Abstract
Objective and design
Sodium lauryl sulfate (SLS) is a known irritant. It releases pro-inflammatory mediators considered pivotal in inflammatory pain. The sensory effects of SLS in the skin remain largely unexplored. In this study, SLS was evaluated for its effect on skin sensory functions.
Subjects
Eight healthy subjects were recruited for this study.
Treatment
Skin sites were randomized to topical SLS 0.25, 0.5, 1, 2% and vehicle for 24 h. Topical capsaicin 1% was applied for 30 min at 24 h after SLS application.
Methods
Assessments included laser Doppler imaging of local vasodilation and flare reactions, rating of spontaneous pain, assessment of primary thermal and tactile hyperalgesia, and determination of secondary dynamic and static hyperalgesia.
Results
SLS induced significant and dose-dependent local inflammation and primary hyperalgesia to tactile and thermal stimulation at 24 h after application, with SLS 2% treatment eliciting results comparable to those observed following treatment with capsaicin 1%. SLS induced no spontaneous pain, small areas of flare, and minimal secondary hyperalgesia. The primary hyperalgesia vanished within 2–3 days, whereas the skin inflammation persisted and was only partly normalized by Day 6.
Conclusions
SLS induces profound perturbations of skin sensory functions lasting 2–3 days. SLS-induced inflammation may be a useful model for studying the mechanisms of inflammatory pain.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Topical application of skin irritants, such as sodium lauryl sulfate (SLS), has been used for years in dermatological research for standardized evaluation of skin susceptibility to irritants [1]. No studies thus far have examined the sensory properties of SLS-induced inflammation. However, several lines of circumstantial evidence indicate that SLS may modulate pain responses in the skin. In vitro studies with human keratinocytes, as well as studies in animal skin, have shown that SLS induces the release or up-regulation of a variety of cytokines, including several considered pivotal in peripheral pain modulation [2, 3], including TNF-α, IL-1α, IL-6, IL-8, IL-10, and several chemokines [4–7]. SLS also induces the release of pro-inflammatory cytokines and chemokines in human skin as shown by superficial lymph vessel drainage techniques [8], the modified skin window technique [9], and macroscopic and microscopic evaluations [10, 11]. Finally, topical application of SLS to human skin induces distinct vasodilation and increased vasodilator responses to heat [1, 12]. The aim of the present study was to characterize sensory reactions of topical SLS in human skin.
Materials and methods
Participants
Eight healthy, male volunteers (aged 19–44 years) participated in the study. All participants received oral and written information about the trial and provided oral and written informed consent. The study was approved by The Central Denmark Region Committees on Biomedical Research Ethics and the Danish Data Protection Agency.
Induction of cutaneous inflammation
Six skin sites in total, three sites on each of the volar aspects of the forearms, were randomized to receive SLS 0.25, 0.5, 1, 2%, vehicle, or capsaicin (positive control) in a Latin Square design. SLS (99% purity) was prepared as isotonic solutions in phosphate buffer with a pH of 7.4 (Skanderborg Pharmacy, Skanderborg, Denmark). The buffer was used as vehicle. All reagents were applied to the skin as 200 μl aliquots in 18 mm Finn chambers with paper filter discs (Epitest Ltd, Oy, Finland). The chambers with SLS and vehicle were covered by non-occlusive dressings and removed the next day (24 h). Capsaicin 1% in alcohol (Aarhus University Pharmacy, Aarhus, Denmark) was applied for 30 min in a Finn chamber as described above at 24 h after SLS application.
Clinical grading of skin reactions
Grading of the SLS-induced inflammatory reactions was performed using the Standardization Group of the European Society of Contact Dermatitis (ESCD) simple scoring for subacute SLS irritant reactions [1]. Based on the extent of erythema, roughness/contour, scaling, edema and fissures, irritant reactions are given a score of 0, 0.5, 1, 2, 3 or 4. ESCD scoring was performed by the same observer at all visits, with the observer blinded to the randomization code. Quantification of erythema (local vasodilation and flare reactions) was performed at each capsaicin site, but ESCD scoring was not.
Spontaneous pain intensity
Subjects rated spontaneous pain intensity on an 11-point numeric rating scale from 0 to 10, where 0 represented no pain at all, and 10 represented the most imaginable pain. Subjects were asked to rate their pain at each visit and to report the highest level of spontaneous pain between visits.
