reported that venous blood samples were collected into tubes without anticoagulant. The serum was separated by centrifugation after clotting and stored at −80 °C until assayed. A mixture of 8.1 % sodium dodecyl sulfate, 20 % acetic acid, and 0.9 % thiobarbituric acid was added to 0.2 ml of serum, and then distilled water was added to the mixture to bring the total volume up to 4 ml. This mixture was incubated at 95 °C for 1 h. Following incubation, the tubes were left to cool under cold water and 1 ml of distilled water plus 5 ml of n-butanol/pyridine (15:1, v/v) were added, followed by mixing. The samples were centrifuged at 4000g for 10 min. The supernatants were then removed and serum calprotectin was evaluated with a commercially available kit [26]. Sugulle et al. reported a method which was comparable to that of Braekke et al. [25]. The remaining studies did not report the exact methods used for calprotectin measurement.
The systolic blood pressure of preeclamptic patients was significantly higher among patients suffering from preeclampsia (Table 2). An exception to this observation was the study of Braekke et al. which reported differences which were close, but did not reach statistical significance (p = 0.07) [24]. Three studies reported that the gestational age at delivery significantly differed among preeclamptic and healthy control parturient (Table 2) [24, 25, 30]. Concurrently, the birth weight of infants was also different.
We observed that all included studies reported that the serum calprotectin levels were significantly elevated among preeclamptic patients (p < 0.05). Feng et al. also reported that the levels of calprotectin were higher among women with severe preeclampsia compared to those with mild preeclampsia (p = 0.01) [27].
Discussion
Tissue hypoxia is present among women suffering from preeclampsia. This hypoxia causes neutrophil activation, which is primarily triggered by soluble mediators of inflammation [16]. Systemic inflammation and leucocyte activation/degranulation have been already proposed as potential pathways which contribute to the pathogenesis of preeclampsia [31]. Calprotectin is secreted during the degranulation process and can be found in the maternal serum. The role of calprotectin in the immune system was extensively investigated through the past 30 years and it was described as a calcium-binding protein which is abundant in the cytosol of neutrophil cells and which can also be found in lower concentrations in monocytes, tissue macrophages and eosinophils [32, 33]. Calprotectin was mentioned as a factor which blocks the intake of metal ions (zinc, manganese and iron), which are essential for the proliferation of pathogens [34]. Its biological functions include immune regulation, regulation of cell proliferation and induction of apoptosis [35, 36]. Previously Feng et al. suggested that the placental expression of calprotectin is increased and they attributed this effect to endothelial damaged which was the result of tissue hypoxia [27]. This protein has been previously found in the amniotic fluid of women with premature rupture of the membranes and in placental samples of pregnant women with HELLP (hemolytic anemia, elevated liver enzymes, low platelets count) syndrome [37, 38]. However, the pathophysiology which explains the increased levels of calprotectin among these diseases has not been elucidated yet.
Previous researchers have suggested that the increased levels of calprotectin may inhibit the activity of MMPs and thus interplay with other factors during placentation [30, 39, 40]. The findings of the present systematic review suggest that it might be an effective screen marker for preeclampsia because it is significantly elevated among women suffering from this disease regardless of its severity. Another study which was excluded from the present systematic review also reported that serum calprotectin was raised among women with preeclampsia [783 (478–928) vs. 618 (343–887) μg/l, p = 0.001] [23]. The same researchers also observed that calprotectin was higher in patients with severe preeclampsia compared to those with mild preeclampsia (p = 0.037). However, certain aspects remain to date unclear and preclude its adoption in current clinical practice. First of all none of the included studies followed the calprotectin levels during the pregnancy course. Moreover, the majority of included studies did not investigate if cases with severe preeclampsia had significantly higher levels of calprotectin compared to cases with mild preeclampsia. Therefore, it remains unclear if it can also be used as a marker of disease progression and of disease-specific antenatal complications. Another important aspect which needs further clarification is the actual interval between calprotectin elevation and the clinical presence of preeclampsia. The studies included in the present systematic review investigated the levels of calprotectin during the third trimester. However, several other factors which are implicated in the pathogenesis of the disease seem to rise from the first trimester [41]. In this context, it would be prudent to investigate the levels of this protein during the first and second trimesters to evaluate its effectiveness as an early pregnancy marker. Lastly, none of the included studies actually proposed the introduction of specific cut-off levels. Future studies in the field should also address this issue.
Strengths and weaknesses of our study
Our study is based on a meticulous review of the literature and presents thorough information on the quality of included studies and summarizes their limitations and their methodological heterogeneity. However, the significant discrepancies in the interpretation of statistical analysis of individual studies precluded meta-analysis of results.
