Abstract
Objective
We investigated the inhibitory effects of quercetin and kaempferol treatment on the suppression of immunoglobulin E (IgE)-mediated allergic responses in relation to intestinal epithelium barrier function in RBL-2H3 and Caco-2 cells.
Methods
RBL-2H3 cells as a model of intestinal mucosa mast cells were treated with flavonols followed by IgE-anti-dinitrophenyl sensitization. The extent of degranulation and the release of pro-inflammatory cytokines were measured. Caco-2 cells were stimulated with interleukin (IL)-4 or IgE-allergen with or without flavonol pretreatment and changes in the expression of CD23 mRNA and mitogen-activated protein kinase (MAPK), and chemokine release were determined.
Results
Flavonols inhibited the secretion of allergic mediators in RBL-2H3 cells and suppressed the CD23 mRNA expression and p38 MAPK activation in IL-4 stimulated Caco-2 cells. Flavonols also suppressed IgE-OVA induced extra signal-regulated protein kinase (ERK) activation and chemokine release.
Conclusions
Quercetin and kaempferol effectively suppressed the development of IgE-mediated allergic inflammation of intestinal cell models.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Incidences of food allergy have increased over the past decade [1]. Although the central mechanisms involved in food-born allergic reactions are known, the target molecular events to alleviate food allergen-induced allergic manifestations are not well understood. The mucus at the inner surface of the gut epithelial cells plays a major role as a barrier to potential foreign antigens. By abrogation of the intestinal epithelium barrier, the specific food allergen easily activates the mast cells that form approximately 2–3% of the cell population within the lamina propria in healthy individuals [2]. Upon allergic responses, this amount can be augmented up to tenfold [3]. Mast cells respond to immunoglobulin E (IgE)/allergen and release bioactive mediators such as IL-4, TNF-α, and histamine into adjacent tissues within a few minutes that induce physiological responses [4]. Compounds which down-regulate the level of these mediators have been considered as anti-allergic agents in clinical settings.
There is strong evidence that mast cell mediators can also accelerate the transport of IgE and allergen in the intestinal barrier [4]. Interleukin (IL)-4 assists to produce more T-helper (Th) 2 type cytokines [5, 6] and controls the IgE transepithelial transport in intestinal epithelium. CD23, the low affinity IgE receptor present in intestinal epithelium, is shown to contain the IL-4 enhancer element [7] indicating that IL-4 is involved in the capture of the IgE/allergen. Consistent with this finding, IL-4 has been shown to up-regulate the CD23 expression through the p38 MAPK activation in rodent and human epithelial cells [8, 9]. In addition, CD23 could trigger ERK MAPK activation and subsequently the up-regulation of epithelial chemokines such as IL-8 and MIP-3α, which infiltrate other immune cells and double inflammatory responses [10, 11]. In light of these facts, it is conceivable that an increase of the barrier function of intestinal epithelium elicits the prevention of allergy.
Interests in plant food-derived flavonoids has increased greatly due to their antioxidant value [12] and anti-inflammatory capacity [13], along with the possible beneficial implications in human health. It has been reported that the average daily US diet contains approximately 210 mg of flavonoids [14]. Quercetin and kaempferol (Fig. 1) in the human diet represents 70% of the flavonoid intake [15, 16]. Many plant components are theorized to be gut protective [17–19], however, limited information is available for their function to protect intestinal barrier.
RBL-2H3 rat basophilic leukemia cell line has been widely used to investigate the IgE-mediated signals [20] due to their expression of high affinity IgE receptor, FcεRI, and ability to release chemical mediators in response to the cross-linking of FcεRI. RBL-2H3 is also similar to bone-marrow derived mast cells and to mucosal mast cell, the latter of which may be involved in inflammation of intestine. A human colon adenocarcinoma cell line, Caco-2 cells were a widely employed model for functional and molecular analysis of intestinal epithelium, because these cells are highly polarized with a well-formed brush border and tight junction. Caco-2 cells have been shown to express several differentiated markers typical of adult enterocytes and behave like the small intestine [21].
The objective of this study was to examine the inhibitory effects of quercetin and kaempferol on IgE-mediated allergic responses in relation to intestinal epithelium barrier function using two in vitro intestinal epithelium models.