Assessment of local vasodilation and flare reactions
Skin blood flow was assessed at each skin site by laser Doppler Imaging (LDI) (MoorLDI2-IR, Moor Instruments Ltd., Devon, UK). Data were analyzed off-line using the manufacturer’s software package. An 18 mm circular region of interest (ROI), corresponding to the size of the Finn chamber, was applied to all images, guided by the corresponding black and white scanning photos. The intensity of local vasodilation was calculated as mean skin blood flow in arbitrary units within the ROI. The LDI images were also used for calculation of flare areas. Mean skin blood flow plus two standard deviations was calculated in normal-appearing skin and used as a low threshold for calculation of the flare area. The reported flare area included the site of stimulation (18 mm diameter, 2.55 cm2).
Tactile pain threshold
The tactile pain threshold (TPT) was determined by applying graded von Frey filaments (Touch-Test, North Coast Medical Inc, CA, USA) within the inflamed skin sites with approximately twofold increasing weights of 8, 15, 26, 60, and 100 g. TPT was determined as the filament that induced a sensation of pain following at least 3 of 6 repeated stimulations at 0.5 Hz performed within the 18 mm skin area [13]. If the subjects experienced no pain in response to the 8 g filament, the value was recorded as 4 g. If no pain was observed following stimulation with the 100 g filament, the threshold was recorded as 200 g in the subsequent analyses.
Heat pain threshold
The heat pain threshold (HPT) was determined within the inflamed skin sites using a computer-controlled thermode and the method of limits (TSA Pathway, Medoc, Israel). The thermode was prepared with isolated rubber, leaving a circular surface 18 mm in diameter for heat stimulation. Baseline temperature was 32°C, followed by a heat ramp of 1°C/s to a maximum of 50°C. The participant controlled the heating device and switched off heating when the heat became painful [14]. The mean of three tests was used in the analysis.
Secondary pin-prick hyperalgesia and allodynia
Sensory pin-prick hyperalgesia was assessed with a von Frey filament of 26 g applied along eight linear paths arranged radially around each skin site. The stimulation was initiated outside the inflamed skin, where no pain was experienced, and continued centripetally in 5-mm steps at a rate of 0.5 Hz towards the center of the site until the subject reported a definite change in sensation. The border was marked on the skin and the mapping was traced onto a clear acetate sheet. The area was calculated as the product of the longest diameters and the perpendicular diameter. The area of allodynia was assessed by a cotton wool tip using the same procedure as outlined above.
Experimental protocol
Assessments of sensory and inflammatory responses were performed within 0.2–2 h after removal of the capsaicin chamber on the first experimental day. Finn chambers above SLS sites were removed 45 min prior to the assessment at 24 h [1]. All subjects were studied on the same time of day at each visit. Measurements were performed in the same order at each visit, starting with verbal assessments, followed by non-invasive measurements and finally mechanical and thermal pain threshold assessments. The subjects were equipped with a blindfold during all pain evaluations. The experiments were performed in a temperature-controlled room held at 23–24°C. Skin temperature was similar among skin sites and constant across visits (data not shown).
Descriptive and analytical statistics
Data were analyzed by repeated measures analysis of variance (ANOVA) using the variables ‘time’ and ‘drug’ (GraphPad Prism version 5.02, GraphPad Software Inc., CA, USA). Significant differences within these factorial groups were localized by post-hoc Bonferroni test. Clinical score data were analyzed using non-parametric, repeated measurement ANOVA (Friedman tests) for statistical differences within SLS concentrations at 24 h. The time course of clinical scoring was also analyzed using Friedman tests with individual tests for each of four SLS concentrations. In the latter analyses, the p value was corrected by the Bonferroni method to adjust for multiple comparisons. Values of p < 0.05 were considered significant. All figures are depicted as mean ± standard error of the mean (if not reported otherwise).
Results
Clinical evaluation of inflammation
SLS induced significant and dose-related inflammation (p = 0.0001). Median ESCD score at 24 h was 0.25 (total range 0–1), 0.5 (0–2), 0.75 (0.5–2), 2 (0.5–2), and 2.5 (0.5–3) with vehicle, 0.25, 0.5, 1, and 2% SLS, respectively. Capsaicin sites showed intense erythema (Fig. 1a) immediately after the application but no other components of the ESCD score. At all subsequent visits, the capsaicin sites were indistinguishable from normal adjacent skin. All vehicle sites scored zero at 48 and 72 h. In contrast, SLS-induced inflammation was maintained throughout the observation period with median ESCD scores of 1, 2, and 3 for SLS 0.5, 1, and 2%, respectively, at both 48 and 72 h.
