Abstract
Objectives
Accurate and timely diagnosis and prognosis of sepsis remain challenging. A combination of markers, as opposed to single ones, may improve the prognosis, and therefore survival. This study compared the effectiveness of routinely used biomarkers of sepsis alone and in combination for the prediction of outcome in rats with abdominal sepsis.
Methods
Rats were subjected to sepsis induced by cecal ligation and puncture (CLP). Seventeen biomarkers were detected 12 h after the CLP. Correlation between the biomarkers and outcome of rats was analyzed; the correlated biomarkers were analyzed by logistic regression analysis and the area under the receiver operating characteristic (ROC) curve was computed to compare their performance in the prognosis of sepsis.
Results
A total of 49 rats were eligible for analysis. Body temperature (T), blood urea nitrogen (BUN), creatinine (Cr), alanine aminotransferase (ALT), creatine kinase (CK), interleukin-6 (IL-6) and monocyte chemoattractant protein-1 (MCP-1) levels after the CLP were negatively correlated with the survival outcome, while platelet count (PLT), high-mobility group box protein 1 (HMGB1) and granulocyte–macrophage colony stimulating factor (GM-CCF) were positively correlated with the survival outcome (P < 0.05). Levels of BUN, Cr, IL-6, and GM-CSF after the CLP were independent predictors of outcome according to conditional logistic regression. The sensitivity and specificity of the four selected biomarkers in combination for predicting sepsis outcome were better than single ones (P < 0.05).
Conclusion
A combination of different biomarkers improves the diagnostic accuracy and is more effective in the prognosis of sepsis in rats. Use of BUN, Cr, IL-6, GM-CSF in combination to predict the severity and outcome in rats with abdominal sepsis exhibited acceptable diagnostic characteristics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sepsis is the host-derived systemic inflammatory response syndrome (SIRS) to invasive infections that may result in septic shock and multiple organ failure. Despite the rapid development of modern medical technology, it remains the leading cause of death in critically ill patients, with mortality rates ranging between 30 and 70 % [1]. The diagnosis, severity evaluation and outcome prediction of sepsis are the most complex clinical scenarios that physicians face because of the highly variable and non-specific nature of the signs and symptoms of sepsis [2]. However, accurate diagnosis and prognosis of sepsis are very important for timely and specific treatment. The mortality rates are substantially improved when appropriate antibiotic administration [3] and goal-directed resuscitation [4] are implemented immediately after disease recognition.
Cytokines have been widely assessed as potential biomarkers for sepsis [5]. The three major proinflammatory cytokines, namely tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6) and interleukin-1β (IL-1β), presumably play a critical role as proximal mediators in a wide range of vital downstream processes. They instigate inflammation primarily by initiating cascades of downstream mediators, such as pro- and anti-inflammatory cytokines and chemokines. Monocyte chemoattractant protein-1 (MCP-1), a prototype of CC chemokines, is a potent chemoattractant and regulatory mediator in sepsis. High-mobility group box protein 1 (HMGB1) serves as a late inflammatory mediator in sepsis. Granulocyte–macrophage colony stimulating factor (GM-CSF) plays a critical role in innate immunity, and has been found to be a valuable marker for sepsis.
Clinical and laboratory signs of systemic inflammation or organ dysfunction are sensitive, including changes in body temperature (T), blood urea nitrogen (BUN), creatinine (Cr), lactate dehydrogenase (LDH), alanine aminotransferase (ALT), aspartate aminotransferase (AST), creatine kinase (CK), and routine bloodwork including white cell count (WBC), red cell count (RBC), platelet count (PLT), and hematocrit (HCT). However, their use is limited due to poor specificity for the diagnosis and prognosis of sepsis [1, 6].
A multitude of biomarkers has been proposed in sepsis suitable for diagnosis and prognosis [7]. However, no biomarker has been established sufficiently to be of great help to clinicians in everyday clinical practice. It remains difficult to differentiate sepsis from other SIRS with noninfectious causes. As each biomarker has limited sensitivity and specificity, a combination of biomarkers may improve the diagnosis, prognosis and treatment efficacy, and thereby survival.