Materials and methods
Chemicals and reagents
Anti-dinitrophenyl (DNP) IgE, piperazine N,N′-bis (2-ethanesulfonic acid) (PIPES), dimethyl sulfoxide (DMSO), n-nitrophenyl-N-acetyl-β-d-glucosaminide, kaempferol, and anti-β-actin were purchased from Sigma (St Louis, MO, USA). Dinitrophenylated bovine serum (DNP-BSA) was obtained from Calbiochem (San Diego, CA, USA). Eagle’s minimum essential medium (MEM), fetal bovine serum (FBS), and penicillin and streptomycin mixture solution were obtained from Invitrogen Life Technologies (Carlsbad, CA, USA). The monoclonal antibody and polyclonal antibody for ERK1/2, phospho-ERK1/2(Tyr 202/204), p38 and phospho-p38 were obtained from Cell Signaling Technology (Beverly, MA, USA). Cell culture transwell permeable supports were from Corning Incorporated (Corning, NY, USA). The ECL advance western blotting system was obtained from Amersham Pharmacia Biotech Inc. (Piscataway, NJ, USA). TNF-α, IL-4, IL-8, and MIP-3α (CCL20) ELISA kits were purchased from R&D Systems (Minneapolis, MN, USA).
Cell culture
RBL-2H3 cells were grown in Eagles’ minimal essential medium containing 10% (v/v) heat-inactivated fetal bovine serum, 2 mmol/l l-glutamate, 100 U/ml penicillin–streptomycin. Cultures were maintained under 5% CO2 at 37°C in tissue culture flasks. Caco-2 cells (HTB-37; American Type Culture Collection, Manassas, VA, USA) were grown in 75 cm2 culture flasks containing modified Eagle’s medium supplemented with 10% (v:v) fetal bovine serum, and 100 U/ml penicillin–streptomycin.
β-Hexosaminidase secretion in DNP-sensitized RBL-2H3 cells
Degranulation was determined by measuring the release of a granule marker, β-hexosaminidase. RBL-2H3 cells in the MEM containing FBS (10%), penicillin (100 unit/ml), streptomycin (100 μg/ml) were seeded into 24-well plates at a cell density of 104 cells/well. Test samples were added to each well and incubated for 48 h and were sensitized overnight with 0.5 μg/ml DNP-specific IgE. The cells were washed with 500 μl of Siraganian buffer (119 mM NaCl, 5 mM KCl, 0.4 mM MgCl2, 25 mM PIPES, and 40 mM NaOH, pH 7.2) and incubated in 160 μl of Siraganian buffer containing 5.6 mM glucose, 1 mM CaCl2, and 0.1% BSA for 10 min at 37°C. This was followed by the addition of 20 μl of antigen (DNP-BSA, 100 ng/ml) for 20 min to induce allergic reactions. The reaction was stopped by cooling in an ice bath for 10 min. The supernatant (50 μl) was transferred to 96-well plate and incubated with 50 μl of substrate (1 mM p-nitrophenyl-N-acetyl-β-d-glucosaminide) in 0.1 M citrate buffer (pH 4.5) at 37°C for 1 h. The reaction was stopped by adding 200 μl of stop solution (0.1 M Na2CO3/NaHCO3, pH10.0). The test samples were dissolved in DMSO and the solution was added to MEM medium at a final concentration of 0.1%. The absorbance (OD) was measured using a microplate reader at 405 nm. The ratio of OD reflects the β-hexosaminidase release.
Cytokine IL-4 and TNF-α production in DNP-sensitized RBL-2H3 cells
RBL-2H3 cells were plated in 24-well plates at a density of 5 × 104 cells per well. Quercetin at 5 or 10 μM were added and incubated for 48 h and cells were sensitized with monoclonal DNP-specific IgE (10 μg/ml) for 12 h. They were then washed twice with Tyrode’s buffer and stimulated at 37°C with DNP-BSA. IL-4 and TNF-α secretion into supernatant were measured 6 h after stimulation with DNP-BSA using an ELISA detection kit, according to the guidelines of the manufacturer.
