Abstract
Objective and design
Sepsis refers to severe systemic inflammation in response to invading pathogens. To understand the molecular events that initiate the systemic inflammatory response, various inflammatory mediators were analyzed in neonatal sepsis samples and compared with normal samples.
Materials and methods
We initially measured the levels of the various classical inflammatory mediators such as acute phase proteins [C-reactive protein (CRP) and procalcitonin (PCT)], granule-associated mediators (NE, MPO and NO), proinflammatory cytokines [tumour necrosis factor-α (TNFα), IL-1β and IL-6), antiinflammatory cytokines (IL-10 and IL-13) and chemokines [IL-8 and monocyte chemotactic protein (MCP-1)] and novel cytokines (IL-12/IL-23p40, IL-21 and IL-23) using ELISA. We also used the human inflammation antibody array membrane to profile the inflammatory proteins that are involved in neonatal sepsis.
Results
There were significantly higher levels of CRP (5.4 ± 0.70 mg/L), PCT (1.500 ± 0.2400 μg/L); NE (499.2 ± 22.01 μg/L), NO (54.22 ± 3.131 μM/L); TNFα (396.6 ± 37.40 pg/mL), IL-1β (445.3 ± 34.25 pg/mL), IL-6 (320.9 ± 43.38 pg/mL); IL-8 (429.5 ± 64.08 pg/mL) MCP-1 (626.25 ± 88.91 pg/mL), IL-10 (81.80 ± 9.45 pg/mL), IL-12/IL-23p40 (30.25 ± 0.6 pg/mL), IL-21 (8,263.3 ± 526.8 pg/mL) and IL-23 (6,083 ± 781.3 pg/mL) in neonates with sepsis compared to normal. The levels of MPO (21.20 ± 3.099 ng/mL) were downregulated, whereas there was no change in IL-13 (188.7 ± 10.63 pg/mL) levels in septic neonates when compared with normal. Using the human inflammation antibody array membrane, we detected the presence of 17 inflammatory proteins such as IL-3, IL6R, IL12p40, IL-16, TNFα, TNFβ, TNF R1, chemokines I-309, IP-10 (IFN-γ inducible protein 10), MCP-1, MCP-2, MIP 1β (macrophage inflammatory protein), MIP-1δ, eotaxin-2, growth factors TGFβ1 (transforming growth factor beta), PDGF (platelet derived growth factor), and cell adhesion molecule ICAM-1 (intracellular adhesion molecule) that were upregulated whereas RANTES which was downregulated in neonatal sepsis.
Conclusion
The simultaneous secretion and release of multiple mediators such as proinflammatory cytokines and chemokines, cell adhesion molecules, and growth factors were found to be involved in the initiation of systemic inflammation in neonatal sepsis. Therefore, measuring the concentration of multiple mediators may help in the early detection of neonatal sepsis and help to avoid unnecessary antibiotic treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neonatal sepsis is a highly inflammatory disease that culminates in septic shock and multiple organ failure due to uncontrolled activation of the inflammatory system in response to pathogens [1]. The World Health Organization has estimated that 5 million neonatal deaths a year and about a quarter of global neonatal deaths occur in India, which has a neonatal mortality of 4 per 1,000 live births. The immune system of neonates is not completely developed, and therefore they are more prone to infection. Initiation of an effective immune response is provided by innate immune cells (macrophages and dendritic cells) in neonates. Cytokines produced by macrophages and dendritic cells (DC) are essential during host defense mechanisms in response to pathogens [2–6].
Cytokines play a vital role in the development of sepsis in neonates [7–10]. A pathogenic stimulus triggers the mononuclear phagocytes to secrete various proinflammatory cytokines, which in turn promote the production of secondary inflammatory mediators by neutrophils and T cells [11, 12]. Several studies have demonstrated that the levels of proinflammatory cytokines such as TNFα, IL-1β, IL-6 and IL-8 are highly elevated in the peripheral blood plasma of neonates with early-onset sepsis. In addition to phagocytosis, neutrophils also release pre-formed mediators that are stored in their granules, such as elastase and myeloperoxidase, by the process of degranulation. During the onset of sepsis, an enormous inflammatory reaction occurs in the preliminary phase, which is followed by an inconsistent antiinflammatory response. However, excessive production of proinflammatory mediators in sepsis overwhelms the antiinflammatory signaling. While inflammatory responses are beneficial and protective at a moderate level, excessive inflammation and acute phase response is detrimental, as it leads to tissue damage and organ dysfunction [13]. Though several studies have reported the few inflammatory mediators released during the course of neonatal sepsis, the identification of new mediators would increase the chances of better understanding the complex pathophysiological events of neonatal sepsis and the initiation and mechanism involved in the spread of systemic inflammatory response in neonates. Furthermore, the factors underlying the strong early systemic inflammatory responses are unclear.