With this background in mind, we undertook the present study to determine the discriminative power of combining multiple biomarkers routinely used and easily obtained to predict the severity and outcome in rats with abdominal sepsis.
Materials and methods
Animals
Male 8-week-old specific pathogen-free Sprague–Dawley rats, each weighing 200–250 g, were purchased from the Laboratory Animal Center of Central South University (Changsha, China). The rats were allowed to acclimate for 7 days under standard temperature and light/dark cycles before use. The rats had access to chow and water ad libitum throughout the study. All procedures were performed with the approval of the Institutional Animal Ethics Committee and conformed to Guidelines of Laboratory Animal Care and Use Committee at Xiangya School of Medicine, Central South University.
Sepsis model
Cecal ligation and puncture (CLP) appears to be a reliable and clinically relevant animal model of the human septic condition because the procedure produces an endogenous polymicrobial infection similar to peritonitis and sepsis in clinical practice. In this study, CLP was performed with some modification as described by Wichmann et al. [8]. The rats were first anesthetized by intraperitoneal injection of sodium pentobarbital (80 mg/kg of body weight), and a 20 mm midline incision was made to expose the cecum. The cecum was mobilized and ligated below the ileocecal valve with the blood flow of the cecum preserved, then the ligated cecum was punctured with an 18-gauge needle, and patency of punctures was assured by gentle expression of the fecal material. The cecum was then replaced in its normal intra-abdominal position and the wound closed with a running suture. In the sham control rats, the cecum was exposed and the bowel was massaged as described above, but it was not ligated or punctured. All rats received saline solution (0.9 % intraperitoneally, 24 ml/kg of body weight) for fluid loss immediately after the surgery.
Experimental design
The experiment was divided into three parts. During the first part, CLP was performed in 49 male Sprague–Dawley rats to observe the 72 h mortality rate and ensure the sepsis model. During the second part, 42 male Sprague–Dawley rats were grouped into the sham-operated group as control (7 rats) and the CLP groups (35 rats, 7 rats each, for 2, 6, 12, 18 and 24 h after the CLP). Rats were killed at different time-points after the CLP. At end-point, blood was drawn from the heart, and tissues from the liver (the left lobe), lungs (the right upper lobe), kidneys, heart and brain were harvested for histology. Serum levels of all cytokines and morphological changes in the organs at each time-point after the CLP were observed to determine the optimal time-point to mimic the time delay typically seen in humans between the onset of sepsis and the presentation to a physician. Finally, in the 49 male Sprague–Dawley rats with CLP, samples were collected at the optimal time-point after the CLP to detect all the biomarkers. In the meantime, 20 male Sprague–Dawley rats had sham operation as control group. And the last point, outcome of rats was observed. In a pilot experiment, the main parameters in normal rats determined in the current study were found to be highly constant over time. Thus, the time course of normal control was not included in this study. The experimental reproducibility was ensured by performing CLP in small groups of rats (n = 11–12) at the same time for 4 days by the same person.
Sampling and hematology test
During the second part of the experiment, blood was drawn from the heart at the time of rats’ end-point. To collect peripheral blood in the third part of the experiment, the distal tail of rats was clipped (5 mm) and 800 μL blood was drawn into a pipette at the optimal post-CLP time-point determined by the second part of the experiment. The rats were not killed at the time of sampling. Blood (33 μL) was immediately diluted (1:3) in EDTA (169 mM tripotassium salt) for the blood routine test; the blood left was centrifuged (15 min/3,000g at 4 °C) and serum was deprived. Fresh serum (67 μL) was used to assess the biochemical parameters by the Olympus AU5400 Automatic Biochemical Analyzer (Olympus, Japan) by using commercially available clinical assay kits. The serum left was stored at −80 °C prior to cytokines analysis.
