Abstract
Background
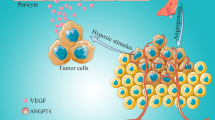
Angptl4 is a secreted protein involved in the regulation of vascular permeability, angiogenesis, and inflammatory responses in different kinds of tissues. Increases of vascular permeability and abnormality changes in angiogenesis contribute to the pathogenesis of tumor metastasis, ischemic-reperfusion injury. Inflammatory response associated with Angptl4 also leads to minimal change glomerulonephritis, wound healing. However, the role of Angptl4 in vascular permeability, angiogenesis, and inflammation is controversy. Hence, an underlying mechanism of Angptl4 in different kind of tissues needs to be further clarified.
Methods
Keywords such as angptl4, vascular permeability, angiogenesis, inflammation, and endothelial cells were used in search tool of PUBMED, and then the literatures associated with Angptl4 were founded and read.
Results
Data have established Angptl4 as the key modulator of both vascular permeability and angiogenesis; furthermore, it may also be related to the progression of metastatic tumors, cardiovascular events, and inflammatory diseases. This view focuses on the recent advances in our understanding of the role of Angptl4 in vascular permeability, angiogenesis, inflammatory signaling and the link between Angptl4 and multiple diseases such as cancer, cardiovascular diseases, diabetic retinopathy, and kidney diseases.
Conclusions
Taken together, Angptl4 modulates vascular permeability, angiogenesis, inflammatory signaling, and associated diseases. The use of Angptl4-modulating agents such as certain drugs, food constituents (such as fatty acids), nuclear factor (such as PPARα), and bacteria may treat associated diseases such as tumor metastasis, ischemic-reperfusion injury, inflammation, and chronic low-grade inflammation. However, the diverse physiological functions of Angptl4 in different tissues can lead to potentially deleterious side effects when used as a therapeutic target. In this regard, a better understanding of the underlying mechanisms for Angptl4 in different tissues is necessary.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
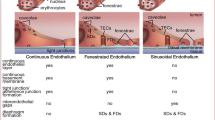
In the whole body, one of the main roles of endothelial cells is to function as gatekeepers or an endothelial barrier that regulates transport of solutes, large molecules, and cells across the vessel wall. In response to the extracellular environment, the disrupted endothelial barrier causes vascular permeability changes. Vascular permeability is a highly coordinated process that is regulated and maintained through three compartments including paracellular junctions, transcellular pathways and heterotypic (inflammatory cells and mural cells) interactions. Mural cells include pericytes, smooth muscle cells and macrophages [1]. In recent years, several researches have focused on the contribution of pericytes as important partners in the control of vascular permeability, such as the blood–brain barrier [2]. Besides, transcellular pathways play important roles in the control of vascular permeability, such as channels, vesiculo-vacuolar organelles (VVOs) and caveolae [3–5]. In a study of Miyawaki-Shimizu et al., it was found that siRNA knockdown of Cav-1 resulted in an increased lung vascular permeability [6]. Furthermore, maintenance of barrier function also requires the integrity of endothelial junctions between contacting endothelial cells. In endothelial cell architecture, there are three types of junctions: the adherent junctions (AJs); the tight junctions (TJs) and the gap junctions. Emerging data has indicated that the communication between adherent junctions and TJs is important for permeability regulation [1]. However, there is no evidence that gap junctions participate in vascular permeability. Normally, moderate vascular permeability contributes the fluid exchanges between blood and tissues. On the other hand, increases of vascular permeability are related to several diseases, such as inflammatory diseases, ischemia–reperfusion injury and tumor metastasis. Therefore, to keep vascular permeability homeostasis, endothelial cell integrity must be maintained.
There are several agents and signaling pathways that regulate vascular permeability including inflammatory mediators, vascular endothelial growth factor (VEGF) and angiopoietin (Ang) Tie receptor signaling. Histamine, thrombin and bradykinin exposure, as inflammatory mediators, are pointed out to result in an increase in vascular permeability. These processes are involved in Rho activation [7]. Besides, VEGF activates multiple signaling pathways downstream of VEGFR2 that have been implicated in vascular permeability [8, 9]. Furthermore, Tie receptors and their ligands are critical regulators of vascular maturation. Ang1/Tie2 signaling has been reported to inhibit VEGF-mediated vascular permeability through several downstream signaling cascades [10]. Angiopoietin-like protein (ANGPTL4) shares structural and functional similarity with angiopoietins and also plays an important role in vascular permeability.
The ANGPTL proteins (ANGPTL1-8) are secreted proteins, which belong to the ANG-like family and have been found in both humans and mice, except for Angptl5. The human ANGPTL4 gene is well conserved among different species and shares ~77 % amino acid sequence similarity with the mouse [11]. The human ANGPTL4 gene is located on chromosome 19p13.3 and encodes a glycoprotein with a molecular mass of ~45–65 KDa. Similar to other members of this family, Angptl4 contains a N-terminal coiled-coil structure and a C-terminal fibrinogen-like domain [12]. Currently, Angptl4 is considered to be an orphan ligand because information regarding its potential binding partner is lacking [13]. However, the function of this protein is regulated by agonists, such as peroxisome proliferators-activated receptors (PPARs), glucocorticoid receptors and protein kinase C (PKC) agonists, and inhibitors (such as angiotensin blockers, resistin, leptin and insulin) of known receptors and inflammatory signaling molecules [14, 15]. In mice, Angptl4 is expressed in adipose tissue, liver, skeletal muscle, heart, skin and the intestine. Whereas, In humans, Angptl4 is produced by several tissues including liver, blood plasma, placenta, small intestine, heart and adipose tissue [16, 17] This is mediated by glucocorticoid and nuclear hormone receptors PPARs, which stimulate Angptl4 expression via a PPAR-response element in the human and mouse ANGPTL4 gene. Besides, the expression of Angptl4 in cadiomyocytes, endothelial cells and tumor tissues is elevated by hypoxic conditions. Furthermore, transforming growth factor β (TGFβ) can also elevate Angptl4 expression in primary tumor cells to prime metastasis [18].
