Abstract
Objective
Colon cancer is a common malignant neoplasm causing huge morbidity and mortality worldwide. Current therapeutic interventions are unsatisfying, which necessitates novel chemopreventive strategies. The present study was intended to elucidate the chemopreventive efficacy of hesperidin against azoxymethane (AOM)-induced mouse colon carcinogenesis.
Materials and methods
Swiss albino mice were subjected to intraperitoneal injections of AOM once a week for 3 consecutive weeks. Hesperidin treatments were provided in the initiation or post-initiation phases. The number and multiplicity of aberrant crypt foci (ACF), tumor incidence and antioxidant status were determined. Histopathological analyses, proliferating cell nuclear antigen (PCNA) index and modulations in the expression of inflammatory markers such as nuclear factor kappa B (NF-κB), inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) were studied.
Results
Hesperidin treatments significantly inhibited the number and multiplicities of AOM-induced ACF and tumor incidence. Hesperidin reduced oxidative stress parameters and enhanced antioxidant status. A marked decrease in the PCNA index was evident on hesperidin administration. Hesperidin treatments caused a prominent downregulation of NF-κB and its target molecules iNOS and COX-2, thereby combating inflammation.
Conclusion
This study proves the chemopreventive efficacy of hesperidin against the deleterious traits of colon carcinogenesis including accelerated proliferation, inflammation and persistent oxidative stress.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colon cancer ranks third amongst the most common fatal cancers in both men and women. Approximately 5 % of the western population develops colon malignancy during their lifetime. The statistics of colon cancer are sobering, with 101,340 new cases and 49,380 deaths in United States during 2011 [1]. Alarmingly, the incidence rate in south central Asia has been rising slowly during the last three decades [2]. Despite treatment with chemotherapy and surgery, death rates are high, together with adverse side effects [3]. The etiology of colon cancer is multifactorial; it occurs sporadically and is inherited in only 5 % of cases. A close association between dietary factors and the risk of colon cancer is apparent from many studies. Alcohol consumption, western dietary habits, increased fat intake and reduced carbohydrate are recognized as key contributing factors for colon cancer. Many studies have reported the mitigating role of dietary molecules in curbing various diseases including cancers. Interventions in the accelerated events of colon carcinogenesis by plant-based agents seem promising [4]. Fruits and vegetables remain a huge source of polyphenolics possessing various biological properties, among which flavonoids play an indispensable role [5]. Flavonoids are an interesting class of polyphenols displaying a wide range of antioxidant, anti-inflammatory and anti-carcinogenic properties [6]. Several researchers have documented the promising role of a diet rich in fruits and vegetables in combating the risk of colon cancer [7, 8]. This prompted us to investigate the potential efficiacy of a widely present citrus bioflavonoid, hesperidin, against colon carcinogenesis.
The beneficial properties of citrus bioflavonoids against various diseases including cancers have previously been reported [9]. Hesperidin [3′,5,7-trihydroxy-4′-methoxyflavanone-7-(6-α-l-rhamnopyranosyl-β-d-glucopyranoside)] (Fig. 1), a flavanone glycoside comprising an aglycone hesperetin and an attached disaccharide rutinose, is found abundantly in citrus fruits [10]. The antioxidant and radical scavenging properties of hesperidin are widely documented [11]. The potential of hesperidin to inhibit tumorigenesis in various cancer systems has gained considerable interest regarding its anticancer effectiveness [12, 13].
Oxidative stress is a consequence of cellular redox changes due to imbalance between reactive oxygen species (ROS) and the antioxidant defense. The harmful effects of ROS are counteracted by the endogenous antioxidant system comprising superoxide dismutase (SOD), catalase (CAT), reduced glutathione (GSH), glutathione peroxidase (GPx), glutathione reductase (GR), vitamin C and vitamin E [14]. An effective rodent model of colon carcinogenesis induced by azoxymethane (AOM) which mimics human colon carcinogenesis was used in this study [15]. Previous evidence proved the involvement of oxidative stress in AOM-mediated colon carcinogenesis accompanied by diminished antioxidants [16, 17]. Thus, appropriate antioxidant interventions with ability to reduce the stress parameters together with a significant restoration of the altered antioxidant status in colon carcinogenic conditions are needed.
Aberrant crypt foci (ACF) are putative preneoplastic lesions of colonic neoplasia. They appear in the early stages of colon cancer and subsequently develop into polyps, adenomas and eventually to carcinomas. AOM-induced ACF are considered to be a potent biomarker for examining the chemopreventive potential of novel agents [18]. Enhancement of colonic proliferation plays a early role in the progression of colon carcinogenesis. Proliferating cell nuclear antigen (PCNA) is a cell cycle protein actively involved in cell proliferation and expressed during the G1 and S phases of the cell cycle, and is widely used as a reliable proliferative marker to determine the anti-proliferative efficacy of natural or synthetic agents. Highly proliferating cancerous cells exhibit enhanced expressions of PCNA. The immunoreactivity of PCNA is directly related to the proliferative index of the cells, which makes it an attractive and reliable proliferative marker [19]. Nuclear factor kappa-B (NF-κB), a highly regulated transcription factor, plays an indispensable role in mediating inflammatory responses. NF-κB resists apoptosis and becomes a good target for treating many inflammatory diseases including cancers [20]. Furthermore, NF-κB activates downstream molecules such as inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2) which favor tumor progression. iNOS-mediated production of nitric oxide (NO) and COX-2-mediated prostaglandin (PG) biosynthesis are actively involved in inflammation. Over-expression of iNOS and COX-2 leads to DNA damage, post-translational modifications, increased proliferation and reduced apoptosis [21, 22]. Numerous studies have reported the aberrant increase of iNOS and COX-2 in colon malignancies, which makes them vital targets [23].