Measurement of CD23 mRNA expression in IL-4 stimulated Caco-2 cells
Caco-2 cells were chosen for this study because they express a higher baseline CD23. Cells grown in plates were treated with 10 or 20 μM quercetin and 10 ng/ml IL-4 for 24 h. The cells were washed twice with cold PBS (pH 7.2). Isolation of total RNA from the intestine was carried out using TRizol reagent according to the instructions of the manufacturer. RT-PCR was carried out using Onestep RT-PCR kit (iNtRON, Daejeon, Korea). Primers were synthesized by Bioneer (Daejeon, Korea).The mixture of 20 μl of PCR product containing loading buffer was subjected to 1.8% (w/v) agarose gels. Gels were stained with ethidium bromide and detected under UV light. For quantitative analysis, Versa Doc Image analyzer (Bio Rad, Mississauga, ON, Canada) was used.
Measurement of p38 and ERK 1/2 MAPK expression in Caco-2 cells
P38 and ERK1/2 MAPK were analyzed by western blot.
For the measurement of p38 MAPK, plate-grown Caco-2 cells were treated with 20 μM quercetin for 24 h and then with 10 ng/ml IL-4 for 20 min. For the measurement of ERK1/2 MAPK, Caco-2 cells grown on permeable supports were used as an experimental model to test the allergic effect of IgE-Ag. To determine the MAPK expression by stimulation with allergen-IgE complex stimulation, cells were grown on the transwell plate. To form the immune complex, NP-OVA (1 μg/ml) was incubated with IgE anti-NP (1 μg/ml) at 37°C for 60 min. The cells were pretreated with 20 μM quercetin and then stimulated with an IgE-Ag complex onto the basal side of filter support for 60 min. The cells were harvested by centrifugation and lysed in a lysis buffer. The lysates were sonicated for 90 s and centrifuged at 13,000×g for 15 min. The total protein in the supernatant of the cell lysates was measured by the Bio-Rad protein assay (Bio-Rad Laboratories, Mississauga, ON, Canada). Then 30 μg of protein for each sample were migrated on a 12% SDS-PAGE and transferred electrophoretically onto a PVDF membrane. After blocking in blocking solution (PBS with 0.1% Tween 20 and 4% skim milk), the specific anti-p38 MAPK, anti-phospho-p38 MAPK, anti-ERK 1/2 MAPK, anti-phospho-ERK1/2 MAPK were incubated with the membrane at a dilution of 1:1,000 for 24 h at 4°C. The secondary HRP-conjugated anti-rabbit antibody was incubated with the membrane for 1 h at a 1:3,000 dilution. An enhanced chemilumiscence detection method was used to detect the target protein phosphorylated p38 MAPK using the Advanced ECL kit. The integrity of the band was quantified by Versa Doc and Quantity one program (Bio Rad, Mississauga, ON, Canada).
IL-8 and MIP-3α production in OVA-IgE complexes stimulated Caco-2 cells
To determine IL-8 and MIP-3α production, the Caco-2 cells were polarized on filter supports and treated with 20 μM flavonols followed by stimulation with allergen-IgE complexes on the basal side of the transwell. IL-8 and MIP-3α chemokine secretion into the basal transwell were measured 24 h after stimulation with IgE-Ag complexes using ELISA detection kit (R&D, Mineapolis, MN, USA), according to guidelines of the manufacturer.
Statistical analysis
Data are expresses as the mean ± standard deviation. Differences were considered significant at P < 0.05. Data were analyzed by a Duncan’s multiple range test. All analyses were performed using the SAS statistical packages (SAS Institute, Cary, NC).
Results
Anti-allergic activity of flavonols in RBL-2H3 cells
To evaluate the anti-allergic activity of quercetin, the degree of degranulation in mast cells was determined by measuring β-hexosaminidase that is usually released along with histamine when antigen-induced mast cell degranulation occurs. Results showed that treatment with quercetin at 10 μM for 48 h significantly inhibited degranulation induced by DNP-BSA from a RBL-2H3 sensitized with anti-DNP IgE (Fig. 2a).