The function of Angptl4 appears to be tissue dependent. Originally discovered in the liver as pivotal regulators of lipid metabolism, Angptl4 is also involved in vascular permeability, angiogenesis and inflammatory signaling. Compared to the pathological state, podocytes have a lower expression in normal conditions. Recently, it has been demonstrated that overexpression of Angptl4 in podocytes results in the development of the nephritic syndrome. This process is associated with characteristic changes including large-scale selective proteinuria, loss of glomerular basement membrane (GBM) charge and diffuse fusion of podocyte foot processes, which are caused by up-regulated Angptl4. This result indicates that Angptl4 may have effects on the barrier function of GBM [19]. Using Angptl4 knockout mice and overexpression mice, it was demonstrated that Angptl4 inhibits the lung metastasis of metastatic Lewis lung carcinoma (3LL) cells and B16F0 melanoma cells by alteration of vascular permeability [20]. However, in a study of Huang et al. [21] it was demonstrated that Angptl4 produced by tumor tissue bound to integrinα5β1, VE-cadherin and claudin-5, which are major components of the adherens and tight junction proteins in the endothelium. This process results in a disruption of the endothelium. Knockdown of Angptl4 suppressed tumor growth and angiogenesis in the model of glioma [22]. Furthermore, using Angptl4 knockout mice, it has been demonstrated that Angptl4 regulates vascular permeability and angiogenesis in retinal via endothelial cell junction organization during development and in pathological conditions [23]. These results indicate that Angptl4 plays an important role in vascular permeability and angiogenesis. However, the role of Angptl4 in vascular permeability and metastasis is controversial because lacking of understanding of how ANGPTL4 modulates vascular permeability [21].
Taken together, these data have established Angptl4 as a key modulator of both vascular permeability and angiogenesis; furthermore, it also may be related to the progression of metastatic tumors, cardiovascular events and inflammatory diseases. This view focuses on recent advances in our understanding of the role of Angptl4 in vascular permeability, angiogenesis, inflammatory signaling and the link between Angptl4 and multiple diseases such as cancer, cardiovascular diseases, diabetic retinopathy, kidney disease (Fig. 1).
ANGPTL4 is master regulator of vascular permeability and angiogenesis
The full-length Angptl4 protein undergoes proteolytic processing by proprotein convertases at the linker region (Lys168 and Lev169 in humans, Lys170 and Met171 in the mouse), producing the nAngptl4 and the carboxyl terminus of Angptl4. The cleavage of Angptl4 seems to be tissue dependent. Angptl4 is present in mouse blood plasma in several forms of around 30 kDa. In humans, the liver secretes the cleaved nAngptl4, which is associated with lipid metabolism, whereas adipose tissue secretes the full-length form. Therefore, the proteolytic processing and perhaps oligomerization may be important for Angptl4 function [24]. Interestingly, the carboxyl terminus of Angptl4 that is mainly produced by endothelial cells is a master regulator of vascular permeability and angiogenesis [25–27].
Angptl4 mRNA was detected in both epithelial cells and endothelial cells such as podocytes in kidney, human pulmonary microvascular endothelial cells (HPMVECs), human umbilical vein endothelial cells (HUVECs) [28, 29]. Among them, endothelial cells are major effector cells of Angptl4 on vascular permeability and angiogenesis. Until now, there has been controversy about the role of angptl4 in vascular permeability and angiogenesis. Some studies have demonstrated that Angptl4 inhibits vascular permeability and angiogenesis. It has been demonstrated that Angptl4 markedly inhibits the proliferation, chemotaxis and tubule formation of endothelial cells. Moreover, it has been recognized that Angptl4 prevents vascular leakiness that is induced by VEGF [30]. Furthermore, another study has demonstrated that the carboxyl terminus of Angptl4 inhibits VEGF-induced cell proliferation, migration, and tubule formation in HUVECs [31]. It was recognized that this effect was via suppression of the Raf/MEK/ERK signaling pathway [29]. In addition, a recent study has found that tumor-derived Angptl4 suppresses in vitro vascular tube formation and proliferation of human umbilical vascular endothelial cells, possibly through the MEK pathway [32].