Discussion
Current evidence suggests that serum calprotectin is significantly raised among women with preeclampsia during the third trimester. However, to date there is no evidence of a clear cut-off which could effectively diagnose this disorder. Furthermore, it remains unclear whether this abnormal finding becomes evident earlier in pregnancy. In this context, the conduct of future research in the field becomes necessary to reach firm conclusions regarding its use as a potential screening and surveillance marker during the pregnancy course of women at risk of developing preeclampsia.
References
Ronsmans C, Graham WJ. Maternal mortality: who, when, where, and why. Lancet. 2006;368:1189–200.
Thornton C, Dahlen H, Korda A, Hennessy A. The incidence of preeclampsia and eclampsia and associated maternal mortality in Australia from population-linked datasets: 2000–2008. Am J Obstet Gynecol. 2013;208(476):e1–5.
Lisonkova S, Sabr Y, Mayer C, Young C, Skoll A, Joseph KS. Maternal morbidity associated with early-onset and late-onset preeclampsia. Obstet Gynecol. 2014;124:771–81.
Lisonkova S, Joseph KS. Incidence of preeclampsia: risk factors and outcomes associated with early- versus late-onset disease. Am J Obstet Gynecol. 2013;209:544 e1–12.
Jelin AC, Cheng YW, Shaffer BL, Kaimal AJ, Little SE, Caughey AB. Early-onset preeclampsia and neonatal outcomes. J Matern Fetal Neonatal Med. 2010;23:389–92.
Raymond D, Peterson E. A critical review of early-onset and late-onset preeclampsia. Obstet Gynecol Surv. 2011;66:497–506.
Naljayan MV, Karumanchi SA. New developments in the pathogenesis of preeclampsia. Adv Chronic Kidney Dis. 2013;20:265–70.
Chaiworapongsa T, Chaemsaithong P, Yeo L, Romero R. Pre-eclampsia part 1: current understanding of its pathophysiology. Nat Rev Nephrol. 2014;10:466–80.
Wang A, Rana S, Karumanchi SA. Preeclampsia: the role of angiogenic factors in its pathogenesis. Physiology (Bethesda). 2009;24:147–58.
Verdonk K, Visser W, Steegers EA, Kappers M, Danser AH, van den Meiracker AH. New insights into the pathogenesis of pre-eclampsia: the role of angiogenesis-inhibiting factors. Ned Tijdschr Geneeskd. 2011;155:A2946.
Zhou Y, McMaster M, Woo K, Janatpour M, Perry J, Karpanen T, et al. Vascular endothelial growth factor ligands and receptors that regulate human cytotrophoblast survival are dysregulated in severe preeclampsia and hemolysis, elevated liver enzymes, and low platelets syndrome. Am J Pathol. 2002;160:1405–23.
Venkatesha S, Toporsian M, Lam C, Hanai J, Mammoto T, Kim YM, et al. Soluble endoglin contributes to the pathogenesis of preeclampsia. Nat Med. 2006;12:642–9.
Sahay AS, Sundrani DP, Joshi SR. Regional changes of placental vascularization in preeclampsia: a review. IUBMB Life 2015.
Laresgoiti-Servitje E. A leading role for the immune system in the pathophysiology of preeclampsia. J Leukoc Biol. 2013;94:247–57.
Lok CA, Jebbink J, Nieuwland R, Faas MM, Boer K, Sturk A, et al. Leukocyte activation and circulating leukocyte-derived microparticles in preeclampsia. Am J Reprod Immunol. 2009;61:346–59.
Mellembakken JR, Aukrust P, Olafsen MK, Ueland T, Hestdal K, Videm V. Activation of leukocytes during the uteroplacental passage in preeclampsia. Hypertension. 2002;39:155–60.
Striz I, Trebichavs.ky I. Calprotectin—a pleiotropic molecule in acute and chronic inflammation. Physiol Res. 2004;53:245–53.
Nilsen T, Sunde K, Larsson A. A new turbidimetric immunoassay for serum calprotectin for fully automatized clinical analysers. J Inflamm (Lond). 2015;12:45.
Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JPA, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700.
Wells GA, Shea B, O’Connel D, Peterson J, V W, Losos M, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis. www.ohri.ca/programs/clinical_epidemiology/oxford.asp, 2011.
Liosi S, Briana DD, Gourgiotis D, Boutsikou M, Baka S, Marmarinos A, et al. Calprotectin in human cord blood: relation to perinatal parameters and restricted fetal growth. J Perinat Med. 2010;38:523–6.
Staff AC, Braekke K, Johnsen GM, Karumanchi SA, Harsem NK. Circulating concentrations of soluble endoglin (CD105) in fetal and maternal serum and in amniotic fluid in preeclampsia. Am J Obstet Gynecol. 2007;197(176):e1–6.
Akcum S, Zulfikaroglu E, Eserdag S, Ozcan U. Plasma Calprotectin Levels in Preeclamptic Normotensive Pregnant and Nonpregnant Women. 2014.