Morphological analysis
For histopathological analysis, the liver (the left lobe), lungs (the right upper lobe), kidneys, heart and brain were fixed in 4 % paraformaldehyde for 24 h. The samples were dehydrated using increasing concentrations of ethanol (30–100 %), then placed in xylene for 3 h and embedded in paraffin wax overnight. Sections (4 μm thickness) were cut and placed on slides. To deparaffinize the sections, the slides were placed in xylene and decreasing concentrations of ethanol (100–70 %). For histological staining, slides were stained with hematoxylin and eosin (H&E). Sections were examined for organ damage under 200× magnification in a blinded manner with respect to the type of experimental group by two independent pathologists. The histological criteria for the organ damage were visualization of non-specific inflammation, including inflammatory cell infiltration, substantial and interstitial cellular degeneration and necrosis, blockage or rupture of capillary and so on.
Enzyme-linked immunosorbent assay (ELISA)
Serum cytokines were measured using commercially available ELISA kits (Quantikine, R&D Systems, Inc., Minneapolis, MN) specific for the cytokines in accordance with the manufacturer’s recommendations.
Statistical analysis
Descriptive statistics were expressed as mean ± SE, median and inter-quartile range (Q1–Q3). Post-CLP analysis of all markers in the rats was compared between the survival and the non-survival groups. Student t test was used to compare the means of the continuous variables with assured normality. Otherwise, the Mann–Whitney U test was used.
Relationship between all variables and outcome of rats was analyzed by Spearman’s correlation analysis. Variables with statistical significance in the correlation analysis were included in the multivariate analysis by applying a multiple logistic regression based on backward elimination of variables.
Discrimination of prognostic performance between the combinations of markers and single marker was analyzed by the area under a receiver operating characteristic (ROC) curve. Areas under the ROC curve (AUC) were compared by a nonparametric approach. The prognostic performances including sensitivity, specificity, overall correctness, and positive and negative predictive values of the composite markers were compared with the performances of all single markers using the AUC.
All tests were two sided, and P < 0.05 was considered statistically significant. The data were analyzed with the SPSS16.0 version.
Results
Mortality
A sepsis model was successfully established by CLP in rats. CLP was performed on 49 male Sprague–Dawley rats to observe the 72 h mortality rate to ensure the sepsis model. The rats died early from 6 h after the CLP, the 72 h mortality rate was 61.2 %. Most rats died between 12 and 24 h after the CLP, and the mortality rate was 42.9 %, as shown in Fig. 1.
Time-point determination
As shown in Fig. 2, the serum cytokine levels changed significantly after the CLP. We chose 12 h after the CLP as the time-point for physiologic parameters and blood sampling for the following reasons: (1) All cytokine levels increased significantly at 12 h after the CLP compared with those of the sham-operated group (P < 0.05 or P < 0.01), and most of the cytokines reached the peak at 12 h after the CLP. (2) Morphological changes in the organs at 12 h after the CLP were observed as shown in Fig. 3 by H&E staining. (3) Most rats died between 12 and 24 h after the CLP and we wanted to mimic the time delay typically seen in humans between the onset of sepsis and the presentation to a physician.
Change of serum cytokines in rats with sepsis after the CLP. a Expression levels of TNF-α increased significantly at 12 h after the CLP, declined at 18 h after the CLP, and rose again at 24 h after the CLP. b Expression levels of IL-1β began to rise at 6 h after the CLP, reached the peak value at 12 h after the CLP, declined at 18 h after the CLP and returned to the baseline at 24 h. c Expression levels of IL-6 began to rise at 2 h after the CLP, reached the peak value at 12 h after the CLP, and declined at 24 h after the CLP. d Expression levels of GM-CSF increased significantly at 12 h after the CLP. E Expression levels of HMGB1 increased significantly from 12 to 24 h after the CLP. The cytokines were detected by ELISA. Asterisk versus control (0 h, the sham-operated group), P < 0.05, n ≥ 3; double asterisks versus control, P < 0.01, n ≥ 3
Morphological changes of organs in rats with sepsis at 12 h after the CLP (HE staining ×200). Morphological changes in the lungs (the right upper lobe), liver (the left lobe), kidneys, heart and brain at 12 h after the CLP compared with those of the sham-operated group. Nonspecific inflammatory changes were observed at 12 h post-CLP in all the organs, including inflammatory cell infiltration, substantial and interstitial cellular degeneration and necrosis, and blockage or rupture of capillary
Post-CLP serum levels of markers
Cecal ligation and puncture was performed on 49 male Sprague–Dawley rats. According to the last outcome (survival or non-survival) at 72 h after the CLP, the rats were divided into a survival and a non-survival group. None of the rats died within 12 h post-CLP, 30 rats died between 12 and 72 h post-CLP, the other 19 rats survived. Individual baseline values and median levels of all biomarkers in the sham group (n = 20) or CLP groups at 12 h after the CLP were shown in Table 1. Significant differences between the sham and the CLP groups (both the survival and the non-survival group) were found in the serum levels of many markers (including BUN, Cr, IL-6, GM-CSF, and so on) except RBC and HCT at 12 h after the CLP. Significant differences between the survival and the non-survival group were found in the serum levels of several markers at 12 h after the CLP. In the non-survival group, the levels of serum BUN, Cr, LDH, ALT, CK, IL-6 and MCP-1 were significantly higher, while the PLT and serum levels of HMGB1 and GM-CSF at 12 h after the CLP were significantly lower than those of the survival group (P < 0.05 or P < 0.01) (Table 1).