Effects of quercetin and kaempferol on β-hexosaminidase and cytokines release in RBL-2H3 cells. RBL-2H3 cells (5 × 104 cells per well) were treated with 1 and 10 μM of quercetin and kaempferol for 48 h. Cells were sensitized with monoclonal DNP-specific IgE (10 μg/ml) for 12 h. The secretion of IL-4 and TNF-α were measured at 1 h after stimulation with DNP-BSA using ELISA. Each value represents mean ± SD (n = 4). Bars with letters (a, b, c, d, e) are significantly different from each other at P < 0.05 as determined by Duncan’s multiple range test
Cytokines including IL-4 and TNF-α are critical for allergic inflammation. Accordingly, the antigen-stimulated secretion of TNF-α and IL-4 were also determined. As shown in Fig. 2b, c, quercetin and kaempferol inhibited the secretion of TNF-α and IL-4 in antigen-stimulated RBL-2H3 cells.
Inhibitory effect of flavonols on CD 23 mRNA and p38 MAPK protein expression in IL-4 stimulated Caco-2 intestinal epithelial cells
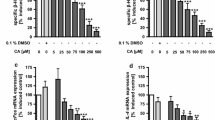
When IL-4 is elevated in allergic condition, allergen-IgE complexes are captured by CD 23 on the luminal side of the intestinal epithelium and transported into the mucosa. Hence, we evaluated the effect of quercetin on IL-4 induced CD23 mRNA expression in Caco-2 intestinal epithelial cells. Results showed that IL-4 treated cells increased the CD23 mRNA expression by 40% as compared to the level of control cells. Both quercetin and kaempferol at 10 and 20 μM significantly inhibited the expression of CD23 mRNA (Fig. 3).
Effect of quercetin and kaempferol on the expression of CD23 mRNA in IL-4 stimulated Caco-2 cells. Cells grown in plates were treated with 10, 20 μM quercetin or kaempferol and then stimulated with 10 ng/ml IL-4 for 24 h. CD23mRNA expression was determined and values were normalized to GAPDH of samples. Each value represents mean ± S.D (n = 3). Bars with letters (a, b) are significantly different from each other at P < 0.05 as determined by Duncan’s multiple range test. GAPDH glyceraldehydes-3-phosphate dehydrogenase
The alteration of the p38 MAPK signal transduction pathway which is known to regulate the CD23 expression was also determined. As shown in Fig. 4, treatment with 10 ng/ml IL-4 for 20 min increased the phosphorylation of p38 MAPK in these cells, while 20 μM quercetin or kaempferol significantly inhibited the IL-4 induced activation of p38 MAPK.
Effects of quercetin and kaempferol on phosphorylated p38 MAPK protein expression in IL-4 stimulated Caco-2 cells. Plate-grown caco-2 cells were treated with 20 μM quercetin and 20 μM kaempferol for 24 h and then with 10 ng/ml IL-4 for 20 min. The expression of p38 MAPK protein expression was determined by western blotting. Values were normalized to the β-actin content of samples and expressed as mean ± SD (n = 3). Bars with letters (a, b) are significantly different from each other at P < 0.05 as determined by Duncan’s multiple range test
Inhibitory effects of flavonols on ERK activation and chemokine release in IgE-Ag complex-stimulated Caco-2 intestinal epithelial cells
Transport of IgE and IgE-antigen immune complexes into lamina propria lead to release of chemokines. The effect of quercetin and kaempferol on the activation of the ERK1/2 protein that regulates chemokine release was measured using Caco-2 cells grown on permeable supports. As shown in Fig. 5, 0.4 μM OVA-IgE complex treatment on the basal transwell for 30 min increased the phosphorylation of ERK protein in Caco-2 cells. Phosphorylation of ERK protein was inhibited by 20 μM quercetin and kaempferol treatment. Treatment with quercetin and kaempferol also decrease the release of IL-8 and MIP-3α by 0.4 μM IgE-OVA complexes for 24 h (Fig. 6).
Effects of quercetin and kaempferol on ERK1/2 MAPK protein expression in OVA-IgE complexes-stimulated Caco-2 cells. The cells were pretreated with 20 μM quercetin and 20 μM kaempferol and then stimulated with OVA-IgE complexes for 60 min. The expression of ERK1/2 protein was determined by western blotting. Values were normalized to the β-actin content of samples and expressed as means ± SD (n = 3). Bars with letters (a, b, c) are significantly different from each other at P < 0.05 as determined by Duncan’s multiple range test
Effects of quercetin and kaempferol on IL-8 and MIP-3α level in OVA-IgE complexes-stimulated Caco-2 cells. Caco-2 cells were polarized on filter supports and treated with 20 M quercetin and 20 μM kaempferol followed OVA-IgE complexes stimulation. MIP-3α chemokine secretion into the basal transwell was measured 24 h after the stimulation. Each value represents mean ± SD (n = 4). Bars with letters (a, b, c) are significantly different from each other at P < 0.05 as determined by Duncan’s multiple range test
Discussion
We tested a hypothesis that quercetin and kaempferol possibly suppress the allergic responses through the inhibition of an allergic inflammation in the intestinal epithelium by maintaining the barrier.