Therefore, we aimed to study the factors that influence the systemic inflammatory response in neonatal sepsis. Here in our study we analyzed various inflammatory mediators, such as acute phase proteins, granule-associated mediators, proinflammatory and antiinflammatory cytokines and chemokines in the plasma of septic neonates and compared with normal. In addition, we also employed human inflammation protein array to find out the key inflammatory mediators that are involved in the pathogenesis of neonatal sepsis.
Materials and methods
Patient characteristics
This study was performed on newborns who were hospitalized with signs of clinical sepsis at NICU (Neonatal Intensive Care Unit) of SRM hospital, Chennai, and CMC hospital, Chengalpet. It involved a total of 118 neonates with early-onset sepsis and 61 normal samples. Inclusion criteria were positive clinical signs of sepsis and/or history of factors associated with increased risk for infection. Clinical signs of sepsis were defined as the presence of three or more of the following categories of clinical signs: apnea, tachypnea (>60/min), nasal flaring, retraction, cyanosis, respiratory distress-bradycardia (<100/min), tachycardia (>180/min), hypotonia, seizures-poor skin colour, capillary refilling time longer than 2 s, irritability and lethargy. The main causes of sepsis in this study population were due to premature rupture of membrane and vertical transmission of infection from mother to child.
Sample collection
This study was carried out from January 2009 to November 2012. Neonates diagnosed with early onset sepsis were enrolled in this study (mean age 2.4 days). Peripheral blood was collected from septic neonatal samples with the approval of Institutional Ethical Committee and informed consent was obtained from parent/guardian of all the subjects enrolled for this study. Normal samples include neonates of the same age, sex and weight without any signs of infection. Septic samples were collected at the time of onset of clinical symptoms of sepsis and before initiation of any antibiotic therapy. Peripheral blood was obtained by vein puncture or drawn from a dwelling arterial or venous line. The blood was collected aseptically in pre-coated EDTA tubes. A total of 179 samples from both male and female neonates were collected. The blood samples were centrifuged at 1,700×g for 15 min at 4 °C. The plasma was harvested and stored at −20 °C until further analysis.
Estimation of acute phase proteins and granule-associated mediators
Acute phase proteins such as procalcitonin (BRAHMS LIA) and C-reactive protein (Diagnostics Biochem Canada Inc) and granule-associated mediators like neutrophil elastase (Biovendor), myeloperoxidase (Calbiochem) and nitric oxide (Cloangen) in peripheral blood plasma were estimated by fully automated assays and carried out according to manufacturers’ instructions.
Measurement of proinflammatory and antiinflammatory cytokines and chemokines by ELISA
The levels of proinflammatory and antiinflammatory cytokines and chemokines such as TNFα, IL-1β, IL-6(e-Bioscience), IL-21 (R&D systems), IL-12/IL-23p40 (R&D systems), MCP-1(Antigenix America), IL-8 (e-Bioscience), IL-10 (e-Bioscience) and IL-13 (e-Bioscience) in peripheral blood plasma were estimated by ELISA according to manufacturers’ instruction.
Human inflammatory protein profiling by membrane array technique
Using Human Inflammation Antibody Array 3 (Raybiotech, USA), we analyzed the expression of inflammatory proteins in 12 sepsis samples and compared this to 12 normal samples. Briefly, the array membranes were incubated with blocking buffer at 25 °C for 30 min, followed by incubation with the 1 ml of threefold diluted patient’s plasma sample at 25 °C for 120 min. After washing, the membranes were incubated with biotin-conjugated antibodies at 25 °C for 120 min followed by the addition of horseradish peroxidase–conjugated streptavidin at 25 °C for 120 min. The membranes were developed with chemiluminescence substrate; images were taken using fluorchem Q, and the spots were analyzed as per manufacturer's instructions.