The present study was intended to explore the anticipated role of hesperidin against AOM-induced mouse colon carcinogenesis. Briefly, effects of hesperidin on the alterations in the oxidative stress parameters were estimated. The potential of hesperidin in combating ACF and tumor incidence was examined. Immunohistochemical analysis of PCNA was carried out to determine the anti-proliferative efficacy of hesperidin. Confocal microscopic analysis of NF-κB and COX-2, along with immunohistochemical analysis of iNOS and corresponding western blotting analyses, were performed in order to determine the inhibitory effect of hesperidin against NF-κB-mediated inflammatory responses.
Materials and methods
Chemicals
Azoxymethane and hesperidin were purchased from Sigma Chemicals Co., St. Louis, MO, USA. The rabbit polyclonal primary antibodies for PCNA and iNOS were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). The rabbit polyclonal antibody for NF-κB p65 was a generous gift from Dr. Irfan Rahman, Rochester University, USA. The rabbit polyclonal antibody for COX-2 was a generous gift from Dr. Asatara Kantasewi, Thailand. Secondary antibodies of anti-rabbit origin with horseradish peroxidase (HRP) or fluorescein isothiocyanate (FITC) were purchased from Bangalore Genei, India, Ltd. All other chemicals used were of high grade unless otherwise specified.
Animals and maintenance condition
Male Swiss albino mice weighing 25–30 g were used in this study. They were housed in separate cages and acclimatized for a week before the start of the experiment. They were maintained under standard temperature and humidity on a 12-h light/dark cycle with access to food and water ad libitum. The experiments were designed and conducted according to the guidelines of the committee for the purpose of control and supervision of experiments on animals (CPCSEA). Approval from the institutional animal ethics committee was obtained (IAEC Approval No. 02/09/2010).
Carcinogen and treatment drug administration
For the induction of colon cancer, Swiss albino mice received intraperitoneal injections of AOM at a dose of 15 mg/kg body weight once a week for three consecutive weeks. AOM was suspended in 0.9 % NaCl. Control mice received intraperitoneal injections of saline. Hesperidin (25 mg/kg body weight) was dissolved in 0.5 % carboxymethyl cellulose (CMC) and administered orally by gavage daily.
Treatment regimen
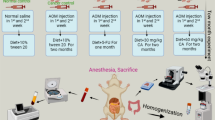
To study the efficacy of hesperidin against AOM-induced colon carcinogenesis, 50 mice were allocated into five groups with ten animals each. The diagrammatic representation of the experimental setup is shown in Fig. 2. Group I animals received intraperitoneal injections of physiological saline and served as control animals. Group II animals were administered AOM (15 mg/kg body weight) intraperitoneally once a week for 3 consecutive weeks. Group III animals were administered AOM as in group II and treated with hesperidin orally (25 mg/kg body weight) from a week before the AOM induction and continuing until the final dose of AOM, thus serving as the initiation group. Group IV animals were administered AOM as in group II and treated with hesperidin orally (25 mg/kg body weight) starting from a week after the AOM induction and continuing until the end of the experimental period, thus serving as the post-initiation group. Group V animals received the same dose of hesperidin alone for the entire period. The initial body weight of all groups of animals was recorded. At the end of the experimental period, the final body weight of all animals was recorded. Blood was collected and used for the estimation of biochemical parameters. All groups of animals were sacrificed by cervical dislocation. The liver from each animal was removed and weighed. The colon was excised quickly, flushed with 0.9 % NaCl solution, slit open longitudinally and examined for tumors. Homogenates of colon tissues were prepared in appropriate homogenizing buffer and were used for further assays.
Diagrammatic representation of the experimental protocol. Mice were allocated into five groups with ten animals in each group. Animals in Group I served as control animals. Animals in Group II received intraperitoneal injections of AOM once weekly for three consecutive weeks. Animals in Group III received AOM injections as in Group II and were administered hesperidin 1 week before the start of the first AOM injection and continued until the final exposure of AOM; this served as the initiation group. Animals in Group IV received AOM injections as in Group II and were administered hesperidin 1 week after the end of last AOM injection and continued until the end; this served as the post-initiation group. Animals in Group V served as drug control and were administered hesperidin alone for the entire experimental period
Determination of aberrant crypt foci
The analysis of ACF was performed by the method of Bird [24]. The colon was slit open longitudinally and placed on a strip of filter paper with its luminal surface open and exposed. Another filter paper was placed on the top of the luminal surface. This setup was fixed in 10 % formalin overnight. The fixed colonic sections were stained with 0.2 % methylene blue for 5 min. The sections were placed on a slide with the mucosal surface facing upwards and observed under a light microscope at 40× magnification. The number of ACF observed per colon and the number of aberrant crypts observed in each focus were recorded.
Biochemical parameters
The protein concentration was determined by the method of Lowry et al. [25] using bovine serum albumin (BSA) as standard. The level of lipid peroxidation (LPO) was measured in colon and plasma by the method of Ohkawa et al. [26] and the level of hydroxyl radicals (OH·) was estimated by the method of Cederbaum and Cohen [27]. Enzymic antioxidants such as SOD [28], CAT [29], GPx [30] and GR [31] and non-enzymic antioxidants including GSH [32], vitamin C [33] and vitamin E [34] were estimated as described.
Histopathological examination
The colonic tissues with tumors were grossly located and harvested from experimental mice. The tissues were fixed in 10 % formalin, routinely processed and embedded in paraffin. Sections of 4 μm thickness were prepared. The sections were stained with hematoxylin and eosin and viewed under light microscopy to document the histological changes.