RBL-2H3 is considered similar to bone-marrow derived mast cells and to mucosal mast cells (MMC), the latter of which may be involved in the inflammation of the intestine. This study investigated whether quercetin suppresses the release of chemical mediators in IgE-DNP sensitized RBL-2H3 cells as a model of the mucosal mast cells of the intestinal epithelium. Beta-hexosaminidase has frequently been employed as a marker for examining the degranulation process of mast cells [22]. The results showed that quercetin and kaempferol at 10 μM reduced degranulation from anti-IgE stimulated degranulation in RBL-2H3. Quercetin had been reported to inhibit histamine release from rat connective tissue mast cells [23] and mucosal mast cells [24], as well as from human lung and intestinal mast cells [25]. Mast cells also produce cytokines including TNF-α and IL-4, both of which play a pathologic role in the onset of various allergic disease including atopic dermatitis, atopic rhinitis, and asthma. Several drug candidates that down-regulate IL-4 and TNF-α levels have been studied for possible use as anti-allergic agents in clinical settings. Our study exhibited that cells treated with quercetin and kaempferol decreased the level of IL-4 and TNF-α. Human umbilical cord blood-derived cultured mast cells (hCBMCs) preincubated with the quercetin and kaempferol at 100 μM, followed by activation with anti-IgE, released a significantly less amount of IL-6, IL-8 and TNF-α, which were 82–93% of the control values [26]. Quercetin, the flavonoid component in Gingko biloba, inhibited LPS-induced TNF-α, IL-6, IL-1β transcription by inhibiting activation of ERK1/2 and p38 MAPK in macrophages [27]. In sensitized human mast cells, kaempferol and quercetin at 10 μM significantly inhibited the release of TNF-α and kaempferol showed higher inhibition rate compared to quercetin. Recent studies have discussed the possible structural requirements for the inhibitory action of flavonols on releasing chemical mediators. The flavonoid basic structure is comprised of two phenolic pyran (A and B) or pyrone ring (C) in the middle. The inhibitory effect of flavonols requires the presence of the C ring of an oxy group at position 4, a double bond between carbon atoms 2 and 3 and a hydroxyl group in position 3 [28]. The catechol (o-dihydroxy) group in the B ring, as in quercetin, confer further potent inhibitory ability, as does the presence of the hydroxyl group on kaempferol. Mastuda et al. [29] also clarified that the flavonols with a phenol type moiety (the 4′-hydroxyl group) or catechol type moiety (the 3′,4′-dihydroxyl groups) at the B ring exhibit a higher activity compared to those with pyrogallo type moiety (the 3′,4′,5′-trihydroxyl groups) at the B ring which is in agreement with this study. The anti-allergic effect of quercetin and kaempferol may also be related to inhibition of protein kinase C, phopholipase A2, along with the inhibition of cyclooxygenase (COX) and 5-lipoxygenase (5-LOX).
IL-4 can be detected at intestinal mucosal surfaces as well as in serum from atopic patients [30]. CD23, the low-affinity receptor for IgE, is expressed by human enterocytes [8, 9, 31] and is induced by allergen sensitization and by the cytokine IL-4[9, 32]. When IL-4 is elevated in an allergic condition, allergen-IgE complexes are captured by CD23 on the luminal side of the intestinal epithelium and transported into the mucosa, where they bind to FcεRI on the mast cells, causing allergic inflammation and local IgE class switching in B cells and IgE synthesis. Hence, this study evaluated the effect of quercetin and kaempferol on IL-4 induced CD23 mRNA expression in Caco-2 intestinal epithelial cells. The results showed that both quercetin and kaempferol inhibited the IL-4 induced CD23 mRNA expression.