Statistical analysis
The difference in estimated parameters between the neonates categories were analyzed by student t test. All parameters were analyzed at 95 % confidence intervals and p value of <0.05 was considered to be statistically significant. Statistical analysis of the data was performed using GraphPad Prism version 5.00 for Windows, GraphPad Software, San Diego, California, USA.
Results
Clinical features
Table 1 shows the clinical and demographic features of the 118 septic and 61 normal neonates and mothers. Respiratory distress and birth asphyxia was found in most of the cases. The mean gestational age was found to be 35.36 weeks and the mean birth weight was 2.55 kg. The blood culture test shows that the predominant microorganisms infecting these neonates were Klebsiella spp. (38 %), Pseudomonas spp. (12 %), Staphylococcus aureus (11 %), Acetobacter spp. (11 %), E. coli (11.1 %) and Enterococcus spp. (6.3 %). Though Group B Streptococcus infection was the major causative agent in western countries, we have not observed any infection with GBS.
Levels of acute phase proteins
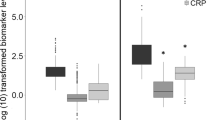
Acute-phase proteins are produced primarily by the liver in response to infection or tissue injury. The circulating levels of many acute phase proteins such as CRP and PCT are enhanced during sepsis and SIRS. In order to find out the concentration of these acute phase proteins we have measured their levels and found them to be higher in neonatal sepsis compared with normal as shown in Fig. 1.
Levels of acute phase proteins (CRP and PCT) and granule-associated mediators (MPO, NE and NO). The amounts of CRP, PCT, MPO, NE and NO were estimated in the peripheral blood plasma of normal and septic neonates by ELISA. Data are represented as mean ± standard error mean. Statistical analysis was done using Student's t test at 95 % confidence level; p values of <0.05 are considered statistically significant
Levels of granule-associated mediators
In order to understand the involvement and to quantify the secreted levels of granule-associated mediators such as neutrophils elastase (NE), myeloperoxidase (MPO) and nitric oxide (NO), the levels of these mediators were individually estimated in septic neonates and compared with normal samples. Though, the levels of NE and NO were significantly up-regulated in septic neonates, MPO was highly downregulated as shown in Fig. 1.
Profiling of inflammatory proteins by antibody array technique
To further profile the inflammatory proteins that are involved in neonatal sepsis, and to get a better insight about the pattern of expression of various cytokines and chemokines, we have used a human inflammation antibody array technique which can detect 40 different inflammatory proteins simultaneously in a single sample. Using this antibody array, we have found that 17 different inflammatory proteins out of 40 were expressed in neonatal sepsis samples. These proteins are cytokines such as IL-3, IL-6R, IL-12p40, IL-16, TNFα, TNFβ, TNF-RI; chemokines like IL-8, IP-10, I-309, Eotaxin-2, MCP-1, MCP-2, MIP-1β, MIP-1α; growth factors TGFβ1, PDGF and cell adhesion molecule ICAM 1 which are upregulated in sepsis samples whereas RANTES which was remarkably downregulated in septic neonates compared with normal (Fig. 2).
Representative figure showing the human inflammatory protein array profile of neonatal samples a plasma of normal sample b plasma of neonatal sepsis sample. Human inflammatory protein array revealed that 17 inflammatory proteins such as IL-3, IL-6R, IL-12p40, IL-16, TNFα, TNFβ, TNF-RI; IL-8, IP-10, I-309, Eotaxin-2, MCP-1, MCP-2, MIP-1β, MIP-1α; TGFβ1, PDGF and ICAM 1 were upregulated but RANTES was downregulated in septic neonates compared to normal. c Densitometry analysis for the differentially regulated inflammatory proteins for the normal and sepsis samples were given. Statistical analysis was performed using student's t test at 95 % confidence level; p values of <0.05 are considered statistically significant
Levels of proinflammatory and antiinflammatory cytokines and chemokines
We have also analyzed various proinflammatory and antiinflammatory cytokines and chemokines to find out whether any imbalance in the inflammatory network exists in neonatal sepsis. For this we measured the levels of various proinflammatory cytokines such as TNFα (396.6 pg/ml), IL-1β (445.3 pg/ml) and IL-6 (320.9 pg/ml) and antiinflammatory cytokines such as IL-10 (81.8 pg/ml) and IL-13 (188.7 pg/ml). Surprisingly, we found that were significantly increased in neonatal sepsis than normal samples except IL-13 where there was no significant difference in both sepsis and normal samples.