On the other hand, Angptl4 seems to be a proangiogenic factor. In an early study, it was reported that Angptl4 was induced by ischemia and recognized as a proangiogenic factor in renal cell carcinoma [33]. Moreover, Cazes et al. [34] have demonstrated that full length Angptl4 induced by hypoxia accumulated in the subendothelial extracellular matrix (ECM) of hypoxia endothelial cells through heparin and, therefore, reduced endothelial cells adhesion. In addition, it has been proved that Angptl4 modulates the balance between triglyceride-rich lipoproteins and chylomicrons via inhibiting endothelial lipase and lipoprotein lipase anchored at the surface of endothelial cells [34]. Moreover, knockdown Angptl4 resulted in increased lipolysis products such as oxidized fatty acids that lead to endothelial cells inflammation [34]. These processes may be associated with endothelial cells growth and migration. VEGF, as one of the angiogenic factors, plays an important role in permeability. Using Angptl4 knockout mice, it has been demonstrated that the dissociation of VEGFR2/VE-cadherin complexes caused adherens junction disruption. The same authors also found that knockdown Angptl4 led to severe destabilization of the VEGFR2/VE-cadherin complex because of Src kinase phosphorylation enhancement. The disrupted adherens junctions are correlated with increased vascular permeability and eventually lead to reperfusion injuries in acute myocardial infarction [35]. Besides, Angptl4 is highly expressed in retinal vascular plexus induced by hypoxia. In proliferative retinopathies, hypoxia-induced angiogenesis is involved in disruption of the vascular barrier. Perdiguero et al., have demonstrated that Angptl4 modulates sprouting, branching and maturation of the retinal vascular plexus. In the Angptl4 knockout mice, endothelial cell differentiation was altered in the microvascular plexus. Furthermore, it has been proved that Angptl4 mediates the permeability of the retinal vascular and protects vessels from oxygen-induced obliteration. This phenomenon indicates that Angptl4 controls vascular permeability and angiogenesis via endothelial cell junction organization and pericyte coverage during development and in pathological conditions [23]. Vascular junctions have been recognized as significant modulators of vascular permeability. It has been demonstrated that the tumor-secreted c-terminal fibrinogen-like domain of angiopoietin-like 4 (cAngptl4) modulates vascular integrity through disruption of VE-cadherin and claudin-5. This indicates that Angptl4 promotes tumor metastasis by increasing vascular permeability [21].
Angptl4 as one regulator of inflammatory signaling
Additionally, recent studies have indicated that Angptl4 is involved in regulating inflammatory signaling. It is well-known that Angptl4 is a gene involved in glucose and lipid metabolism. The hypoxia level can stimulate the expression and secretion of Angptl4 in human adipocytes. Therefore, oxygen may negatively regulate Angptl4 mRNA expression in adipocytes [36]. Furthermore, the hypoxia of adipose tissue causes chronic inflammation and macrophage infiltration in white adipose tissue [36]. Based on the previous results, we predicate that Angptl4 may have a connection with inflammation.
Increased lipid uptake is associated with a stimulation of inflammatory related genes. In a study of Lichtenstein et al., it has been demonstrated that Angptl4 that is induced by chyle protects against severe proinflammatory effects of saturated fat through inhibiting fatty acid uptake into mesenteric lymph node macrophages. They also found that the level of inflammatory factors such as IL-6 was elevated in Angptl4 knockout mice on a high-saturated-fat diet. In addition, infiltrates of neutrophils and macrophages were observed in the liver of Angptl4 knockout mice [37]. This result indicates that Angptl4 is involved in the inflammation.
Toll-like receptor activators are believed to be regulators of Angptl4. In a study of Brown et al. [38] it has been found that the level of Angptl4 is increased in the hypothalamic, pituitary, cortical and adipose tissues following treating with LPS in mice. Furthermore, using antagonists of TLR4 signaling pathways, it was concluded that the expression of Angptl4 may be partially regulated by NF-KB and the P38MAPK pathways in N1-neuronal and 3T3-L1 adipocyte cells [38]. Another study also found that LPS increased the expression of Angptl4 in heart, muscle, and adipose tissues [39]. However, LPS did not increase the expression of Angptl4 in either muscle or adipose tissues in TLR4 knockout mice; this result indicated that the LPS effect may be mediated through the TLR4 pathway. The same authors also found that one of TLR2 activators (zymosan) increases the expression of Angptl4 in liver, heart, muscle, and adipose tissues. Besides, several cytokines such as interleukins, TNF-α, IFN-γ have been pointed out to be involved in Angptl4 expression [40]. However, a direct effect of Angptl4 on inflammatory pathways needs to further investigated.
Angptl4 in tumor growth and metastasis
Tumor metastasis depends on an increased vascular leakiness and on the steps of intravasation and extravasation, which are involved in the migration of tumor cells across the disrupted endothelium. Several tumor-derived products have been pointed out to increase vascular permeability. Tumor secreted products recognize the tumor microenvironment, and, therefore, lead to corrupting normal neighboring cells and recruiting them for tumorigenesis. It is well known that endothelial cell activation in tumor growth and metastasis is regulated by several factors such as growth factors, proinflammatory cytokines, chemokines and derivatives of arachidonic acid.