SLS-induced dose-related local vasodilation (a) and flare reactions (b) (p < 0.0001). Local vasodilation with SLS 2% was comparable with reactions induced by capsaicin 1% at 24 h, whereas flare reactions were more severe with capsaicin. Capsaicin-induced flare reactions were absent and local vasodilation had normalized to vehicle response levels by 48 h. The results of post-hoc analysis of vehicle responses versus individual concentrations of SLS and capsaicin are shown by asterisks (*p < 0.05, **p < 0.01, ***p < 0.001). Values are mean ± SEM of eight subjects
Spontaneous pain
Topical SLS did not induce spontaneous pain. The mean pain rating from the time of application of SLS to the final assessment at 72 h did not exceed a numeric value greater than 1 for any concentrations of SLS at any time point. Mean pain intensity was 0.3 ± 0.2 for SLS 2% at 24 and 48 h and zero for all subjects at 72 h. In contrast, capsaicin 1% for 30 min induced moderate spontaneous pain in most subjects. The maximum pain in subjects was observed during the application or immediately after removal of the chamber; the mean pain intensity was 3.6 ± 0.7. This value decreased to 0.4 ± 0.3 at 48 h, and no spontaneous pain was reported in any patient at 72 h.
Local vasodilation
SLS induced a statistically significant, dose-related local vasodilation (Fig. 1a, p < 0.0001). Post-hoc analysis showed significantly increased local vasodilation with SLS 1 and 2% versus vehicle response at 24 h. Local vasodilation at 24 h with 2% SLS (589 ± 51 AU) was not significantly different from the reaction observed with 1% capsaicin (690 ± 52 AU). The reaction peaked at 48 h for all SLS doses, and then slowly declined at 72 h where SLS 0.5–2% maintained a significant vasodilation as compared to vehicle responses. Local vasodilation was measured at Day 6 in six of eight subjects and showed persistent, significant vasodilation with SLS 1% (264 ± 106 AU) and 2% (403 ± 130 AU), whereas SLS 0.25% (89 ± 11 AU) and 0.5% SLS (105 ± 22 AU) were not significantly different from vehicle responses (74 ± 12 AU). Local vasodilation was prominent with capsaicin at 24 h but had normalized to vehicle response levels at subsequent visits.
Flare reactions
SLS induced a significant dose-related flare (Fig. 1b, p = 0.00002). Post-hoc analyses showed that SLS 2%, but not lower concentrations of SLS, induced significant reactions. SLS 2% flares were 8.4 ± 2.8 cm2 at 24 h and maintained significantly above vehicle responses up to 72 h. No flare to SLS 2% was observed in 5 of 6 subjects on Day 6. Topical capsaicin 1% for 30 min induced a flare of 22.6 ± 3.6 cm2 which was significantly larger than corresponding reaction induced by SLS 2%. No flare to capsaicin was observed in any subjects at subsequent visits.
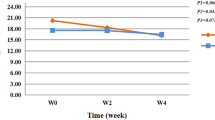
Tactile pain threshold
SLS induced a statistically significant, dose-related decline in TPT (Fig. 2a, p = 0.0004). Post-hoc tests showed that 1 and 2% SLS induced significant decreases in TPT at 24 h, which was maintained at 48 h with 2% SLS. No significant SLS-induced tactile hyperalgesia was observed at 72 h. Capsaicin induced significant tactile hyperalgesia on the first experimental day; non-significant hyperalgesia was seen at 48 h; and responses were comparable to vehicle sites at 72 h.
Measurement of tactile pain threshold (TPT) and heat pain threshold (HPT) following topical application of SLS for 24 h. TPT was studied by applying von Frey filaments with varying weights (a) and HPT was studied with a heated thermode and the method of limits (b). SLS-induced a statistically significant, dose-related decrease in TPT (p = 0.0004) and HPT (p = 0.0004). The results of post-hoc analysis of vehicle responses versus individual concentrations of SLS and capsaicin are shown by asterisks (*p < 0.05, **p < 0.01; ***p < 0.001). Values are mean ± SEM of eight subjects
Heat pain threshold
SLS induced a statistically significant and dose-related reduction in HPT (Fig. 2b, p = 0.0004). HPT was reduced by 1.2 ± 1.3, 4.4 ± 1.5, 5.7 ± 1.1, 6.7 ± 1.3, and 6.5 ± 1.0°C with SLS 0.25, 0.5, 1, 2%, and capsaicin 1%, respectively, compared to vehicle responses at 24 h. Responses with all SLS concentrations but 0.25% were significant different from vehicle responses. Significant heat pain hyperalgesia was maintained at 48 h with SLS 1–2% and with SLS 2% at 72 h. Six of eight subjects were also examined for heat hyperalgesia at Day 6, when the heat pain threshold at any SLS site was similar to that at vehicle sites. Notably, capsaicin-induced heat pain hyperalgesia was evident at 24 h but not at subsequent visits.