Production of chemokines is essential for host defense against bacteria but the overproduction of these mediators leads to multiorgan dysfunction and death. Therefore, we measured the levels of potent chemoattractants like IL-8 and MCP 1 and found them to be upregulated in septic neonates compared with normal. Figure 3 shows the levels of the various cytokines and chemokines in neonatal sepsis.
Amounts of various inflammatory cytokines and chemokines. Amounts of proinflammatory cytokines (IL-6, IL-8, TNFα, IL-21, IL-23 and IL-12p40) and anti-inflammatory cytokines (IL-10 and IL-13) and chemokines (MCP-1 and IL-8) in the peripheral blood plasma of normal and septic neonates were determined by ELISA. Data are represented as mean ± standard error mean. Statistical analysis was done using student's t test at 95 % confidence level; p values of <0.05 are considered statistically significant
Quantification of novel cytokines
Recently it has been reported that novel cytokines such as IL-12/23p40, IL-21 and IL-23 play an important role in infection and inflammation. In order to determine the role of these cytokines in neonatal sepsis, we have measured their concentration in the plasma of neonatal sepsis samples. The levels of IL-12/IL-23p40 (30.25 pg/ml), IL-21 (8263 pg/ml) and IL-23 (6083 pg/ml) were found to be significantly upregulated in septic samples compared with normal as shown in Fig. 4.
Concentration of novel cytokines (IL-21, IL-23 and IL-12p40) in the peripheral blood plasma of normal and septic neonates was determined by ELISA. Data are represented as mean ± standard error mean. Statistical analysis was performed using Student's t test at 95 % confidence level; p values of <0.05 are considered statistically significant
Discussion
Innate immune cells act as a first line of host defense—particularly neutrophils and macrophages, which on activation upon pathogen entry produces a number of proinflammatory cytokines and chemokines and other soluble mediators to eliminate the pathogens. The release of these mediators into circulation results in the development of systemic inflammation in adults and infants. These phagocytes produce a number of toxic chemicals like superoxide radicals, hydrogen peroxide and HOCl, which are not only involved in pathogen-killing mechanisms but also contribute to host tissue damage [1, 14, 15]. Though several studies have been documented in adult sepsis, few studies were reported in neonatal sepsis, related to the regulation of various inflammatory mediators. Here in our study we investigated the profiles of various inflammatory mediators that are involved in the regulation of systemic inflammation in neonatal sepsis.
Acute phase proteins are produced as part of an immediate response to infection. Several studies have shown that both CRP and PCT concentration increased appreciably in systemic bacterial infection during both early and late onset neonatal sepsis and is reported to be used as a marker for early detection of sepsis [16, 17]. We have also demonstrated that CRP and PCT concentrations are increased significantly in septic neonates compared to normal.
Antimicrobial enzymes such as elastase, nitric oxide synthase, myeloperoxidase and superoxide dismutase that are stored in the cytoplasmic granules are released upon degranulation for the clearance of pathogen [13, 18–24]. Furthermore, PMN-elastase is a sensitive and rapidly responding indicator of the neonatal systemic bacterial infection and nitric oxide has a role in host defense by killing pathogens. However, excessive levels of NO which is due to the over production of nitric oxide synthase leads to hypotension, cardiodepression and vascular hyporeactivity in septic shock. Also, MPO deficiency in PMN from neonates may contribute to their susceptibility to bacterial infection and systemic inflammatory state due to poor bacterial clearance. Consistent with previous reports, we have also observed the upregulation of NE and NO, which predisposes neonates to systemic inflammatory state while the downregulation of MPO accounts for poor bacterial clearance in neonatal sepsis.