Angptl4, as a secreted protein, is associated with angiogenesis and vascular permeability. Numerous studies have demonstrated that Angptl4 is involved in tumor growth and metastasis such as lung cancer, hepatic carcinoma, breast cancer, kidney cancer and gastric cancer. However, the role of Angptl4 in tumor formation remains controversial. Early studies demonstrated that ANGPTL4 prevented tumor growth and metastasis by inhibiting vascular leakiness. In a study of Galaup et al. [20] it was found that ANGPTL4 remarkably prevented metastasis through the inhibition of vascular permeability in the mice which were injected with metastatic Lewis lung carcinoma cells and B16F0 melanoma cells. The underlying mechanism of its role in tumor metastasis was believed to be inhibition of endothelial cell adhesion, migration and sprouting caused by ECM-bound form of ANGPTL4 [31]. On the hand, Angptl4 was believed to be a pro-angiogenic and pro-metastasis factor. It has been demonstrated that tumor-derived ANGPTL4 promotes cell survival and tumor growth through the PI3K/PKB and ERK signaling pathway [41]. Furthermore, the same phenomenon was also observed in renal cell carcinoma [33]. It was found that the mRNA and protein levels of ANGPTL4 expression were increased in renal cell carcinoma, though there was no ANGPTL4 expression in other benign and malignant tumors of the kidney cells [33]. These findings indicate that ANGPTL4 may be a diagnostic marker of renal cell carcinoma. This effect of Angptl4 on tumor growth was believed to be involved in promoting sprouting of vascular endothelial cells caused by Angptl4 during ischemia [33]. Furthermore, the promotion effect of Angptl4 on tumor metastasis was also found in breast cancer, gastric cancer, esophageal squamous cell carcinoma, colorectal cancer and Kaposi’s sarcoma [42–44]. In a study of Ma et al., it was found that ANGPTL4 up-regulated by the viral G protein-coupled receptor promoted angiogenesis and vascular permeability in Kaposi’s sarcoma. The underlying mechanism that Angptl4 promotes tumor metastasis was recognized and that the C-fibrinogen-like domain of ANGPTL4 activates specific integrins and disrupts connection of cell–cell and cell–matrix to modulate vascular permeability, thereby promoting tumor metastasis. In addition, Angptl4 has been recognized as one of the genes that is involved in breast cancer to lung metastasis. It has been demonstrated that TGFβ-induced Angptl4 disrupts vascular endothelial cell–cell junctions, increases the permeability of the lung capillaries, therefore promoting the steps of metastasis [18]. Recently, in a study of Takada et al. [32] it was found that the wild-type Angptl4 inhibits gastric cancer metastasis, whereas the mutant ANGPTL4 with a somatic 21-bp deletion in exon 1 is associated with the progression of gastric cancer. Taken together, the role of Angptl4 in tumor growth and metastasis is controversial and the underlying mechanism for regulation of Angptl4 on tumor needs to be further elucidated.
Angptl4 in inflammatory diseases
Inflammatory disease is a type of inflammatory mediator increase caused by infection or non-specific infection. Increasing evidence indicates the possible involvement of Angptl4 in the development of inflammatory disease such as minimal change disease (MCD), wound healing, and low-grade chronic inflammation.
Minimal change disease
Each glomerulus in the kidney is composed of capillary loops constructed by fenestrated endothelium and foot processes of podocytes. A GBM is produced by both cell types including endothelial cells, mesangial cells, parietal epithelial cells of the Bowman’s capsule, and podocytes. Podocytes are highly specialized epithelial cells that line the urinary surface of the glomerular capillary and are involved in capillary permeability. The role of podocytes in the kidney mainly modulates hydrostatic ultrafiltration of blood plasma and retains vital proteins. Disruption of podocytes' function results in proteinuria and, possibly, renal failure.
Angptl4, also called fasting-induced adipose factor (FIAF), was introduced as a novel adipokine influencing glucose and lipid homeostasis. It has been demonstrated that serum Angptl4 levels are significantly increased in end-stage renal disease and involved in markers of renal function in control subjects. Angptl4 is well known for its role in the regulation of plasma triglyceride concentrations. Furthermore, hypertriglyceridemia is a characteristic of the nephritic syndrome seen in MCD. In a study of Clement et al., it has been demonstrated that ANGPTL4 expression is up-regulated in podocytes from experimental rodent models of nephritic syndrome and human MCD and, therefore, results in the secretion of hyposialylated ANGPTL4 into the glomerular capillary wall and proteinuria in MCD rats. They also found that podocyte-specific overexpression of ANGPTL4 (NPHS2-Angptl4) in rats led to large-scale albuminuria, loss of GBM charge and fusion of podocyte foot processes. However, adipose tissue-specific overexpression of Angptl4 (aP2-Angptl4) only increases circulating concentrations of ANGPTL4, but does not lead to proteinuria; this result indicates that proteinuria in MCD requires glomerular production of ANGPTL4. In addition, feeding NPHS2-Angptl4 rats the sialic acid precursor N-Acetyl-D-mannosamine (ManNAc), results in a decline in albuminuria. Furthermore, from a clinical perspective, sialic acid precursors might provide increased efficacy in treating MCD [19, 45]. However, the upstream mechanisms that cause increased podocyte ANGPTL4 expression are not known and need to be further elucidated.
Wound healing
Normal wound healing needs to go through several processes of inflammation, re-epithelialization, matrix remodeling and related cellular activity events [46]. Upon skin injury, keratinocytes use various signaling molecules to promote reepithelialization for efficient wound closure. Angptl4, secreted by keratinocytes, plays an important role in wound healing. Using Angptl4 knockout mice, it has been demonstrated that wound reepitheliazation is delayed with impaired keratinocytes migration. They also found that suppression expression of Angptl4 using si-RNA led to impaired migration associated with diminished integrin-mediated signaling β1 and β5, which is important for cell migration. Later, the same authors found that Angprl4 was a novel matri-cellular protein whose expression was up-regulated by PPARβ/δ in response to inflammation during wound healing. Keratinocytes secreted by Angptl4 interacted with vitronectin and fibronectin in the wound bed and regulated the availability of the local ECM by delaying their proteolytic degradation. In addition, Angptl4 specifically interacts with integrins β1 and β5 that reside on the surface of wound keratinocytes which activate integrin-mediated intracellular signaling and accelerate the wound healing process. Using Angptl4 knockout mice, it has been demonstrated that wound re-epithelialization was delayed [34, 47]. These results may be attributed to the impairment of Angptl4-deficient keratinocytes.