IL-4 also induces activation of p38 MAPK in murine B- and T-lymphoid cell lines [33]. Recent publications have provided evidence that the p38 MAPK pathway is involved in intestinal barrier damage. Inhibitors of p38 MAPK improved the integrity of the intestinal barrier and reduced the production of proinflammatory cytokines due to epithelial damages. P38 MAPK inhibitors also suppressed the transport of IgE and IgE-antigen immune complexes across the intestinal barrier. Tu et al. (2006) [34] provided the evidence that p38 MAPK is involved in the pathway regulating expression of CD23 and that inhibition of this enzyme by an inhibitor of p38 MAPK can eliminate the transepithelial transport of both IgE and its immune complexes. Our study indicated that phosphorylated p38 MAPK was up-regulated by IL-4 and was inhibited by quercetin and kaempferol at 20 μM, suggesting that quercetin and kaempferol as p38 MAPK inhibitor may exert inhibitory effects on CD23 expression, thereby allowing suppression in the transport of IgE and IgE-antigen immune complexes across the intestinal barrier.
In addition to the function as an IgE capture mechanism, CD23 expressed on human intestinal epithelial cells could trigger the up-regulation of epithelial chemokines [11]. Once the IgE-Ag complex by CD23 have breached the epithelial barrier, these can then induce the release of chemokines such as MIP-3α and IL-8 from the epithelium. The importance of chemokines in the development of allergic inflammation is huge and this is emphasized by studies showing that experimental allergic disease cannot be induced in animals with specific chemokine deficiencies [35, 36]. These chemokines can recruit both inflammatory cells and adaptive immune cells such as DCs, T cells, and B cells. MIP-3α is a chemoattractant for immature dendritic cells, memory T lymphocytes and B lymphocytes, whereas IL-8 is a chemoattractant for neutrophils and eosinophils, and has been shown to be increased in late-phase allergic inflammation. Moreover, it has been reported that MIP-3α expression by immunostaining on intestinal biopsy specimens from subjects with IgE sensitization to foods was increased [11] and was constitutively expressed under inflammatory conditions in epithelium overlying lymphoid follicles in the gastrointestinal tract. In our study, the effects of quercetin and kaempferol on the release of chemokine in stimulation of IgE-Ag complex on the basolateral membrane were determined. The results showed that quercetin at 20 μM blocked IgE-Ag complexes induced IL-8 and MIP-3α release. Nanua et al. (2006) [37] suggested that pretreatment with quercetin reduced TNF-α induced IL-8 release by attenuating a PI3K/Akt-dependent pathway in airway epithelial cell. Quercetin has also been noted to inhibit the induction of IL-8 and MCP-1 by TNF-α in cultured human synovial cells [26]. ERK activation was necessary for downstream of chemokine release. The results showed that pre-treated with 20 μM quercetin and kaempferol for 24 h reduced IgE-OVA complex induced phosphorylated ERK protein expression.
Together, these in vitro results support a paradigm in which inhibition of CD23 expression by quercetin and kaempferol resulted in an abrogation of ERK activation in response to IgE-Ag and significant inhibition of chemokines secretion, which possibly reduce development of allergic inflammation including degranulation and a release of mediators including IL-4 and TNF-α from infiltrated mast cells. The present study suggests the possible use of quercetin and kaempferol in preventing IgE-mediated allergic inflammation. Because mast cells are included in the pathology of other inflammatory bowel diseases, the protective effect of these flavonols can be also anticipated in a wide range of anti-inflammatory therapy. In our study, the inhibitory mechanisms of flavonol are examined mainly for restoring intestinal barrier function of the epithelium. However, recent studies have shown strong evidence of antigen presentation and IgE-allergen transport changes in submucosal counterparts in allergic responses [38, 39]. Thus, further studies addressing direct effects of flavonols on the suppression of allergic responses will provide greater understanding of the mechanisms of action involved.
References
Kim JW, Lee JH, Hwang BY, Mun SH, Ko NY, Kim do K, et al. Morin inhibits Fyn kinase in mast cells and IgE-mediated type I hypersensitivity response in vivo. Biochem Pharmacol. 2009;77:1506–12.
Bischoff SC, Wedemeyer J, Herrmann A, Meier PN, Trautwein C, Cetin Y, et al. Quantitative assessment of intestinal eosinophils and mast cells in inflammatory bowel disease. Histopathology. 1996;28:1–13.