Immunohistochemical analysis of PCNA and iNOS
Immunohistochemical analyses of PCNA and iNOS were performed in the paraffin-embedded colon tissue sections of 4 μm thickness. The tissue sections were rehydrated first in xylene followed by graded ethanol solutions. The slides were blocked with 5 % BSA in TBS (Tris-buffered saline) for 2 h. The sections were then immunostained with rabbit polyclonal primary antibodies for PCNA and iNOS, diluted (1:500) as recommended with 5 % BSA in TBS. The slides were incubated overnight at 4 °C. After washing the slides three times with TBS, the sections were incubated with HRP-conjugated anti-rabbit secondary antibody, diluted 1:2,000 with 5 % BSA in TBS, and incubated for 2 h at room temperature. Sections were then washed with TBS and incubated for 5–10 min in a solution of 0.02 % diaminobenzidine containing 0.01 % hydrogen peroxide. The sections were counter-stained with hematoxylin, dehydrated and mounted. The slides were visualized under a light microscope.
Confocal microscope analysis of NF-κB and COX-2
Paraffin-embedded tissue sections were processed and immunostained with rabbit polyclonal primary antibodies for NF-κB and COX-2, diluted (1:500) as recommended with 5 % BSA in TBS. The slides were incubated overnight at 4 °C. After washing the slides three times with TBS, the sections were incubated with FITC-conjugated anti-rabbit secondary antibody, diluted 1:40 with 5 % BSA in TBS, and incubated for 2 h at room temperature. The sections were washed with TBS and incubated with nucleus-specific counter-stain propidium iodide (Sigma, St. Louis, MO, USA) to highlight cell nuclei. Slides were visualized under a confocal microscope (Leica TCS-SP2 XL) using excitation/emission wavelengths of 529 nm/620 nm for PI and 494 nm/525 nm for FITC.
Protein extraction and Western blotting
The colonic tissues of the control and experimental groups of animals were homogenized in homogenizing buffer (135 mM NaCl, 20 mM Tris, 2 mM EDTA and 1 mM PMSF, pH 7.4). The homogenates were centrifuged at 10,000 rpm for 15 min at 4 °C. The supernatants were recovered and protein concentration was estimated. Equal amounts of protein samples were separated on 12 % sodium dodecyl sulfate–polyacrylamide gel electrophoresis. Separated proteins were electrophoretically transferred to polyvinylidene fluoride membrane (Millipore, USA). The membrane was blocked with 5 % BSA in Tris–Tween buffered saline for 1 h at room temperature, and incubated with respective primary antibodies (rabbit polyclonal-NF-κB, rabbit polyclonal-iNOS and rabbit polyclonal-COX-2) overnight at 4 °C. The membrane was then incubated with anti-rabbit horseradish peroxidase-conjugated secondary antibody (Bangalore Genei, India) for 2 h at room temperature. Protein antibody complexes were detected by the addition of diaminobenzidine as a substrate.
Statistical analysis
All the data were evaluated using SPSS v.16.0 software. Hypothesis testing methods included one-way analysis of variance (ANOVA) followed by least significant difference (LSD) test. P < 0.05 was considered to indicate statistical significance. All the results were expressed as mean ± SD for 10 mice in each group.
Results
Effect of hesperidin on body weight, liver weight and tumor incidence
The AOM induction resulted in a prominent decrease in the body weight gain percentage (%) of Group II animals compared to the control animals (Group I). Hesperidin treatments (Group III and IV) significantly increased the body weight gain percentage as compared to Group II animals. Concurrently, the AOM induction increased the liver weights of Group II animals compared to Group I animals. The liver weights of hesperidin-supplemented animals (Group III and IV) were lower than those of Group II animals. No marked variations were observed between the body and liver weight profiles of Group V and Group I animals (Table 1). The AOM challenges elicited colonic tumors in Group II animals. The anti-tumorigenic potential of hesperidin was evident by the reduction in the incidence and number of tumors in Group III and IV animals. No tumors were found in the control animals (Group I) and those administered hesperidin alone (Group V) (Table 2).
Hesperidin prevents AOM-induced ACF formations
Strong incidence of ACF along with visible tumors was seen in the colon of AOM-induced animals (Group II). The frequency and crypt multiplicity of the colonic ACF were markedly decreased on hesperidin supplementation (Group III and IV) compared with Group II animals. With the two different hesperidin treatment protocols, the inhibition of ACF was more pronounced in the protocol comprising hesperidin treatment at the initiation phase (Group III) rather than at the post-initiation phase (Group IV). No incidence of ACF was found in control animals (Group I) and those administered hesperidin alone (Group V) (Table 3).
Hesperidin restored the AOM-induced antioxidant status
The AOM inductions elevated the levels of LPO and OH· in Group II animals. Hesperidin treatments (Group III and IV) produced significant reductions in both LPO and OH·, as shown in Figs. 3 and 4, respectively. Treatment with hesperidin at the initiation phase (Group III) showed higher inhibitions than at the post-initiation phase (Group IV). No significant changes in the levels of LPO and OH· were observed between Group I animals and Group V animals.
Levels of lipid peroxidation (LPO) in colonic tissues and plasma of control and experimental groups of animals. The values are expressed as mean ± SD. Comparisons: aAOM versus control; bHES + AOM versus AOM; cAOM + HES versus AOM; nsnon-significant. The values are statistically significant at P < 0.05. LPO: μmoles of MDA released/mg of protein. Plasma: nmol/ml of MDA released per mg protein (AOM azoxymethane, HES hesperidin; Group I: control, Group II: AOM, Group III: HES + AOM, Group IV: AOM + HES, Group V: HES)
Levels of hydroxyl radicals (OH·) in colonic tissues and plasma of control and experimental groups of animals. The values are expressed as mean ± SD. Comparisons: aAOM versus control; bHES + AOM versus AOM; cAOM + HES versus AOM; nsnon-significant. The values are statistically significant at P < 0.05. OH: ng/mg protein (AOM azoxymethane, HES hesperidin; Group I: control, Group II: AOM, Group III: HES + AOM, Group IV: AOM + HES, Group V: HES)
The status of enzymic (SOD, CAT, GPx and GR) and non-enzymic (GSH, vitamins C and E) antioxidants in control and experimental groups of animals are shown in Tables 4 and 5. The AOM induction (Group II) resulted in marked decreases in the enzymic activities of SOD, CAT, GPx and GR in colonic tissues as compared to Group I animals. The same scenario was observed with the non-enzymic antioxidant status, comprising GSH, vitamin C and vitamin E. In marked contrast, hesperidin administrations (Group III and IV) elevated the antioxidant status compared to Group II animals. The antioxidant efficacy of hesperidin was more pronounced when administered at the initiation phase than at the post-initiation phase. The Group I and V animals showed optimal levels of antioxidants, with no significant difference between them.