Secondary pin-prick hyperalgesia and allodynia
Data on secondary hyperalgesia are shown in Table 1. Capsaicin induced large areas of pin-prick secondary hyperalgesia at 24 and 48 h in most subjects, with fading proportions of responders at 72 h. Capsaicin induced allodynia (dynamic hyperalgesia) in most subjects at 24 h but not at later time points. In comparison, even the highest concentrations of SLS induced secondary hyperalgesia in no more than 50% of the subjects studied; the involved area was notably smaller than that at capsaicin sites. Due to the low number of respondents, no statistical analyses were performed.
Discussion
SLS, a well-known inducer of irritant contact dermatitis, was evaluated for its effect on skin sensory functions. The results show that SLS induces no spontaneous pain but induces sustained inflammatory reactions and primary hyperalgesia to mechanical impact and heat. Hyperalgesia vanished within 2–3 days, despite persistent clinical and objective measurements of skin inflammation. To the best of our knowledge, this report is the first study demonstrating hyperalgesia during the course of SLS inflammation.
Standardized models of inflammation and inflammatory pain are useful for screening anti-inflammatory and analgesic drugs. Experimental skin models of inflammatory pain have been described in detail in rodents [15]. Capsaicin is widely used, but mainly neurogenic in nature, and the effects are short-lasting. Mustard oil was used previously with similar results. Subacute or long-lasting human hyperalgesia models are probably more relevant for studies of the mechanisms that underlie inflammatory or neuropathic pain. The limited human models available include UVB-inflammation [16, 17], thermal injuries elicited by contact-heat [13] and freezing [18, 19], and non-inflammatory hyperalgesia induced by nerve growth factor [20]. The results of our study indicate that SLS-induced inflammation may be a novel model of inflammatory pain. Despite the relatively small number of subjects (in accordance with the estimate obtained prior to the study in a sample size calculation), the data were consistent; a dose–response relationship was established, and the extent of variability was acceptable.
SLS induced notable localized inflammation with intensity of clinical inflammation and changes in skin perfusion as reported in previous studies [1, 21, 22]. Topical SLS did not elicit spontaneous pain. In comparison, infusion of SLS into the skin via microdialysis fibers has been shown to induce immediate pain and release of prostaglandin E2 [23], likely due to either a direct toxic effect or activation of acid-sensing nociceptors by the low pH of most SLS solutions. Here, we show that SLS induces significant, dose-related thermal and tactile hyperalgesia. The reduction in thermal pain sensitivity with SLS 2% at 24 h was 6–7°C as compared to vehicle sites. This result was similar to the pattern observed at capsaicin sites. There are no prior investigations on mechanical or thermal pain thresholds in the context of SLS inflammation.
Although SLS 2% and capsaicin induced comparable increases in local vasodilation as well as thermal and tactile hyperalgesia at 24 h, the mechanisms of action are most likely different. Capsaicin is known specifically to activate TRPV1 receptors on C-fibers at the site of stimulation, with retrograde excitation causing axon reflexes. Flare reactions as well as secondary pin-prick hyperalgesia and allodynia were observed with capsaicin, but corresponding reactions were modest with SLS, even at the highest concentrations. Secondary pin-prick hyperalgesia and brush-evoked allodynia were not found consistently at SLS sites. These findings are consistent with previous studies showing that painful input to the spinal cord is essential for central sensitization and the development of secondary mechanical hyperalgesia [24]. The lack of spontaneous pain, notable hyperalgesia within the inflamed skin site, and the lack of secondary hyperalgesia are all comparable with findings related to UVB-inflammation [25]. SLS-induced inflammation most likely involves some degree of retrograde C-fiber activation because flares with SLS 2% were approximately 3–4 cm in diameter at 24 h. It is unlikely that this relatively large area of vasodilation is caused by SLS contamination outside the 18 mm Finn chambers. Development of flare reactions has no definite relation to pain because the flare has been observed following intradermal injection of a variety of compounds causing no pain [26]. Further research will be necessary to verify an association between the comparable time course of a flare reaction and reduced primary sensory thresholds within the inflamed skin site.