Accumulating evidence suggests that the proinflammatory cytokines such as IL-6, IL-1β and TNFα levels increased rapidly upon exposure to bacterial antigens and are usually known to precede increase in CRP. Martin et al. [25] showed that serum IL-6, IL-8, and TNFα level were all higher in septic neonates than controls. Here in our study we have also found that the levels of these cytokines TNFα, IL-1β and IL-6 were highly enhanced. Furthermore, it is demonstrated that febrile children with positive blood cultures had increased Th1/Th2 cytokine levels, particularly IL-6 and IL-10. We also showed that IL-10 levels are significantly increased in septic neonates and may predict the severity of infection in neonates with sepsis [26–30]. Although the levels of IL-13 have been shown to increase during sepsis [27, 28], we were not able to observe any significant changes in septic neonates, which may be due to the time of collection of blood being not optimal for determining the concentration of IL-13.
While a large number of studies have been published based on few cytokines and chemokines measurements in neonatal sepsis, a limited number of works using inflammation protein array membrane approaches is available [31, 32]. Profiling the inflammatory proteins by protein array membrane technique has an added value over single cytokine analysis, since it allows detection of several cytokines simultaneously in the same sample [33]. By employing such a system, we were able to detect the presence of several inflammatory mediators in neonatal sepsis. Firstly, we were able to detect 17 proteins which are over expressed in neonatal sepsis compared to control samples. These inflammatory proteins include cytokines such as IL-3, IL-6R, IL-12p40, IL-16, TNFα, TNFβ, TNF- RI; chemokines like IL-8, IP-10, I-309, Eotaxin-2, MCP-1, MCP-2, MIP-1β, MIP-1α; growth factors TGFβ1, PDGF and cell adhesion molecule ICAM 1. Though 17 proteins are over expressed, RANTES, a potent chemoattractant of neutrophils and monocytes is highly down-regulated, which is consistent with previous reports [34]. Defective RANTES secretion by neonatal immune cells has also been linked to the enhanced susceptibility of neonates to bacterial or viral infections [8]. Healthy term neonates are capable of producing high levels of RANTES during the first month of life which represents the functional maturation of the neonatal immune system whereas low levels of RANTES may show the immaturity of the immune system in neonates.
Secondly, we found not only several cytokines and chemokines have contributed to the pathogenesis of sepsis but also growth factors and cell adhesion molecules. High levels of adhesion molecules have been found in inflammatory diseases and infection [16, 35–41]. For the initiation of systemic inflammation, leukocytes adhere to the endothelial cells and initiate the cytokine cascade towards increased production of IL-6, IL-8 and other chemokines. While production of chemokines is essential for host defense against bacteria, overproduction of these mediators has been shown to play an important role in the pathogenesis of sepsis [42, 43]. As reported in previous studies we also found that the levels of chemoattractants such as IL-8 and MCP-1 increased in neonatal sepsis.
Furthermore, as has been reported, IL-21 and IL-23 has been found to play a key role in infection, inflammation and tissue damage, and was also found to be important in the differentiation of Th17 cells [25, 44–49]; we observed that novel cytokines such as IL-12/IL-23p40, IL-21 and IL-23 were significantly higher in septic neonates compared to normal samples. Previous reports suggest that the p40 subunit of IL-12 was found to be induced by bacterial infection and plays an important role in host defense [50]. Hence, the upregulation in neonatal sepsis may show that they may be one of the factors contributing to the spread of inflammatory response.
Among the neonates enrolled in this study, there were six non-survivors. Correspondingly the levels of the mediators measured were highly elevated in these cases compared to others. We also found that though there was no significant difference in the levels of the mediators analyzed between preterm and term, we found a slight variation in the levels of MCP-1 and IL-12p40 with a mean difference of 100 and 12 pg/ml respectively.
In summary, in our current investigation, we have shown the differential regulation of various inflammatory mediators which in turn may contribute to the progression and pathogenesis of sepsis in neonates. As it is evident from our study there is an imbalance in the inflammatory network, that is, the production of both proinflammatory and antiinflammatory mediators were found to be highly enhanced. Furthermore, we have observed that not only the involvement of cytokines and chemokines but also other factors such as cell adhesion molecule (ICAM-I) and growth factors (TGFβ and PDGF) in neonatal sepsis. These mediators are released as an immediate response to infection and may contribute to the systemic inflammatory response. Thus, simultaneous secretion and release of multiple mediators stimulate the rapid progression of bacterial infection to systemic inflammation in neonates.