Hesperidin ameliorated AOM-induced histopathological changes
The hematoxylin and eosin stained colonic tissue sections were subjected to histopathological examination. Group I animals possessed normal mucosal and submucosal layers with no signs of abnormalities (Fig. 5a, f). The AOM induction caused severe deteriorations with colonic tumors in Group II animals. The mucosal layers of Group II animals were highly thickened and densely packed with inflammatory cell infiltrates with enlarged nuclei and hyperchromatism. They possessed disintegrated cryptal structures with aberrant crypts together with massive necrotic destructions of epithelium (Fig. 5b, g). Supplementation with hesperidin at the initiation and post-initiation phases (Group III and IV animals) displayed normal crypts (Fig. 5c, d) with a notable decrease in the mucosal thickening with scattered infiltration of cells (Fig. 5h, i). Group V animals showed normal architecture of cryptal cells and mucosa similar to Group I animals (Fig. 5e, j).
Histopathological observations of colon tissues of control and experimental groups of animals stained with hematoxylin and eosin (scale bar 50 μm). Series a–e shows the colonic cryptal architecture and series f–j shows the mucosal and submucosal layers. a Colon of Group I animals showing normal crypts. f Group I animals possess normal mucosal and submucosal layers. b Colon of Group II animals showing disintegrated cryptal structures, with loss of epithelial integrity (encircled area pinpointed by solid arrow). g Group II animals possess densely packed inflammatory infiltrates in mucosal layer (encircled area pinpointed by solid arrow). c Colon of Group III animals showing restored cryptal morphology (solid arrows). h Mucosa of Group III animals showing scattered inflammatory infiltrates with normal mucosal folds (solid arrows). d Colon of Group IV animals showing normal crypts (solid arrows). i Mucosa of Group IV showing thickened and scattered inflammatory infiltrates. e Group V animals showing normal colonic architecture. j Mucosa of Group V animals has normal folds with no inflammatory cell infiltrates. (CR crypts, M mucosa, SM submucosa)
Hesperidin inhibits colonic cell proliferation
Representative microscope images of colonic tissue sections probed for the proliferative marker PCNA are presented in Fig. 6. A clear increase in PCNA-positive cells in the colonic tissue sections of Group II animals was observed (Fig. 6b) compared to Group I animals (Fig. 6a). Hesperidin administration markedly decreased PCNA-positive cells in the colonic tissues of Group III and IV animals. Hesperidin administration at the initiation phase showed higher efficacy (Fig. 6c) than at the post-initiation phase (Fig. 6d). Group V animals showed normal expressions of PCNA, the same as Group I animals (Fig. 6e). The average number of PCNA-positive cells across 20 random fields in each group of animals is represented in the bar graph (Fig. 6f).
Representative photographs of immunohistochemical staining of PCNA in colonic sections of control and experimental groups of animals (scale bar 50 μm). a Group I animals showing normal PCNA index. b Group II animals showing increased PCNA-positive cells (encircled area pinpointed by solid arrow). c and d Group III and IV animals showing decreased PCNA-positive cells as indicated by the solid arrows. e Group V animals showing normal expressions of PCNA. f PCNA-positive cells were quantified by averaging positive cells across 20 randomly selected fields in a blinded manner. The values are expressed as mean ± SD. Comparisons: aAOM versus control; bHES + AOM versus AOM; cAOM + HES versus AOM; nsnon-significant. The values are statistically significant at P < 0.05 (AOM azoxymethane, HES hesperidin; Group I: control, Group II: AOM, Group III: HES + AOM, Group IV: AOM + HES, Group V: HES)
Hesperidin attenuates AOM-induced inflammation
As inflammation plays a vital role in enhancing colon carcinogenesis, the ability of hesperidin to inhibit the key inflammatory mediators NF-κB, iNOS and COX-2 was studied. Representative confocal images of colon tissues probed for NF-κB are shown in Fig. 7. A substantial increase in NF-κB-positive cells in AOM-induced animals was evidenced by enhanced fluorescence compared to the control animals. The expression of NF-κB was significantly decreased in animals which received hesperidin either at the initiation or at post-initiation phases compared with the AOM-induced animals. NF-κB-positive cells were almost absent in control groups of animals. The colon tissues were further probed for other inflammatory markers such as iNOS and COX-2. Representative photographs of immunohistochemical staining showed a clear increase in iNOS-positive cells in Group II animals compared to Group I animals (Fig. 8b). Hesperidin treatments (Groups III and IV) significantly inhibited the expressions of iNOS in the colonic tissues (Fig. 8c, d) compared to Group II animals. Both Group I and V animals showed negligible expressions of iNOS (Fig. 8a, e). The iNOS-positive cells across 20 random fields in each group of animals are presented in the bar graph (Fig. 8f). AOM induction also increased COX-2 expressions in Group II animals compared to Group I animals. COX-2 immunoreactivity was almost absent in Group I and V animals. COX-2-positive cells were significantly decreased in the hesperidin-treated groups of animals (Groups III and IV) (Fig. 9). To further validate the expressions of NF-κB, iNOS and COX-2 in control and experimental groups of animals, Western blotting analysis was carried out. Representative immunoblots for NF-κB (65 kDa), iNOS (130 kDa) and COX-2 (70 kDa) expressions are shown in Fig. 10a. The protein expression pattern clearly showed overexpressions of NF-κB, iNOS and COX-2 in AOM-induced animals (Fig. 10a, lane 2). Hesperidin treatments significantly decreased the expressions of these inflammatory mediators (Fig. 10a, lanes 3 and 4). No significant differences were observed in the protein expression patterns of NF-κB, iNOS and COX-2 between animals administered hesperidin alone and control groups (Fig. 10a, lanes 5 and 1). The quantitative analysis of NF-κB, iNOS and COX-2 expressions in each lane is depicted in Fig. 10b. β-Actin was used as internal control.