Correlation analysis
Blood urea nitrogen, Cr, ALT, CK, IL-6 and MCP-1 levels at 12 h post-CLP were negatively correlated with the rat survival outcome; while PLT, HMGB1 and GM-CSF at 12 h post-CLP were positively correlated with the survival outcome (Table 2).
Logistic regression analysis and ROC curves
To evaluate the significance of each marker and choose the best combination of markers for the prognosis of sepsis, logistic regression was used to analyze the above markers correlated with the sepsis outcome. The following biomarkers were identified by multivariate analysis as having independent prognostic significance: BUN, Cr, IL-6, and GM-CSF. Regression coefficients of these biomarkers (Table 3) were used to calculate a logit of death for each rat with sepsis as follows:
The odds ratio (OR) of each biomarker indicated that high serum levels of BUN, Cr and IL-6 were risk factors for septic rats, while a high serum level of GM-CSF was a protective factor.
Discrimination of prognostic performances
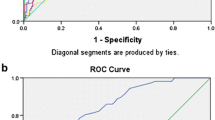
To compare the prognostic performances between the composite markers in combination and the four single markers, ROC and AUC were analyzed (Fig. 4). The specificity, sensitivity, positive and negative predictive value, and AUC of the four markers in combination were better than any single marker in predicting the sepsis outcome (Table 4).
ROC curves of single marker and markers in combination. ROC curves were drawn with a single marker such as BUN, Cr, IL-6, GM-CSF or with two markers and four markers in combination for predicting the outcome of rats with sepsis. AUCs from any single marker or combination of two or four markers were much better than the reference AUC (P < 0.01). AUC with four markers in combination is 0.920, which indicating an excellent diagnostic performance
Discussion
The overall 72 h mortality rate was 61.2 % in rats with sepsis induced by CLP, which was consistent with previous studies of the lethally septic rat model. This investigation revealed that nine clinical and laboratory biochemical parameters and cytokines of systemic inflammation were significantly correlated with 72 h mortality of rats with sepsis, including BUN, Cr, ALT, CK, IL-6, MCP-1, PLT, HMGB1 and GM-CSF. In addition, we found levels of BUN, Cr, IL-6, and GM-CSF at 12 h after the CLP could predict the outcome and established a logit of death equation containing the four markers according to backward conditional logistic regression. AUC of the 4-marker combination was 0.920 (0.854–0.985), indicating its effectiveness in prognostic performances (Table 4). This study confirmed the idea that in evaluating prognosis and predicting outcome of sepsis, the combination of several markers would be a better approach than single ones.