Bischoff SC, Kramer S. Human mast cells, bacteria, and intestinal immunity. Immunol Rev. 2007;217:329–37.
Bischoff SC. Physiological and pathophysiological functions of intestinal mast cells. Semin Immunopathol 2009.
Bischoff SC, Sellge G, Manns MP, Lorentz A. Interleukin-4 induces a switch of human intestinal mast cells from proinflammatory cells to Th2-type cells. Int Arch Allergy Immunol. 2001;124:151–4.
Lorentz A, Schwengberg S, Sellge G, Manns MP, Bischoff SC. Human intestinal mast cells are capable of producing different cytokine profiles: role of IgE receptor cross-linking and IL-4. J Immunol. 2000;164:43–8.
Richards ML, Katz DH. Regulation of the murine Fc epsilon RII (CD23) gene. Functional characterization of an IL-4 enhancer element. J Immunol. 1994;152:3453–66.
Yahong T, Perdue MH. CD23-mediated transport of IgE/immune complexes across human intestinal epithelium: role of p38 MAPK. Am J Physiol Gastrointest Liver Physiol. 2006;291:G532–8.
Yu LC, Montagnac G, Yang PC, Conrad DH, Benmerah A, Perdue MH. Intestinal epithelial CD23 mediates enhanced antigen transport in allergy: evidence for novel splice forms. Am J Physiol Gastrointest Liver Physiol. 2003;285:G223–34.
Chacon P, Vega A, Monteseirin J, El Bekay R, Alba G, Perez-Formoso JL, et al. Induction of cyclooxygenase-2 expression by allergens in lymphocytes from allergic patients. Eur J Immunol. 2005;35:2313–24.
Li H, Chehade M, Liu W, Xiong H, Mayer L, Berin MC. Allergen-IgE complexes trigger CD23-dependent CCL20 release from human intestinal epithelila cells. Gastroenterology. 2007;133:1905–15.
Terao J. Dietary flavonoids as antioxidants. Forum Nutr. 2009;61:87–94.
Tunon MJ, Garcia-Mediavilla MV, Sanchez-Campos S, Gonzalez-Gallego J. Potential of flavonoids as anti-inflammatory agents: modulation of pro-inflammatory gene expression and signal transduction pathways. Curr Drug Metab. 2009;10:256–71.
Chun OK, Floegel A, Chung SJ, Chung CE, Song WO, Koo SI. Estimation of antioxidant intakes from diet and supplements in US adults. J Nutr 2009.
de Vries JH, Janssen PL, Hollman PC, van Staveren WA, Katan MB. Consumption of quercetin and kaempferol in free-living subjects eating a variety of diets. Cancer Lett. 1997;114:141–4.
Hertog MG, Feskens EJ, Hollman PC, Katan MB, Kromhout D. Dietary antioxidant flavonoids and risk of coronary heart disease: the Zutphen Elderly Study. Lancet. 1993;342:1007–11.
Amasheh M, Schlichter S, Amasheh S, Mankertz J, Zeitz M, Fromm M, et al. Quercetin enhances epithelial barrier function and increases claudin-4 expression in Caco-2 cells. J Nutr. 2008;138:1067–73.
Suzuki T, Hara H. Quercetin enhances intestinal barrier function through the assembly of zonula occludens-2, occludin, and claudin-1 and the expression of claudin-4 in Caco-2 cells. J Nutr. 2009;139:965–74.
Amasheh M, Andres S, Amasheh S, Fromm M, Schulzke JD. Barrier effects of nutritional factors. Ann NY Acad Sci. 2009;1165:267–73.
Choi OH, Adelstein RS, Beaven MA. Secretion from rat basophilic RBL-2H3 cells is associated with diphosphorylation of myosin light chains by myosin light chain kinase as well as phosphorylation by protein kinase C. J Biol Chem. 1994;269:536–41.
Menconi MJ, Salzman AL, Unno N. Acidosis induces hyperpermeability in Caco-2BBe cultured intestianl epithelial monolayers. Am J Physiol. 1997;272:G1007–21.
Choi OH, Kim JH, Kinet JP. Calcium mobilization via sphingosine kinase in signalling by the Fc epsilon RI antigen receptor. Nature. 1996;380:634–6.