Confocal microscope analysis of NF-κB expressions in colonic sections of control and experimental groups of animals. Tissue sections were immunostained with the anti-NF-κB antibody and a FITC-conjugated secondary antibody (green). Tissue sections were counter-stained with PI (red) to stain nuclei (scale bar 50 μm). Slides were visualized under a confocal microscope (Leica TCS-SP2 XL) using excitation/emission wavelengths of 529 nm/620 nm for PI and 494 nm/525 nm for FITC. Expressions of NF-κB in colonic tissues of Group II, III and IV animals are indicated by white arrows (AOM azoxymethane, HES hesperidin; Group I: control, Group II: AOM, Group III: HES + AOM, Group IV: AOM + HES, Group V: HES)
Representative photographs of immunohistochemical staining of iNOS in colonic sections of control and experimental groups of animals (scale bar 50 μm). a Group I animals showing negligible expression of iNOS. b Group II animals showing an aberrant increase in iNOS-positive cells (encircled area pinpointed by solid arrow). c and d Group III and IV animals showing decreased iNOS-positive cells as indicated by the solid arrows. e Group V showing negligible expression of iNOS similar to that of control. f iNOS-positive cells were quantified by averaging positive cells across 20 randomly selected fields in a blinded manner. The values are expressed as mean ± SD. Comparisons: aAOM versus control; bHES + AOM versus AOM; cAOM + HES versus AOM; nsnon-significant. The values are statistically significant at P < 0.05 (AOM azoxymethane, HES hesperidin; Group I: control, Group II: AOM, Group III: HES + AOM, Group IV: AOM + HES, Group V: HES)
Confocal microscope analysis of COX-2 expression in colonic sections of control and experimental groups of animals. Tissue sections were immunostained with the anti-COX-2 antibody and a FITC-conjugated secondary antibody (green). Tissue sections were counter-stained with PI (red) to stain nuclei (scale bar 50 μm). Slides were visualized under a confocal microscope (Leica TCS-SP2 XL) using excitation/emission wavelengths of 529 nm/620 nm for PI and 494 nm/525 nm for FITC. Expressions of COX-2 in colonic tissues of Group II, III and IV animals are indicated by white arrows (AOM azoxymethane, HES hesperidin; Group I: control, Group II: AOM, Group III: HES + AOM, Group IV: AOM + HES, Group V: HES)
Immunoblot analysis of NF-κB, iNOS and COX-2 in colonic tissues of control and experimental groups of animals. β-Actin served as internal control. a Lane 1 control, Lane 2 AOM-induced group, Lane 3 hesperidin-treated group (initiation phase), Lane 4 hesperidin-treated group (post-initiation phase), Lane 5 group administered hesperidin alone . b Quantitative data representing the corresponding protein levels assessed using densitometry. Y axis represents relative intensity (arbitrary units). Each column represents the mean ± SD. Hypothesis testing methods included one-way analysis of variance (ANOVA) followed by least significant difference (LSD). Comparisons: aAOM versus control; bHES + AOM versus AOM; cAOM + HES versus AOM; nsnon-significant. The values are statistically significant at P < 0.05 (AOM azoxymethane, HES hesperidin)
Discussion
Our current study demonstrated for the first time the extensive role of hesperidin in suppressing AOM-mediated colon carcinogenesis. Hesperidin exhibited promising inhibitions against AOM-induced oxidative stress parameters. Hesperidin treatments significantly downregulated the NF-κB-dependent inflammatory responses comprising iNOS and COX-2 activation. Concomitantly, a visible decline in the proliferative marker PCNA on hesperidin treatments further proved the anti-carcinogenic efficacy of hesperidin against AOM-induced colon carcinogenesis.
The AOM induction resulted in ACF incidence in the colon of experimental animals. The AOM-induced ACF are the precursors for microadenomas, adenomas and adenocarcinomas. Though ACF may further develop into tumors, not all of the ACF will do so [35]. At the end of experimental protocol, the colon of AOM-induced animals showed a mixed scenario of early and advanced ACF along with visible tumors. Hesperidin administration, both at initiation and at post-initiation phases, inhibited AOM-induced ACF formations. Hesperidin actively reduced the incidence and crypt multiplicities of aberrant crypts. The inhibitory effect of hesperidin against AOM-induced ACF is consistent with several reports addressing the potential of bioflavonoids to inhibit AOM-mediated ACF formation [36, 37]. In line with previous studies addressing the anti-tumor potential of flavonoids, hesperidin, being a bioflavonoid, was found to possess anti-tumor efficacy when administered either at the initiation phase or the post-initiation phase [38, 39]. Hesperidin supplementation did not show any apparent signs of toxicity to animals in terms of body weight gain profiles.