Chronic low-grade inflammation
Low-grade inflammation is a type of nonspecific and persistent chronic inflammation characterized by increase of nonspecific inflammatory markers such as c-reactive protein (CRP), tumor necrosis factorα (TNF-α), interleukin-1 (IL-1) and VEGF. Until now, atherosclerosis, diabetes and obesity are recognized as chronic low-grade inflammation [48].
It is well-known that the plasma triglyceride level is a risky factor for cardiovascular disease. Patients with hypertriglyceridemia have increased plasma levels of remnants from CMs and VLDL, which penetrates the endothelium, and can lead to the development of atherosclerosis and coronary heart disease. As a typical ANGPTL4 family member, Angptl4 contains an N-terminal coiled-coil domain that modulates lipid metabolism and irreversibly inhibits LPL activity by disrupting LPL dimerization [49]. Furthermore, LPL is produced from different types of cells, such as cardiomyocytes, adipocytes and macrophages. Macrophage foam cells play an important role in atherosclerosis [50]. In a study of Georgiadi et al., it has been demonstrated that recombinant Angptl4 decreased uptake of oxidized low density lipoprotein by macrophages, possibly through lipolysis in dependent and independent mechanisms. Furthermore, Angptl4 was expressed in human atherosclerosis lesions and localized in macrophages. However, in the early study, it was found that Angptl4 deficiency protected ApoE (−/−) against progression of atherosclerosis and suppressed the ability of the macrophages to become foam cells in vitro [51]. These results indicate that Angptl4 is highly correlated with inflammatory changes and progression of atherosclerosis. However, inconsistent results have been reported concerning the effects of Angptl4 on coronary atherosclerosis. In a study of Folsom et al. [52] it has been demonstrated that an Angptl4 loss of function mutation (E40K variant) was involved in the incidence of coronary artery disease. Those carrying at least one 40K variant showed a lower mean triglyceride and 40K carriers had a lower CAD incidence. However, in another study, it was found that 40K carriers had a higher CAD risk independent of triglyceride and HDL-C concentrations but had a significantly lower triglyceride.
Obese subjects often present a low-grade chronic inflammation in the white adipose tissue, which seems to play an important role in the development of obesity-related diseases. It is well known that the inflammation process is related to the hypoxic state. In a study of Quintero et al., it has been found that hyperoxia decreased cell viability, increased intracellular ROS content and then provoked an inflammatory response in adipocytes. Using Angptl4 knockout mice, it has been demonstrated that mice display higher body fat accumulation and are extremely sensitive to systematic inflammation after addition of saturated fatty acids. In addition, Angptl4 knockout mice showed a protection from high-fat-diet-induced obesity, which was attributed to decreased expression of peroxisomal proliferators-activated receptor coactivators [36].
It is well known that Angptl4 is associated with glucose metabolism. However, until now, the mechanism of Angptl4 regulation glucose metabolism is not clear. In patients with type 2 diabetes, levels of angptl4 in serum were significantly lower than those in healthy persons, indicating that low level of angptl4 may be a causative factor of this disease [53]. On the other hand, it has been found that overexpression of Angptl4 could decrease glucose tolerance in liver but not affect glucose level in periphery [54]. In addition, diabetes complication such as diabetic retinopathy is also recognized as low-grade inflammation. Angiogenesis is correlated with disruption of the vascular barrier that leads to plasma leakage, subsequent edema, and vision loss in proliferative retinopathy, such as proliferative retinopathies. ANGPTL4 is a secreted glycoprotein that is induced by hypoxia and regulates context dependent angiogenesis and vascular permeability. It has been well-known that ANGPTL4 is highly expressed in retinal endothelial cells during development and oxygen-induced retinopathy. Using Angptl4 knockout mice, it has been demonstrated that endothelial cells show a defect in sprouting, branching and a delay in the maturation of the retinal vasculature during developmental angiogenesis. Furthermore, pathological neovascularization is impaired in Angptl4 knockout mice in a model of retinopathy of prematurity [23]. These results indicate that loss of Angptl4 impairs sprouting angiogenesis both in developmental and in pathological conditions. Recently, Angptl4 was proved to be an angiogenic factor released from retinal pigment epithelium cells (ARPE) under a high glucose condition. The same authors found that Angptl4 was one of the highest up-regulated genes under high glucose and the recombinant Angptl4 promoted all of the elements of angiogenesis in human retinal endothelial cells [55]. Therefore, Angptl4 may be a potential candidate molecule involved in diabetic retinopathy.
Angptl4 in ischemic reperfusion injury
Restoration of blood supply in ischemia tissue limits the extent of acute infarction, but it also causes microvascular dysfunction and oxidative damage. It has been recognized that increased vascular permeability is an important factor for tissue damage after ischemia. In addition to the role of Angptl4 in lipid metabolism, Angptl4 plays an important role in capillary permeability and ischemic reperfusion injury such as ischaemic stroke and myocardial ischemic reperfusion injury.
A recent study demonstrated that coronary vascular integrity was severely disrupted and junction disconnected more frequently in ANGPTL4 knockout mice with confocal images stained with VE-cadherin and CD31 antibody after 4 h of reperfusion. It has been well-known that ischemia promotes VEGF expression and leads to vascular permeability and edema. Furthermore, they also found that the expression of VEGFR2 and VE-cadherin was decreased and the expression of phosphorylation of Src kinase, which is a downstream signaling pathway of VEGFR2, was increased in the ANGPTL4 knockout mice after 4 h reperfusion until 18 h. Lastly, the phenomenon of an increase in infarct size, no-reflow and post-ischemic inflammation in ANGPTL4 knockout mice was found [35]. These results indicate that hypoxic induction of ANGPTL4 may modulate coronary vascular permeability during acute myocardial infarction. Based on the results, they also found that rhANGPTL4 induced infract size and the area of no-flow decreased and the extent of hemorrhage declined. These results indicate that rhANGPTL4 induced preservation of vascular integrity that reduced infarct size, hemorrhage, and no-reflow, thereby conferring cardioprotection. Therefore, rhANGPTL4 may be a target for treating acute coronary infraction.