Our data revealed that the combination of BUN, Cr, IL-6 and GM-CSF could be used for the prediction of prognosis and outcome of sepsis. Of the four markers involved in the combination, BUN and Cr are both indicators of kidney injury. Severe sepsis and septic shock, are often complicated by acute kidney injury (AKI), and sepsis is also a well-known risk factor for the development of acute renal failure (ARF); 35–50 % of ARF cases in ICUs are due to sepsis [8, 9]. During septic shock, global tissue hypoxia caused by an imbalance between systemic oxygen delivery and oxygen demand results in renal tubular necrosis, multiple organ failure, and increased mortality rate [10, 11]. Robert A. et al. found the feature of CLP-induced ARF in rats is similar to that in humans. In the rats that survived, serum Cr values ranged from 0.4 to 2.3 mg/dL and there was a 27 % mortality rate at 24 h. About 24 % of the rats developed acute renal failure, which is similar to humans as 19–23 % of moderate to severe septic patients develop ARF [12, 13]. The mortality rate of 27 % in the rat model was also similar to that of humans (Fig. 1). Hotchkiss et al. found that the mortality rate in patients admitted to the ICU with sepsis was nearly 30 % [12, 14]. In our data, serum levels of BUN and Cr were significantly higher in the CLP group (both the survival and the non-survival group) than those of the sham group, which was consistent with previous studies. Analysis of the ability to discriminate between outcomes of septic rats identified AUCs of 0.744 (95 % CI 0.619–0.869) for BUN, 0.759 (95 % CI 0.638–0.880) for Cr, and 0.822 (95 % CI 0.716–0.928) for the 2-marker in combination. And the OR was 3.076 (95 % CI 0.848–11.166) for BUN, 3.107 (95 % CI 1.340–7.206) for Cr. These data further illustrated that the monitoring of renal function in sepsis was important for its prognosis and outcome.
Of the four markers involved in the combination, IL-6 and GM-CSF are both cytokines. Cytokines are key mediators in sepsis, and increased plasma and serum levels of those mediators are associated with the intensity of the inflammatory response. However, the usefulness of individual cytokines as prognostic markers is controversial. Cytokine levels were assessed in many studies to evaluate their diagnostic value. Classically, IL-6 is referred to as a cytokine with important prognostic value in sepsis. Among a wide array of cytokines assessed so far, it was found that persistently elevated IL-6 values are associated with both multiple organ failure and high mortality [15, 16]. However, in a study of cytokines in sepsis, the mortality rate was independent of the IL-6 levels [17]. In previous studies it has been shown that high IL-6 levels on admission [18] or subsequently [5] are seen in patients with severe sepsis and septic shock, and sequential measurement of blood IL-6 levels is useful in evaluating the severity and predicting the outcome of the patients with sepsis [19, 20]. Our data also showed that serum levels of IL-6 were significantly higher in CLP group (both the survival and the non-survival group) than those of the sham group, and the measurement of IL-6 levels was of great value for sepsis outcome.
As a growth factor that stimulates the proliferation and differentiation of myeloid cells, GM-CSF plays a key role in the functional maturity of monocytes and neutrophils [21]. Granulocyte–macrophage colony stimulating factor does not stimulate the proliferation of mature cells. However, it modulates mature cells by enhancing antigen presentation, altering leukocyte adhesion, increasing complement and antibody mediated phagocytosis, as well as potentiating cytokine release, all of which would aid host pathogen clearance [21]. It was documented that plasma GM-CSF levels in septic patients were significantly suppressed in patients who died compared with those who survived, and whose levels were comparable to those of healthy control subjects; low plasma GM-CSF levels were associated with adverse consequences for septic patients [22, 23]. The measurement of GM-CSF levels in the plasma of septic patients merits further study for use as a prognostic marker and also to identify the type of immunotherapy the patient may benefit from. Granulocyte–macrophage colony stimulating factor is by far the hematopoietic growth factor that is most widely used for the augmentation of immune responses. Previous studies have suggested that GM-CSF may be useful in reversing the immune paralysis that has been described in the later stages of sepsis [24]. Monocyte dysfunction has been shown to be associated with adverse consequences in septic patients. Therapy with the cytokine growth factor GM-CSF may be required for optimal monocyte function in these patients [25]. In our data, serum levels of GM-CSF were significantly lower in the CLP group (both the survival and the non-survival group) than those of the sham group, and GM-CSF levels were significantly higher in the survival group than the non-survival group. This suggested that GM-CSF might prevent the immune paralysis and GM-CSF levels were negatively correlated with the mortality in rat models of sepsis.