This study may provide a better understanding of the factors that contribute to systemic inflammation in neonatal sepsis and identification of sensitive and specific markers of inflammation would assist in the early detection of neonatal sepsis and help to avoid unnecessary antibiotic therapy and the development of drug-resistant organisms.
References
Kenzel S, Henneke L. The innate immune system and its relevance to neonatal sepsis. Curr Opin Infect Dis. 2006;19(3):264–70.
Cloberty JP, Stark R, Eichenwald E. Manual of neonatal care. Chap 49, 7th ed. Lippincott Williams and Wilkins; 1998.
Pertova A, Metha R. Dysfunction of innate immunity and associated pathology in neonates. Indian J Pediatr. 2007;74:185–91.
Dasari P, Zola H, Nicholson IC. Expression of toll-like receptors by neonatal leukocytes. Pediatr Allergy Immunol. 2011;22(2):221–8.
Medzhitov R, Janeway CA Jr. Innate immunity: the virtues of a nonclonal system of recognition. Cell. 1997;91:295–8.
Hotoura E, Giapros V, Kostoula A, Spirou P, Andronikou S. Tracking changes of lymphocyte subsets and pre-inflammatory mediators in full-term neonates with suspected or documented infection. Scand J Immunol. 2011;73(3):250–5.
Santana RC, Garcia MF, Reyes D, Gonzalez G, Dominguez C, Domenech E. Role of cytokines (interleukin-1beta, 6, 8, tumour necrosis factor-alpha, and soluble receptor of interleukin-2) and C-reactive protein in the diagnosis of neonatal sepsis. Acta Paediatr. 2003;92(2):221–7.
Abdelhamid AE, Chuang SL, Hayes P, Fell JM. In vitro cow’s milk protein-specific inflammatory and regulatory cytokine responses in preterm infants with necrotizing enterocolitis and sepsis. Pediatr Res. 2011;69(2):165–9.
Mokart D, Merlin M, Sannini A, Brun JP, Delpero JR, Houvenaeghel G, Moutardier V, Blache JL. Procalcitonin, interleukin 6 and systemic inflammatory response syndrome (SIRS): early markers of postoperative sepsis after major surgery. Br J Anaesth. 2005;94(6):767–73.
Weinberg GA, D’Angio CT. The search for new diagnostic tests for neonatal sepsis. J Pediatr. 2009;155(5):763–4.
Riedemann NC, Goo RF, Ward PA. Novel strategies for the treatment of sepsis. Nat Med. 2003;9:517–20.
Aziz M, Jacob A, Yang WL, Matsuda A, Wang P. Current trends in inflammatory and immunomodulatory mediators in sepsis. J Leukoc Biol. 2013. doi:10.1189/jlb.0912437.
Miller E. Phagocytosis in the newborn infant. J Ped. 1969;74:255.
Baltimore R, Huie SM, Meek JI, Schuchat A, Brien KLO. Early-onset neonatal sepsis in the era of group B Streptococcal prevention. J Pediatr. 2001;108:1094–8.
Baltimore RS. Perinatal bacterial and fungal infections. In: Jenson HB, Baltimore RS, editors. Ped Inf Dis. Prin Prac. 2nd ed. Philadelphia: WB Saunders Co; 2002. p. 1119–34.
Dollner H, Vatten L, Austgulen R. Early diagnostic markers for neonatal sepsis: comparing C-reactive protein, interleukin-6, soluble tumour necrosis factor receptors and soluble adhesion molecules. J Clin Epidemiol. 2001;54:1251.
Chiesa C, Natale F, Pascone R, Osborn JF, Pacifico L, Bonci E, De Curtis M. C reactive protein and procalcitonin: reference intervals for preterm and term newborns during the early neonatal period. Clin Chimica Acta. 2011;412:1053–9.
Manroe BL, Weinberg AG, Rosenfeld CR, Browne R. The neonatal blood count in health and disease. I. Reference values for neutrophils cells. J Ped. 1979;95(1):89–98.
Tsaka T, Herkner KR. Polymorphonuclear elastase in neonatal sepsis. Clin Chim Acta. 1990;193:103–12.