Vascular injury leads to edema in the brain during stroke. Angptl4, as a regulator of endothelial barrier integrity, might exert a protective effect during ischaemic stroke [56]. Using Angptl4 knockout mice, it has been demonstrated that the expression of VE-cadherin and claudin-5 were reduced. Vascular damage and infarct severity were increased in Angptl4 knockout mice. Furthermore, they also found that treatment with recombinant Angptl4 led to significantly decreased infarct sizes, integrity of tight and adherens junctions were increased and the vascular network was preserved. These results indicate that Angptl4 protects not only the vascular network, but also interendothelial junctions and controls inflammatory response and edema. In addition, it has been demonstrated that Angptl4 protects ischaemic stroke via Src signaling downstream from VEGFR2.
Conclusion
As discussed above, vascular permeability and angiogenesis are involved in the pathogenesis of several diseases, such as tumor metastasis, ischemic-reperfusion injury and inflammation (Table 1). It is conceivable that Angptl4 modulates vascular permeability, angiogenesis, inflammatory signaling and associated diseases. In addition, Angptl4 expression is regulated by several factors. The use of Angptl4-modulating agents, such as certain drugs, food constituents (such as fatty acids), nuclear factor (such as PPARα) and bacteria may treat associated diseases such as tumor metastasis, ischemic-reperfusion injury, inflammation and chronic low-grade inflammation. However, the diverse physiological functions of Angptl4 in different tissues can lead to potentially deleterious side effects when used as a therapeutic target. In this regard, a better understanding of the underlying mechanisms for Angptl4 in different tissues is necessary.
References
Goddard LM, Iruela-Arispe ML. Cellular and molecular regulation of vascular permeability. Thromb Haemost. 2013;109:407–15.
Armulik A, Genové G, Mäe M, Nisancioglu MH, Wallgard E, Niaudet C, et al. Pericytes regulate the blood-brain barrier. Nature. 2010;468:557–61.
Daneman R, Zhou L, Kebede AA, Barres BA. Pericytes are required for blood-brain barrier integrity during embryogenesis. Nature. 2010;468:562–6.
Mehta D. Signalling mechanisms regulating endothelial permeability. Physiol Rev. 2006;86:279–367.
Dvorak AM, Feng D. The vesiculo-vacuolar organelle (VVO). A new endothelial cell permeability organelle. J Histochem Cytochem. 2001;49:419–32.
Miyawaki-Shimizu K, Predescu D, Shimizu J, Broman M, Predescu S, Malik AB. siRNA-induced caveolin-1 knockdown in mice increases lung vascular permeability via the junctional pathway. Am J Physiol Lung Cell Mol Physiol. 2006;290:L405–13.
Thennes T, Mehta D. Heterotrimeric G proteins, focal adhesion kinase, and endothelial barrier function. Microvasc Res. 2012;83:31–44.
Sun Z, Li X, Massena S, Kutschera S, Padhan N, Gualandi L, et al. VEGFR2 induces c-Src signalling and vascular permeability in vivo via the adaptor protein TSAd. J Exp Med. 2012;209:1363–77.
Thibeault S, Rautureau Y, Oubaha M, Faubert D, Wilkes BC, Delisle C, et al. S-nitrosylation of beta-catenin by eNOS-derived NO promotes VEGF-induced endothelial cell permeability. Mol Cell. 2010;39:468–76.
Mammoto T, Parikh SM, Mammoto A, Gallagher D, Chan B, Mostoslavsky G, et al. Angiopoietin-1 requires p190 RhoGAP to protect against vascular leakage in vivo. J Biol Chem. 2007;282:23910–8.
Yao Q, Shin MK, Jun JC, Hernandez KL, Aggarwal NR, Mock FR, et al. Effect of chronic intermittment hypoxia on triglyceride uptake in different tissues. J Lipid Res. 2013;54:1058–65.
Zhu PC, GOH YY, Alison HF. Angiopoietin-like 4: a decade of research. Biosci Rep. 2011;32:211–9.
Valenzuela DM, Griffiths JA, Rojas J, Aldrich TH, Jones PF, Zhou H, et al. Angiopoietins 3 and 4: diverging gene counterparts in mice and humans. Proc Natl Acad Sci USA. 1996;96:1904–9.
Wiesner G, Morash BA, Ur E, Wilkinson M. Food restriction regulates adipose-specific cytokines in pituitary gland but not in hypothalamus. J Endocrinol. 2004;180:R1–6.
Koliwad SK, Kuo T, Shipp LE, Gray NE, Backhed F, Wang JC, et al. Angiopoietin-like 4(ANGPTL4, fasting-induced adipose factor) is a direct glucocorticoid receptor target and participates in glucocorticoid-regulated triglyceride metabolism. J. BiolChem. 2009;284:25593–601.
Kersten S, Mandard S, Tan NS, Escher P, Metzger D, Chambon P, et al. Characterization of the fasting-induced adipose factor FIAF, a novel peroxisome proliferators-activated receptor target gene. J Biol Chem. 2000;275:28488–93.