Free-radical-mediated oxidative stress is known to play crucial roles in carcinogenesis. It causes extensive damage to cell structures, lipids, proteins and nucleic acids. In cases of severe oxidative stress, ROS such as OH· are released in plasma and tissues. LPO is one such consequence of ROS responsible for causing extensive cellular damage. It can be determined by the estimation of its byproduct malondialdehyde (MDA) in plasma and tissues [40]. An aberrant increase in MDA along with OH· in colon carcinogenic conditions has been previously reported [41]. The cell defends itself against ROS by the induction of antioxidants, which include enzymic antioxidants such as SOD, CAT, GPx and non-enzymic antioxidants such as GSH, vitamin C and vitamin E [42]. Physiologically, SOD eliminates the superoxide radicals by converting them into hydrogen peroxide (H2O2) and molecular oxygen (O2). The H2O2 so formed is converted to water by CAT and peroxidases, and thus SOD and CAT work together to eliminate ROS [43]. GSH is a major non-enzymic antioxidant which can directly scavenge free radicals or can act as a substrate for GPx and GST during the detoxification of H2O2 [44]. Together, vitamin C and vitamin E are involved in the cell defense process; the former is a natural free radical scavenger which prevents free radical chain sequence, and vitamin E, a major membrane-bound antioxidant, protects the cell against LPO [45, 46]. In the current study, AOM induction resulted in increased LPO and OH· in both plasma and colon tissues which were considerably decreased by hesperidin supplementation during the initiation phase as well as at the post-initiation phase. This effect may be due to the free radical quenching properties of hesperidin. Furthermore, there were extensive alterations in the overall antioxidant status among the AOM-induced group of animals. The activities of enzymic and non-enzymic antioxidants were significantly lowered in the AOM-induced animals compared to the control group. The decline in the levels of antioxidants might be due to insufficient antioxidants against increased LPO and OH·. Hesperidin supplementation was found to substantially increase the antioxidant status thereby arresting the production of ROS. The antioxidant potential of hesperidin was more pronounced when hesperidin was given during the initiation phase than at the post-initiation phase. These data illustrate the potential of hesperidin to act as an effective antioxidant to combat free-radical-mediated oxidative stress.
Increased proliferation and suppressed apoptosis are the common denominators for enhanced tumorigenesis [47]. The disturbance in normal colonocyte homeostasis leading to uncontrolled proliferation of colonic epithelial cells has been documented previously [48]. Naturally occurring polyphenols are known to reverse these abnormal balances between cell proliferation and apoptosis in cancerous conditions [49]. In this regard, the anti-proliferative efficacy of hesperidin against AOM-induced colonic proliferation was determined by examining the expressions of PCNA, a well-known cell cycle marker protein. The enhanced expressions of PCNA and its significant role as a proliferative marker in colon carcinogenesis has been reported previously [50]. The immunohistochemical analysis showed enhanced expressions of PCNA in AOM-induced animals compared to control animals. This is due to the disturbed proliferation of colonocytes caused by AOM induction. Hesperidin supplementation at both initiation and post-initiation phases caused significant reductions in the PCNA index. The anticipated role of hesperidin in inhibiting cellular proliferation by reducing PCNA expression is in line with previous studies which reported the inhibitory role of flavonoids against PCNA activity [51, 52]. Thus the chemopreventive activity of hesperidin can be attributed to its anti-proliferative potential.
Overwhelming evidence proves chronic inflammation to be a major contributor to the establishment of cancers. Many studies underline the involvement of inflammation and its associated molecules during various stages of colon cancer [53]. NF-κB, a major transcriptional factor, plays a central role in many biological processes including cell proliferation and survival [54]. Furthermore, the constitutive activation of NF-κB aids in the progression of cancers by activating multiple anti-apoptotic and inflammatory signaling pathways [55]. Considering the pivotal role of NF-κB in tumor promotion, progression and maintenance, agents that inhibit its activities are gaining much importance. The abrupt increase in the immunoreactivity of NF-κB is well reported in the case of colon malignancies [56]. In accordance with the previous observations, the present study showed an increased expression of NF-κB in AOM-induced colonic tissues. The hesperidin supplementation inhibited NF-κB expression when administered at the initiation as well as the post-initiation phases. Enhanced NF-κB expression activates downstream inflammatory molecules such as iNOS and COX-2 [57]. The iNOS-mediated production of nitric oxide acts as a key pro-inflammatory molecule accelerating the early stages of tumorigenesis, and the enhanced expression of iNOS is well evidenced in colonic tumors [58]. Cyclooxygenases are the other set of molecules playing pro-inflammatory roles in cancerous conditions. They exist in two isoforms, COX-1 and COX-2, which are involved in prostaglandin (PGE2) synthesis. While COX-1 is constitutive, COX-2 is found to be enhanced in inflammatory conditions. The active role of COX-2 in carcinogenesis, including colon cancer, is been well documented [59]. The inhibition of iNOS and COX-2 could therefore be plausible for the chemoprevention of colon carcinogenesis. Similar to previous studies, AOM-induced colonic tissues clearly exhibited increased iNOS and COX-2 expressions compared to controls. Expression levels of iNOS and COX-2 were negligible in the control group of animals. The hesperidin treatments significantly contributed to the reduction of these inflammatory markers. These data substantiate the anti-inflammatory potential of hesperidin against colon carcinogenesis. Since the underlying causes for colon cancer progression are basically associated with abnormal proliferation and inflammation, modulation of proliferative and inflammatory markers by hesperidin seems a promising chemopreventive approach in combating colon carcinogenesis.
From the above findings, we report the chemopreventive efficacy of hesperidin against AOM-induced mouse colon carcinogenesis. Briefly, hesperidin reduced oxidative stress by enhancing antioxidants and inhibiting ROS. The anti-proliferative role of hesperidin was shown by the reduction of the proliferative marker PCNA. Furthermore, the expressions of inflammatory markers such as NF-κB, iNOS and COX-2 were reduced by hesperidin treatment. In a nutshell, we postulate that the primary mechanism of action of hesperidin occurs through the reduction of colonic cellular proliferation and inhibition of inflammation, along with its antioxidant effects (Fig. 11). Further studies will be required to explore the potential of hesperidin to modulate the key signaling cascades which play active roles in colon carcinogenesis.
Simplified illustration showing the chemopreventive efficacy of hesperidin against AOM-induced colon carcinogenesis. The AOM induction leads to the depletion of cellular antioxidants due to enhanced oxidative stress. AOM induction further leads to enhanced proliferation and inflammation via increased expressions of PCNA, NF-κB, iNOS and COX-2 in the colonic tissues. Treatment with hesperidin attenuates the expressions of these key proteins, thereby suppressing colon carcinogenesis
References
Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics 2011: the impact of eliminating socio economic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–36.