Braekke K, Holthe MR, Harsem NK, Fagerhol MK, Staff AC. Calprotectin, a marker of inflammation, is elevated in the maternal but not in the fetal circulation in preeclampsia. Am J Obstet Gynecol. 2005;193:227–33.
Sugulle M, Kvehaugen AS, Braekke K, Harsem NK, Staff AC. Plasma calprotectin as inflammation marker in pregnancies complicated by diabetes mellitus and superimposed preeclampsia. Pregnancy Hypertens. 2011;1:137–42.
Börekçi B, Aksoy H, Öztürk N, Kadanalı S. Correlation between calprotectin and oxidized LDL in preeclampsia. Turk J Med Sci. 2009;39:191–5.
Feng C, Tao Y, Shang T, Yu M. Calprotectin, RAGE and TNF-alpha in hypertensive disorders in pregnancy: expression and significance. Arch Gynecol Obstet. 2011;283:161–6.
Ramma W, Buhimschi IA, Zhao G, Dulay AT, Nayeri UA, Buhimschi CS, et al. The elevation in circulating anti-angiogenic factors is independent of markers of neutrophil activation in preeclampsia. Angiogenesis. 2012;15:333–40.
Zaid O, Abdel-Maksoud H, Elmagid A, Omnia M. Biochemical effect of pre eclampsia on serum calprotectin, ascorbic acid, Uric Acid and Calcium. Benha Veterinary Med J. 2014;27(2):217–24.
Holthe MR, Staff AC, Berge LN, Fagerhol MK, Lyberg T. Calprotectin plasma level is elevated in preeclampsia. Acta Obstet Gynecol Scand. 2005;84:151–4.
Ramma W, Buhimschi IA, Zhao G, Dulay AT, Nayeri UA, Buhimschi CS, et al. The elevation in circulating anti-angiogenic factors is independent of markers of neutrophil activation in preeclampsia. Angiogenesis. 2012;15:333–40.
Dale I, Brandtzaeg P, Fagerhol MK, Scott H. Distribution of a new myelomonocytic antigen (L1) in human peripheral blood leukocytes. Immunofluorescence and immunoperoxidase staining features in comparison with lysozyme and lactoferrin. Am J Clin Pathol. 1985;84:24–34.
Brandtzaeg P, Dale I, Fagerhol MK. Distribution of a formalin-resistant myelomonocytic antigen (L1) in human tissues. I. Comparison with other leukocyte markers by paired immunofluorescence and immunoenzyme staining. Am J Clin Pathol. 1987;87:681–99.
Nakashige TG, Zhang B, Krebs C, Nolan EM. Human calprotectin is an iron-sequestering host-defense protein. Nat Chem Biol 2015.
Kerkhoff C, Klempt M, Sorg C. Novel insights into structure and function of MRP8 (S100A8) and MRP14 (S100A9). Biochim Biophys Acta. 1998;1448:200–11.
Yui S, Nakatani Y, Mikami M. Calprotectin (S100A8/S100A9), an inflammatory protein complex from neutrophils with a broad apoptosis-inducing activity. Biol Pharm Bull. 2003;26:753–60.
Espinoza J, Chaiworapongsa T, Romero R, Edwin S, Rathnasabapathy C, Gomez R, et al. Antimicrobial peptides in amniotic fluid: defensins, calprotectin and bacterial/permeability-increasing protein in patients with microbial invasion of the amniotic cavity, intra-amniotic inflammation, preterm labor and premature rupture of membranes. J Matern Fetal Neonatal Med. 2003;13:2–21.
Buimer M, Keijser R, Jebbink JM, Wehkamp D, van Kampen AH, Boer K, et al. Seven placental transcripts characterize HELLP-syndrome. Placenta. 2008;29:444–53.
Gallery ED, Campbell S, Arkell J, Nguyen M, Jackson CJ. Preeclamptic decidual microvascular endothelial cells express lower levels of matrix metalloproteinase-1 than normals. Microvasc Res. 1999;57:340–6.
Isaksen B, Fagerhol MK. Calprotectin inhibits matrix metalloproteinases by sequestration of zinc. Mol Pathol. 2001;54:289–92.
Bian Z, Shixia C, Duan T. First-trimester maternal serum levels of sFLT1, PGF and ADMA predict preeclampsia. PLoS One. 2015;10:e0124684.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
The authors report they did not receive funding for the present paper.
Conflict of interest
None for all authors.
Additional information
Responsible Editor: John Di Battista.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Pergialiotis, V., Prodromidou, A., Pappa, E. et al. An evaluation of calprotectin as serum marker of preeclampsia: a systematic review of observational studies. Inflamm. Res. 65, 95–102 (2016). https://doi.org/10.1007/s00011-015-0903-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-015-0903-0