Kersten S, Lichtenstein L, Steenbergen E, Mudde K, Hendriks HJ, Hesselink MK, et al. Caloric restriction and exercises increase plasma ANGPTL4 levels in humans via elevated free fatty acids. Arterioscler Thromb Vasc Biol. 2009;29:969–74.
Padua D, Zhang XHF, Wang Q, Nadal C, Gerald WL, Gomis RR, et al. TGFbeta primes breast tumors for lung metastasis seeding through angiopoietin-like 4. Cell. 2008;133:66–77.
Clement LC, Avila-Casado C, Mace C, Soria E, Bakker WW, Kersten S, et al. Podocyte-secreted angiopoietin-like-4 mediates proteinuria in glucocorticoid-sensitive nephrotic syndrome. Nat Med. 2011;17:117–22.
Galaup A, Cazes A, Le Jan S, Phlippe J, Connault E, CozE Le, et al. Angiopoietin-like 4 prevents metastasis through inhibition of vascular permeability and tumor cell motility and invasiveness. Proc Natl Acad Sci USA. 2006;103:18721–6.
Huang RL, Teo Z, Chong HC, Zhu P, Tan MJ, Tan CK, et al. ANGPTL4 modulates vascular junction integrity by integrin signaling and disruption of intercellular VE-cadherin and claudin-5 clusters. Blood. 2011;118:3990–4002.
Katanasaka Y, Kodera Y, Kitamura Y, Morimoto T, Tamura T, Koizumi F. Epidermal growth factor receptor variant type III markedly accelerates angiogenesis and tumor growth via inducing c-myc mediated angiopoietin-like 4 expression in malignant glioma. Mol Cancer. 2013;25:12–31.
Perdiguero EG, Galaup A, Durand M, Teillon J, Phillippe J, Valenzuela DM, et al. Alteration of developmental and pathological retinal angiogenesis in angptl4-deficient mice. J Biol Chem. 2011;286:36841–51.
Mandard S, Zandbergen F, Tan NS, Escher P, Patsouris D, Koenig W, et al. The direct peroxisome proliferator-activated receptor target fasting-induced adipose factor (FIAF/PGAR/ANGPTL4) is present in blood plasma as a truncated protein that is increased by fenofibrate treatment. J Biol Chem. 2004;279:34411–20.
Ge H, Yang G, Huang L, Motola DL, Pourbahrami T, Li C. Oligomerization and regulated proteolytic processing of angiopoietin-like protein 4. J Biol Chem. 2004;279:2038–45.
Lei X, Shi F, Basu D, Huq A, Routhier S, Day R, et al. Proteolytic processing of angiopoietin-like protein 4 by proprotein convertases modulates its inhibitory effects on lipoprotein lipase activity. J Biol Chem. 2011;286:15747–56.
Mandard S, Zandbergen F, Tan NS, Escher P, Patsouris D, Koenig W, et al. The direct peroxisome proliferator-activated receptor target fasting-induced adipose factor (FIAF/PGAR/ANGPTL4) is present in blood plasma as a truncated protein that is increased by fenofibrate treatment. J Biol Chem. 2004;279:34411–20.
Stapleton CM, Joo JH, Kim Y-S, Liao G, Panettieri RA Jr, Jetten AM. Induction of ANGPTL4 expression in human airway smooth muscle cells by PMA through activation of PKC and MAPK pathways. Exp Cell Res. 2010;316:507–16.
Yang YH, Wang Y, Lam KS, Yau MH, Cheng KK, Zhang J, et al. Supression of the Raf/MEK/ERK signaling cascade and inhibition of angiogenesis by the carboxyl terminus of angiopoietin-like protein 4. Arterioscler Thromb Vasc Biol. 2008;28:835–40.
Ito Y, Olike Y, Yasunaga K, Hamada K, Miyata K, Matsumoto SI, et al. Inhibition of angiogenesis and vascular leakiness by angiopoietin-related protein 4. Cancer Res. 2003;63:6651–7.
Hutchings H, Ortega N, Plouet J. Extracellular matrix-bound vascular endothelial growth factor promotes endothelial cell adhesion, migration, and survival through integrin ligation. FASEB J. 2003;17:1520–2.
Okochi-Takada E, Hattori N, Tsukamoto T, Miyamoto K, Ando T, Ito S, et al. ANGPTL4 is a secreted tumor suppressor that inhibits angiogenesis. Oncogene. 2013; 174 [Epub ahead of print].
Le Jan S, Amy C, Cazes A, Monnot C, Lamande N, Favier J, et al. Angiopoietin-like 4 is a proangiogenic factor produced during ischemia and in conventional renal cell carcinoma. Am J Pathol. 2003;162:1521–8.
Goh YY, Pal M, Chong HC, Zhu P, Tan MJ, Punugu L, et al. Angiopoietin-like 4 interacts with matrix proteins to modulate wound healing. J Biol Chem. 2010;285:32999–3009.
Galaup A, Gomez E, Souktani R, Durand M, Cazes A, Monnot C, et al. Protection aganist myocardial infarction and no-reflow through preservation of vascular integrity by angptl4. Circulation. 2012;125:140–9.
Quintero P, Gonzalez-Muniesa P, Martinez JA. Influence of different oxygen supply on metabolic markers and gene response in murine adipocytes. J Biol Requl Homeost Agents. 2012;26:379–88.
Lichtenstein L, Mattijssen F, de Wi NJ, Georgiadi A, Hooiveld GJ, van der Meer R, et al. ANGPTL4 protects against severe proinflammatory effects of saturated fat by inhibiting fatty acid uptake into mesenteric lymph node macrophages. Cell Metab. 2010;12:580–92.