One of the most important actions for coping with patients with sepsis is to assess the severity of the attack quickly and accurately. Early identification of patients at risk of developing severe sepsis and septic shock potentially improves their care via prompt admission to the ICU. Besides the development of several prognostic systems for severity in sepsis, there are multiple biochemical tests and clinical parameters proposed as single markers for severe or septic shock. Most laboratory assays have significant limitations in clinical sepsis mainly because they are expensive and not widely available, and the results have been controversial. The prognostic systems are usually very complicated, also with some drawbacks, such as difficult and biased clinical data obtainment, controversial results and low sensitivity and/or specificity. BUN, Cr, IL-6 and GM-CSF might be the most economical and easily available parameters because they result from routine patient assessment, and they show good performances as risk factors for the development of severe sepsis.
In conclusion, our data offer new insight on the role of combination markers of BUN, Cr, IL-6 and GM-CSF in the prognosis and outcome of sepsis. Combining information from several markers improves diagnostic accuracy and is more effective in the prognosis and outcome of sepsis in rats. Use of BUN, Cr, IL-6, GM-CSF in combination to predict the severity and outcome in rats with abdominal sepsis exhibits acceptable diagnostic characteristics.
This study has several limitations. First, we only detected the levels of 11 routinely used markers which could be easily measured in rats, while it is possible that other sets of markers including hemodynamic parameters, though difficult to measure in rats, may also have similar or better prognostic value and may be better early markers for sepsis. Second, as to the 12 h time frame, we chose this time-point based on two reasons: (1) We wanted to choose a time-point just before death for physiologic parameters and blood sampling to ensure the accuracy of the prognosis, and most of the rats died between 12 and 24 h after the CLP. (2) We wanted to mimic the time delay typically seen in clinical settings. The 12 h time frame in the clinical setting may be too late to offer optimal therapy for septic patients, and it is possible that other biomarkers may have already changed within 12 h after the injury or septic insult. Third, the serum levels of some biomarkers such as HMGB1 in post-CLP rats were not consistent with previous studies. The serum levels of HMGB1 at 12 h after the CLP in the non-survival group were significantly lower than those of the survival group in our data. We considered one of the possible causes was the different responses in individual rats, and another might be that 12 h after the CLP might not be the best time-point taken for HMGB1 due to its late inflammatory effects. The immune paralysis state of severe sepsis might also be another reason. Finally, we just established one lethal sepsis model by CLP. It is possible that the diagnostic performance of our prognostic equation may vary in different sepsis models.
References
Alberta C, Brun-Buisson C, Goodman SV, Guidici D, Granton J, Moreno R, et al. Influence of systemic inflammatory response syndrome and sepsis on outcome of critically ill infected patients. Am J Respir Crit Care Med. 2003;168:77–84.
Lever A, Mackenzie I. Sepsis: definition, epidemiology, and diagnosis. BMJ. 2007;335:879–83.
Kumar A, Roberts D, Wood KE, Light B, Parrillo JE, Sharma S, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med. 2006;34:1589–96.
Rivers E, Nguyen B, Havstad S, Ressler J, Muzzin A, Knoblich B, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med. 2001;345:1368–77.
Meisner M. Biomarkers of sepsis: clinically useful? Curr Opin Crit Care. 2005;11:473–80.
Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–6.
Pierrakos C, Vincent JL. Sepsis biomarkers: a review. Crit Care. 2010;14:R15.
Bagshaw SM, George C, Dinu I, Bellomo R. A multi-centre evaluation of the RIFLE criteria for early acute kidney injury in critically ill patients. Nephrol Dial Transplant. 2008;23:1203–10.
Ricci Z, Cruz D, Ronco C. The RIFLE criteria and mortality in acute kidney injury: A systematic review. Kidney Int. 2008;73:538–46.
Sakr Y, Vincent JL, Schuerholz T, Filipescu D, Romain A, Hjelmqvist H, et al. Early-versus late-onset shock in European intensive care units. Shock. 2007;28:636–43.