Henson PM. The immunologic release of constituents from neutrophil leukocytes. I. The role of antibody and complement on nonphagocytosable surfaces or phagocytosable particles. J Immunol. 1971;107:1535.
Ohlsson K, Olsson AS. Immunoreactive granulocyte elastase in human serum. Hoppe-Seyler’s Z Physiol Chem. 1978;359:1531.
Terregino C, Lopez B, Karras D, Killian A, Arnold G. Endogenous mediators in emergency department patients with presumed sepsis: are levels associated with progression to severe sepsis and death. Ann Emerg Med. 2000;35:26–34.
Shi YH, Li C, Shen J, Wang S, Qin R, Pan LJ. Plasma nitric oxide level in newborn infants with sepsis. J Ped. 1993;123:436–8.
Spack LP, Griffith HO. Measurement of total plasma nitrite and nitrate in pediatric patients with the systemic inflammatory response syndrome. Crit Care Med. 1997;25:1071–8.
Martin H, Olander B, Norman M. Reactive hyperemia and interleukin 6, interleukin 8, and tumor necrosis factor-α in the diagnosis of early-onset neonatal sepsis. Pediatrics. 2001;108:1–6.
Urbonas V, Eidukaite A, Tamuliene I. Increased interleukin-10 levels correlate with bacteremia and sepsis in febrile neutropenia pediatric oncology patients. Cytokine. 2012;57:313–5.
Takahashi N, Uehara R, Kobayashi M, Yada Y, Koike Y, Kawamata R, Odaka J, Honma Y, Momoi MY. Cytokine profiles of seventeen cytokines, growth factors and chemokines in cord blood and its relation to perinatal clinical findings. Cytokine. 2010;49:331–7.
Caoa YZ, Tua YY, Chen X, Wang BL, Zhong YX, Liu MH. Protective effect of ulinastatin against murine models of sepsis: inhibition of TNF and IL-6 and augmentation of IL-10 and IL-13. Exp Toxicol Pathol. 2012;64(6):543–7.
Schelonka RL, Maheshwari A, Carlo WA, Taylor S, Hansen NI, Schendel DE, Thorsen P, Skogstrand K, Hougaard DM, Higgins RD, NICHD Neonatal Research Network. T cell cytokines and the risk of blood stream infection in extremely low birth weight infants. Cytokine. 2011;53:249–55.
Ng PC, Li K, Leung TF, Wong RP, Li G, Chui KM, Wong E, Cheng FW, Fok TF. Early prediction of sepsis-induced disseminated intravascular coagulation with interleukin-10, interleukin-6, and RANTES in preterm infants. Clin Chem. 2006;52(6):1181–9.
Mera S, Tatulescu D, Cismaru C, Bondor C, Slavcovici A, Zanc V, et al. Multiplex cytokine profiling in patients with sepsis. Apmis. 2011;119(2):155–63.
Andaluz-Ojeda D, Bobillo F, Iglesias V, Almansa R, Rico L, Gandía F, Resino S, Tamayo E, de Lejarazu RO, Bermejo-Martin JF. A combined score of pro- and anti inflammatory interleukins improves mortality prediction in severe sepsis. Cytokine. 2012;57(3):332–6.
Harlan JM. Leukocyte–endothelial interactions. Blood. 1985;65:513–25.
Manoura A, Gourgiotis D, Galanakis E, Matalliotakis E, Hatzidaki E, Korakaki E, Saitakis E, Marmarinos AS, Giannakopoulou C. Circulating concentrations of α- and β-chemokines in neonatal sepsis. Int J Infect Dis. 2010;14(9):e806–9.
Wu YH, Chuang SY, Hong WC, Lai YJ, Chang YL, Pang JH. In vivo and in vitro inhibitory effects of a traditional Chinese formulation on LPS-stimulated leukocyte–endothelial cell adhesion and VCAM-1 gene expression. J Ethnopharmacol. 2012;140(1):55–63.
Cheon H, Yu SJ, Yoo DH, Chae IJ, Song GG, Sohn J. Increased expression of pro-inflammatory cytokines and metalloproteinase-1 by TGFbeta1 in synovial fibroblasts from rheumatoid arthritis and normal individuals. Clin Exp Immunol. 2002;127:547–52.