Pearce FL, Befus AD, Bienenstock J. Mucosal mast cells III. Effect of quercetin and other flavonoids on antigen-induced histamine secretion from rat intestinal mast cells. J Allergy Clin Immunol. 1984;73:819–23.
Penissi AB, Rudolph MI, Piezzi RS. Role of mast cells in gastrointestinal mucosal defense. Biocell. 2003;27:163–72.
Fox CC, Wolf EJ, Kagey-Sobotka A, Lichtenstein LM. Comparison of human lung and intestinal mast cells. J Allergy Clin Immunol. 1988;81:89–94.
Kempuraj D, Madhappan B, Christodoulou S, Boucher W, Cao J, Papadopoulou N, et al. Flavonols inhibit proinflammatory mediator release, intracellular calcium ion levels and protein kinase C theta phosphorylation in human mast cells. Br J Pharmacol. 2005;145:934–44.
Wadsworth TL, Koop DR. Effects of the wine polyphenolic quercetin and resveratrol on pro-inflammatory cytokine expression in RAW 264.7 macrophages. Biochem Pharmacol. 1999;57:941–9.
Min YD, Choi CH, Bark H, Son HY, Park HH, Lee S, et al. Quercetin inhibits expression of inflammatory cytokines through attenuation of NF-kappaB and p38 MAPK in HMC-1 human mast cell line. Inflamm Res. 2007;56:210–5.
Mastuda H, Morikawa T, Ueda K, Managi H, Yoshikawa M. Structural requirements of flavonoids for inhibition of antigen-induced degranulation, TNF-α and IL-4 production from RBL-2H3 cells. Bioorg Med Chem. 2002;10:3123–8.
Hashimoto S, Amemiya E, Tomita Y, Kobayashi T, Arai K, Yamaguchi M, et al. Elevation of soluble IL-2 receptor and IL-4, and nonelevation of IFN-gamma in sera from patients with allergic asthma. Ann Allergy. 1993;71:455–8.
Marshall LA, Hansbury MJ, Bolognese BJ, Gum RJ, Peter RY, Mayer RJ. Inhibitors of the p38 mitogen-activated kinase modulate IL-4 induction of low affinity IgE receptor(CD23) in human monocytes. J Immunol. 1998;161:6005–13.
Montagnac G, Yu LC, Bevilacqua C, Heyman M, Conrad DH, Perdue MH, et al. Differential role for CD23 splice forms in apical to basolateral transcytosis of IgE/allergen complexes. Traffic. 2005;6:230–42.
Hunt AE, Williams LM, Lali FV, Foxwell BMJ. IL-4 regulation of p38 MAPK signaling is dependent on cell type. Cytokine. 2002;18:295–303.
Tu Y, Salim S, Bourgeois J, Di Leo V, Irvine EJ, Marshall JK, et al. CD23-mediated IgE trasnport across human intestinal epithelium: inhibition by blocking sites of translation or binding. Gastroenterology. 2005;129:928–40.
Lucas N. Role of chemokines in the pathogenesis of asthma. Nat Rev Immunol. 2001;1:108–16.
Kaplan AP. Chemokines, chemokine receptors and allergy. Int Arch Allergy Immunol. 2001;124:423–31.
Nanua S, Zick SM, Andrade JE, Sajjan US, Burgess JR, Lukacs NW, et al. Quercetin blocks airway epithelial cell chemokine expression. Am J Respir Cell Mol Biol. 2006;35:602–10.
Yu LC. The epithelial gatekeeper against food allergy. Pediatr Neonatol. 2009;50:247–54.
Li H, Nowak-Wegrzyn A, Charlop-Powers Z, Shreffler W, Chehade M, Thomas S, et al. Transcytosis of IgE-antigen complexes by CD23a in human intestinal epithelial cells and its role in food allergy. Gastroenterology. 2006;131:47–58.
Acknowledgments
This study was supported by the grant (A080664) from the Ministry for Health, Welfare and Family Affairs, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: A. Falus.
Rights and permissions
About this article
Cite this article
Lee, EJ., Ji, GE. & Sung, MK. Quercetin and kaempferol suppress immunoglobulin E-mediated allergic inflammation in RBL-2H3 and Caco-2 cells. Inflamm. Res. 59, 847–854 (2010). https://doi.org/10.1007/s00011-010-0196-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-010-0196-2