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90.
Thompson PA, Gerner EW. Current concepts in colorectal cancer prevention. Expert Rev Gastroenterol Hepatol. 2009;3:369–82.
Wei EK, Giovannucci E, Wu K, Rosner B, Fuchs CS, Willett WC, Colditz GA. Comparison of risk factors for colon and rectal cancer. Int J Cancer. 2004;108:433–42.
Tapas AR, Sakarkar DM, Kakde RB. Flavonoids as nutraceuticals: a review. Trop J Pharm Res. 2008;7:1089–99.
Birt DF, Hendrich S, Wang W. Dietary agents in cancer prevention: flavonoids and isoflavonoids. Pharmacol Ther. 2001;90:157–77.
Boateng J, Verghese M, Shackelford L, Walker LT, Khatiwada J, Ogutu S, Williams DS, Jones J, Guyton M, Asiamah D, Henderson F, Grant L, DeBruce M, Johnson A, Washington S, Chawan CB. Selected fruits reduce azoxymethane (AOM)-induced aberrant crypt foci (ACF) in Fisher 344 male rats. Food Chem Toxicol. 2007;45:725–32.
Nishino H, Tokuda H, Satomi Y, Masuda M, Onozuka M, Yamaguchi S, Takayasu J, Tsuruta J, Takemura M, Ii T, Ichiishi E, Kuchide S, Okuda M, Murakoshi M. Cancer chemoprevention by phytochemicals and their related compounds. Asian Pac J Cancer Prev. 2000;1:49–55.
Benavente OG, Castillo J, Alcaraz M, Vicente V, Del JA, Ortuno A. Beneficial action of Citrus flavonoids on multiple cancer-related biological pathways. Curr Cancer Drug Targets. 2007;7:795–809.
Garg A, Garg S, Zaneveld LJ, Singla AK. Chemistry and pharmacology of the Citrus bioflavonoid hesperidin. Phytother Res. 2001;15:655–69.
Patricia KW, Dalla SS, Mirian S. Antioxidant activity of the flavonoid hesperidin in chemical and biological systems. J Agric Food Chem. 2005;53:4757–61.
Kamaraj S, Ramakrishnan G, Anandakumar P, Jagan S, Devaki T. Antioxidant and anticancer efficacy of hesperidin in benzo(a)pyrene induced lung carcinogenesis in mice. Invest New Drugs. 2009;27:214–22.
Leef KH, Yehb MH, Kao ST, Hung CM, Liu CJ, Huang YY, Yeh CC. The inhibitory effect of hesperidin on tumor cell invasiveness occurs via suppression of activator protein 1 and nuclear factor-kappa B in human hepatocellular carcinoma cells. Toxicol Lett. 2010;194:42–9.
Andriantsitohaina R, Duluc L, Rodriguez JCG, Valle LGD, Garcia MG, Simard G, Soleti R, Su DF, Perez LV, Wilson JX, Laher I. Systems biology of antioxidants. Clin Sci (Lond). 2012;123:173–92.
Heijstek MW, Kranenburg O, Rinkes IHMB. Mouse models of colorectal cancer and liver metastases. Dig Surg. 2005;22:16–25.
Ashokkumar P, Sudhandiran G. Protective role of luteolin on the status of lipid peroxidation and antioxidant defence against azoxymethane-induced experimental colon carcinogenesis. Biomed Pharmacother. 2008;62:590–7.
Beelen VA, Spenkelink B, Mooibroek H, Sijtsma L, Bosch D, Rietjens IM, Alink GM. An n-3 PUFA-rich microalgal oil diet protects to a similar extent as a fish oil-rich diet against AOM-induced colonic aberrant crypt foci in F344 rats. Food Chem Toxicol. 2009;47:316–20.
Wargovich MJ, Brown VR, Morris J. Aberrant crypt foci: the case for inclusion as a biomarker for colon cancer. Cancers. 2010;2:1705–16.
Hall PA, Levison DA, Woods AL, Yu CCW, Kellock DB, Watkins JA, Barnes DM, Gillett CE, Camplejohn R, Dover R, Waseem NH, Lane DP. Proliferating cell nuclear antigen (PCNA) immunolocalization in paraffin sections: an index of cell proliferation with evidence of deregulated expression in some neoplasms. J Pathol. 1990;162:285–94.
Pikarsky E, Neriah YB. NF-κB inhibition: a double-edged sword in cancer? Eur J Cancer. 2006;42:779–84.
Murakami A, Ohigashi H. Targeting NOX, INOS and COX-2 in inflammatory cells: chemoprevention using food phytochemicals. Int J Cancer. 2007;21:2357–63.
Lala PK, Chakraborty C. Role of nitric oxide in carcinogenesis and tumor progression. Lancet Oncol. 2001;2:149–56.
Watanabe K, Kawamori T, Nakatsugi S, Wakabayashi K. COX-2 and iNOS, good targets for chemoprevention of colon cancer. BioFactors. 2000;12:129–33.
Bird RP. Observation and quantification of aberrant crypt foci in murine colon treated with a colon carcinogen: preliminary findings. Cancer Lett. 1987;37:147–51.
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–75.
Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1972;95:351–8.
Cederbaum AI, Cohen G. In: Packer L, editor, Methods in enzymology. San Diego: Academic Press; 1984. pp. 516–522.
Misra HP, Fridovich I. The role of superoxide anion in the autoxidation of epinephrine and a simple assay for superoxide dismutase. J Biol Chem. 1972;247:3170–5.
Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–94.
Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, Hoekstra WG. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179:588–90.
Staal GE, Visser J, Veeger C. Purification and properties of glutathione reductase of human erythrocytes. Biochim Biophys Acta. 1969;185:39–48.
Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta. 1979;4:67–78.
Omaye ST, Urnbull JB, Sauberlich HE. Selected methods for the determination of ascorbic acid in animal cells, tissues and fluids. Methods Enzymol. 1979;62:1–11.