Edwards DR, Leco KJ, Beaudry PP, Atadja PW, Veillette C, Riabowol KT. Differential effects of transforming growth factor-beta 1 on the expression of matrix metalloproteinases and tissue inhibitors of metalloproteinases in young and old human fibroblasts. Exp Gerontol. 1996;31:207–23.
Fava RA, Olsen NJ, Postlethwaite AE, Broadley KN, Davidson JM, Nanney LB, et al. Transforming growth factor beta 1(TGFbeta 1) induced neutrophil recruitment to synovial tissues: implications for TGFbeta-driven synovial inflammation and hyperplasia. J Exp Med. 1991;173(5):1121–32.
Ramsay PL, O’Brian Smith E, Hegemier S, et al. Early clinical markers for the development of bronchopulmonary dysplasia: soluble E-Selectin and ICAM-1. Pediatrics. 1998;102:927.
Endo S, Inada K, Kasai T, et al. Levels of soluble adhesion molecules and cytokines in patients with septic multiple organ failure. J Inflamm. 1995;46:212.
Xanthou M, Fotopoulos S, Mouchtouri A, et al. Inflammatory mediators in perinatal asphyxia and infection. Acta Paediatr Suppl. 2002;91:92.
Ramnath RD, Ng SW, Guglielmotti A, Bhatia M. Role of MCP-1 in endotoxemia and sepsis. Int Immunopharmacol. 2008;8:810–8.
Bozza FA, Salluh JI, Japiassu AM, Soares M, Assis EF, Gomes RN, Bozza MT, Castro-Faria-Neto HC, Bozza PT. Cytokine profiles as markers of disease severity in sepsis: a multiplex analysis. Crit Care. 2007;11(2):R49.
Kantar M, Kültürsay N, Kütükçüler N, Akisü M, Cetingül N, Caglayan S. Plasma concentrations of granulocyte-macrophage colony-stimulating factor and interleukin-6 in septic and healthy preterms. Eur J Pediatr. 2000;159:156–7.
Magudumana O, Ballot DE, Cooper PA, et al. Serial interleukin 6 measurements in the early diagnosis of neonatal sepsis. J Trop Pediatr. 2000;46:267–71.
Yannam GR, Gutti T, Poluektova LY. IL-23 in infections, inflammation, autoimmunity and cancer: possible role in HIV-1 and AIDS. J Neuroimmune Pharmacol. 2012;7:95–112.
Louis S, Dutertre C-A, Vimeux L, Fery L, Henno L, Diocou S, Kahi S, Deveau C, Meyer L, Goujard C, Hosmalin A. IL-23 and IL-12p70 production by monocytes and dendritic cells in primary HIV-1 infection. J Leukoc Biol. 2010;. doi:10.1189/jlb.1009684.
Kollmann TR, Crabtree J, Rein-Weston A, Blimkie D, Thommai F, Wang XY, Lavoie PM, Furlong J, Fortuno ES 3rd, Hajjar AM, Hawkins NR, Self SG, Wilson CB. Neonatal innate TLR-mediated responses are distinct from those of adults. J Immunol. 2009;183(11):7150–60.
Bozza FA, Salluh JI, Japiassu AM, Soares M, Assis EF, Gomes RN, et al. Cytokine profiles as markers of disease severity in sepsis: a multiplex analysis. Crit Care. 2007;11:R49.
Nakano N, Nishiyama C, Kanada S, Niwa Y, Shimokawa N, Ushio H, Nishiyama M, Okumura K, Ogawa H. Involvement of mast cells in IL-12/23 p40 production is essential for survival from polymicrobial infections. Blood. 2007;109:4846–55.
Acknowledgments
This study is supported by the grant from Department of Biotechnology (DBT), Government of India (No. BT/PR12666/BRB/10/723/2009). The authors are thankful to SRM University for their support. Authors are also thankful to staffs of SRM hospital and CMC hospital, Chengalpet for assistance in sample collection.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Artur Bauhofer.
Rights and permissions
About this article
Cite this article
Sugitharini, V., Prema, A. & Berla Thangam, E. Inflammatory mediators of systemic inflammation in neonatal sepsis. Inflamm. Res. 62, 1025–1034 (2013). https://doi.org/10.1007/s00011-013-0661-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-013-0661-9