Brown R, Imran SA, Wilkinson M. Lipopolysaccharide (LPS) stimulates adipokine and socs3 gene expression in mouse brain and pituitary gland in vivo, and in N-1 hypothalamic neurons in vitro. J Neuroimmunol. 2009;209:96–103.
Lu B, Moser A, Shigenaga JK, Grunfeld C, Feingold KR. The acute phase response stimulates the expression of angiopoietin like protein 4. Biochem Biophys Res Commun. 2010;391:1737–41.
Rummel C, Inoue W, Sachot C, Poole S, Hubschle T, Luheshi GN. Selective contribution of interleukin-6 and leptin to brain inflammatory signals induced by systemic LPS injection in mice. J Comp Neurol. 2008;511:373–95.
Zhu P, Tan MJ, Huang RL, Tan CK, Chong HC, Pal M, et al. Angiopoietin-like 4 protein elevates the prosurvival intracellular O2 −:H2O2 ratio and confers anoikis resistance to tumors. Cancer Cell. 2011;19:401–15.
Nakayama T, Hirakawa H, Shibata K, Nazneen A, Abe K, Naqayasu T, et al. Expression of angiopoietin-like 4(ANGPTL4) in human colorectal cancer: ANGPTL4 promotes venous invasion and distant metastasis. Oncol Rep. 2011;25:929–35.
Shibata K, Nakayama T, Hirakama H, Hidaka S, Naqayasu T. Clinicopathological significance of angiopoietin-like protein 4 expression in oesophageal squamous cell carcinoma. J Clin Pathol. 2010;63:1054–8.
Ma T, Jham BC, Hu J, Friedman ER, Basile JR, Molinolo A, et al. Viral G protein-coupled receptor up-regulates angiopoietin-like 4 promoting angiogenesis and vascular permeability in Kaposi’s sarcoma. PNAS. 2010;107:14363–8.
Reiser J. Filtering new facts about kidney disease. Nat Med. 2011;17:44–5.
Werner S, Grose R. Regulation of wound healing by growth factors and cytokines. Physiol Rev. 2003;83:835–70.
Goh YY, Pal M, Chong HC, Zhu P, Tan MJ, Punugu L, et al. Angiopoietin-like 4 interacts with integrins beta1 and beta5 to modulate keratinocyte migration. Am J Pathol. 2010;177:2791–803.
Musso G, Gambino R, Cassader M. Interactions between gut microbiota and host metabolism predisposing to obesity and diabetes. Annu Rev Med. 2011;62:361–80.
Lichtenstein L, Berbée JF, van Dijk SJ, van Dijk KW, Bensadoun A, Kema IP, et al. Angptl4 upregulates cholesterol synthesis in liver via inhibition of LPL- and HL-dependent hepatic cholesterol uptake. Arterioscler Thromb Vasc Biol. 2007;27:2420–7.
Adachi H, Fujiwara Y, Kondo T, Nishikawa T, Ogawa R. Angptl 4 deficiency improves lipid metabolism, suppresses foam cell formation and protects against atherosclerosis. Biochem Biophys Res Commun. 2009;379:806–11.
Romeo S, Pennacchio LA, Fu Y, Boerwinkle E, Tybjaerg-Hansen A, Hobbs HH, et al. Population-based resequencing of ANGPTL4 uncovers variations that reduce triglycerides and increase HDL. Nat Genet. 2007;39:513–6.
Folsom AR, Peacock JM, Demerath E, Boerwinkle E. Variation in ANGPTL4 and risk of coronary heart disease: the atherosclerosis risk in communities study. Metabolism. 2008;57:1591–6.
Jonker JT, Smit JW, Hammer S, Snel M, van der Meer RW, Lamb HJ, et al. Dietary modulation of plasma angiopoietin-like protein 4 concentrations in healthy volunteers and in patients with type 2 diabetes. Am J Clin Nutr. 2013;97:255–60.
Xu A, Lam MC, Chan KW, Wang Y, Zhang J. Angiopoietin-like protein 4 decreases blood glucose and improves glucose tolerance but induces hyperlipidemia and hepatic steatosis in mice. Proc Natl Acad Sci USA. 2005;102:6086–91.
Yokouchi H, Eto K, Nishimura W, Takeda N, Kaburagi Y, Yamamoto S, et al. Angiopoietin-like protein 4 (ANGPTL4) is induced by high glucose in retinal pigment epithelial cells and exhibits potent angiogenic activity on retinal endothelial cells. Acta Ophthalmol. 2013;91:e289–97.
Bouleti C, Mathivet T, Coqueran B, Serfaty JM, Lesaqe M, Berland E, et al. Protective effects of angiopoietin-like 4 on cerebrovascular and functional damages in ischaemic stroke. Eur Heart J. 2013; [Epub ahead of print].
Acknowledgments
This work was supported by the Pudong New Area Health Bureau Foundation of Shanghai PWRd2012-07, the National Natural Science Foundation of China (NO. 81270130) and the National Natural Science Foundation of China(NO.81070056).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible Editor: Ikuo Morita.
L. Guo and S.-Y. Li contributed equally to this work.
Rights and permissions
About this article
Cite this article
Guo, L., Li, SY., Ji, FY. et al. Role of Angptl4 in vascular permeability and inflammation. Inflamm. Res. 63, 13–22 (2014). https://doi.org/10.1007/s00011-013-0678-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-013-0678-0