Desai ID. Vitamin E analysis method for animal tissues. Methods Enzymol. 1984;105:138–43.
Takayama T, Katsuki S, Takahashi Y, Ohi M, Nojiri S, Sakamaki S, Kato J, Kogawa K, Miyake H, Niitsu Y. Aberrant crypt foci of the colon as precursors of adenoma and cancer. N Engl J Med. 1998;339:1277–84.
Miyamoto S, Yasui Y, Ohigashi H, Tanaka T, Murakami A. Dietary flavonoids suppress azoxymethane-induced colonic preneoplastic lesions in male C57BL/KsJ-db/db mice. Chem Biol Interact. 2010;18:276–83.
Gee JM, Hara H, Johnson IT. Suppression of intestinal crypt cell proliferation and aberrant crypt foci by dietary quercetin in rats. Nutr Cancer. 2002;43:193–201.
Kohno H, Tanaka T, Kawabata K, Hirose Y, Sugie S, Tsuda H, Mori H. Silymarin, a naturally occurring polyphenolic antioxidant flavonoid, inhibits azoxymethane-induced colon carcinogenesis in male f344 rats. Int J Cancer. 2002;101:461–8.
Leonardi T, Vanamala J, Taddeo SS, Davidson LA, Murphy ME, Patil BS, Wang N, Carroll RJ, Chapkin RS, Lupton JR, Turner ND. Apigenin and naringenin suppress colon carcinogenesis through the aberrant crypt stage in azoxymethane-treated rats. Exp Biol Med. 2010;23:710–7.
Valko M, Izakovic M, Mazur M, Rhodes CJ, Telser J. Role of oxygen radicals in DNA damage and cancer incidence. Mol Cell Biochem. 2004;266:37–56.
Skrzydlewska E, Stankiewicz A, Sulkowska M, Sulkowski S, Kasacka I. Antioxidant status and lipid peroxidation in colorectal cancer. J Toxicol Environ Health. 2001;64:213–22.
Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2000;160:1–40.
Mates JM, Sanchez JF. Antioxidant enzymes and the implications in pathophysiologic processes. Front Biosci. 1999;4:D339–45.
Roberta M, Roberta DB, Rosaria V, Carmela F, Claudio G. Novel mechanisms of natural antioxidant compounds in biological systems: involvement of glutathione and glutathione-related enzymes. J Nutr Biochem. 2005;16:577–86.
Kojo S. Vitamin C: basic metabolism and its function as an index of oxidative stress. Curr Med Chem. 2004;11:1041–64.
Burton GW, Ingold KU. Auto oxidation of biological molecules: the antioxidant activity of vitamin E and related chain-breaking phenolic antioxidants in vitro. J Am Chem Soc. 1981;103:64–72.
Evan GI, Vousden KH. Proliferation, cell cycle and apoptosis in cancer. Nature. 2001;411:342–8.
Bedi A, Pasricha PJ, Akhtar AJ, Barber JP, Bedi GC, Giardiello FM, Zehnbauer BA, Hamilton SR, Jones RJ. Inhibition of apoptosis during development of colorectal cancer. Cancer Res. 1995;55:1811–6.
Galati G, Teng S, Moridani MY, Chan TS, Brien PJO. Cancer chemoprevention and apoptosis mechanisms induced by dietary polyphenolics. Drug Metabol Drug Interact. 2000;17:311–49.
Kubben FJGM, Haesevoets AP, Engels LGJB, Baeten CGMI, Schutte B, Arends JW, Stockbrugger RW, Blijham GH. Proliferating cell nuclear antigen (PCNA): a new marker to study human colonic cell proliferation. Gut. 1994;35:530–5.
Ravichandran K, Velmurugan B, Gu M, Singh RP, Agarwal R. Inhibitory effect of silibinin against azoxymethane-induced colon tumorigenesis in A/J mice. Clin Cancer Res. 2010;16:4595–606.
Velmurugan B, Singh RP, Agarwal R, Agarwal C. Dietary-feeding of grape seed extract prevents azoxymethane-induced colonic aberrant crypt foci formation in Fischer 344 rats. Mol Carcinog. 2010;49:641–52.
Terzic J, Grivennikov S, Karin E, Karin M. Inflammation and colon cancer. Gastroenterology. 2010;138:2101–14.
Perkins ND. Integrating cell-signalling pathways with NF-kappa B and IKK function. Nat Rev Mol Cell Biol. 2007;8:49–62.
Karin M. NF-κB as a critical link between inflammation and cancer. Cold Spring Harb Perspect Biol. 2009;1:a000141.
Wang S, Liu Z, Wang L, Zhang X. NF-kB signaling pathway, inflammation and colorectal cancer. Cell Mol Immunol. 2009;6:327–34.
Kojima M, Morisaki T, Sasaki N, Nakano K, Mibu R, Tanaka M, Katano M. Increased nuclear factor-κB activation in human colorectal carcinoma and its correlation with tumor progression. Anticancer Res. 2004;24:675–82.
Rao CV. Nitric oxide signaling in colon cancer chemoprevention. Mutat Res. 2004;555:107–19.
Williams CS, Mann M, Dubois RN. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene. 1999;18:7908–16.
Acknowledgments
This work was supported by a fund from the Council of Scientific and Industrial Research (CSIR), New Delhi. We thank Dr. Ramamurthy, Director, Ultra-fast Process Laboratory, University of Madras for his help in confocal imaging.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Liwu Li.
Rights and permissions
About this article
Cite this article
Saiprasad, G., Chitra, P., Manikandan, R. et al. Hesperidin alleviates oxidative stress and downregulates the expressions of proliferative and inflammatory markers in azoxymethane-induced experimental colon carcinogenesis in mice. Inflamm. Res. 62, 425–440 (2013). https://doi.org/10.1007/s00011-013-0595-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-013-0595-2