Lin SM, Frevert CW, Kajikawa O, Wurfel MM, Ballman K, Mongovin S, et al. Differential regulation of membrane CD14 expression and endotoxin-tolerance in alveolar macrophages. Am J Respir Cell Mol Biol. 2004;31:162–70.
Holly MK, Dear JW, Hu X, Schechter AN, Gladwin MT, Hewitt SM, et al. Biomarker and drug-target discovery using proteomics in a new rat model of sepsis-induced acute renal failure. Kidney Int. 2006;70:496–506.
Heresi GA. Acute renal failure and sepsis. N Engl J Med 2004; 351:2347-9; author reply 2347-9.
Hotchkiss RS, Karl IE. The pathophysiology and treatment of sepsis. N Engl J Med. 2003;348:138–50.
Patel RT, Deen KI, Youngs D, Warwick J, Keighley MR. Interleukin 6 is a prognostic indicator of outcome in severe intra-abdominal sepsis. Br J Surg. 1994;81:1306–8.
Panacek EA, Marshall JC, Albertson TE, Johnson DH, Johnson S, MacArthur RD, et al. Efficacy and safety of the monoclonal anti-tumor necrosis factor antibody F(ab,)2 fragment afelimomab in patients with severe sepsis and elevated interleukin-6 levels. Crit Care Med. 2004;32:2173–82.
Gogos CA, Skoutelis A, Lekkou A, Drosou E, Starakis I, Marangos MN, et al. Comparative effects of ciprofloxacin and ceftazidime on cytokine production in patients with severe sepsis caused by gram-negative bacteria. Antimicrob Agents Chemother. 2004;48:2793–8.
Oda S, Hirasawa H, Shiga H, Nakanishi K, Matsuda K, Nakamua M. Sequential measurement of IL-6 blood levels in patients with systemic inflammatory response syndrome (SIRS)/sepsis. Cytokine. 2005;29:169–75.
Yoon DY, Chu J, Chandler C, Hiyama S, Thompson JE, Hines OJ. Human cytokine levels in nonperforated versus perforated appendicitis: molecular serum markers for extent of disease? Am Surg. 2002;68:1033–7.
Remick DG, Bolgos G, Copeland S, Siddiqui J. Role of interleukin-6 in mortality from and physiologic response to sepsis. Infect Immun. 2005;73:2751–7.
Hamilton JA, Anderson GP. GM-CSF Biology. Growth Factors. 2004;22:225–31.
Presneill JJ, Waring PM, Layton JE, Maher DW, Cebon J, Harley NS, et al. Plasma granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor levels in critical illness including sepsis and septic shock: relation to disease severity, multiple organ dysfunction, and mortality. Crit Care Med. 2000;28:2344–54.
Perry SE, Mostafa SM, Wenstone R, McLaughlin PJ. Low plasma granulocyte-macrophage colony stimulating factor is an indicator of poor prognosis in sepsis. Intensive Care Med. 2002;28:981–4.
Nierhaus A, Montag B, Timmler N, Frings DP, Gutensohn K, Jung R, et al. Reversal of immunoparalysis by recombinant human granulocyte-macrophage colony-stimulating factor in patients with severe sepsis. Intensive Care Med. 2003;29:646–51.
Kylanpaa ML, Mentula P, Kemppainen E, Puolakkainen P, Aittomaki S, Silvennoinen O, et al. Monocyte anergy is present in patients with severe acute pancreatitis and is significantly alleviated by granulocyte-macrophage colony-stimulating factor and interferon-gamma in vitro. Pancreas. 2005;31:23–7.
Acknowledgments
The authors would like to thank Weixin Hu for his kind assistance in statistical analysis. This research was supported by National Natural Science Foundation of China (81071594).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Responsible Editor: Artur Bauhofer.
Rights and permissions
About this article
Cite this article
Gao, M., Zhang, L., Liu, Y. et al. Use of blood urea nitrogen, creatinine, interleukin-6, granulocyte–macrophage colony stimulating factor in combination to predict the severity and outcome of abdominal sepsis in rats. Inflamm. Res. 61, 889–897 (2012). https://doi.org/10.1007/s00011-012-0481-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-012-0481-3