The pathophysiology of SLS inflammation and possible mechanism of sensitization of peripheral nociceptors in the skin remain to be clarified. Studies with human skin cells in vitro, animal skin in vivo and human skin have shown that SLS releases a plethora of inflammatory mediators [6], including cytokines such as TNF-α, IL-1β, and IL-6 as well as a variety of chemokines [4–11]. The cellular source of mediators causing nociceptor sensitization in SLS reactions, as well as other human models of inflammatory pain, remains to be elucidated. Keratinocytes are likely the key source of cytokines in SLS and UVB-inflammation [6, 27], but macroscopic and immunohistochemical studies have also revealed pronounced influxes of polymorphonuclear cells as well as lymphocytes at SLS sites [28–30]. There is likely to be a large overlap among key soluble pain-related mediators, including TNF-α, IL-1β, and IL-6, released by SLS, on one hand, and those mediators found to be up-regulated in established human models of inflammatory dermal pain such as UVB-inflammation [27, 31–34] and thermal injuries, on the other [35, 36]. The relative contributions of individual mediators to the induction of inflammation and hyperalgesia during the life cycle of the inflammatory event remain to be clarified. Most investigations in humans are performed after 24 h of application of SLS only, and no studies have performed serial assessments of mediator profiles and concurrent biological responses.
The extent to which the pathophysiology and the time course of sensory perturbations in SLS inflammation differ from those observed in UVB-inflammation and thermal lesions remains to be determined. Comparisons across individual trials can be questionable. For example, inflammatory reactions induced by three times the minimal erythema dose of UVB in healthy subjects measured by laser Doppler flowmetry, as well as the presence and/or magnitude of secondary hyperalgesia, have shown quite different results in trials of apparently similar design [16, 25, 37]. However, comparative studies with several skin models may be relevant in the search for pivotal mediators of hyperalgesia in the skin during the life cycle of inflammation. In our study, hyperalgesia had vanished at Day 6, but the inflammation persisted. The use of several models may improve our understanding of the pathophysiology of pain sensitization in inflammation and eventually lead to the development of effective and targeted therapies.
References
Tupker RA, Willis C, Berardesca E, Lee CH, Fartasch M, Agner T, et al. Guidelines on sodium lauryl sulfate (SLS) exposure tests A report from the Standardization Group of the European Society of Contact Dermatitis. Contact Derm. 1997;37:53–69.
Verri WA Jr, Cunha TM, Parada CA, Poole S, Cunha FQ, Ferreira SH. Hypernociceptive role of cytokines and chemokines: targets for analgesic drug development? Pharmacol Ther. 2006;112:116–38.
Thacker MA, Clark AK, Marchand F, McMahon SB. Pathophysiology of peripheral neuropathic pain: immune cells and molecules. Anesth Analg. 2007;105:838–47.
Spiekstra SW, Toebak MJ, Sampat-Sardjoepersad S, van Beek PJ, Boorsma DM, Stoof TJ, et al. Induction of cytokine (interleukin-1alpha and tumor necrosis factor-alpha) and chemokine (CCL20, CCL27, and CXCL8) alarm signals after allergen and irritant exposure. Exp Dermatol. 2005;14:109–16.
Piguet PF, Grau GE, Hauser C, Vassalli P. Tumor necrosis factor is a critical mediator in hapten induced irritant and contact hypersensitivity reactions. J Exp Med. 1991;173:673–9.
Effendy I, Loffler H, Maibach HI. Epidermal cytokines in murine cutaneous irritant responses. J Appl Toxicol. 2000;20:335–41.
Niwa M, Nagai K, Oike H, Kobori M. Evaluation of the skin irritation using a DNA microarray on a reconstructed human epidermal model. Biol Pharm Bull. 2009;32:203–8.
Hunziker T, Brand CU, Kapp A, Waelti ER, Braathen LR. Increased levels of inflammatory cytokines in human skin lymph derived from sodium lauryl sulphate-induced contact dermatitis. Br J Dermatol. 1992;127:254–7.
de Jongh CM, Lutter R, Verberk MM, Kezic S. Differential cytokine expression in skin after single and repeated irritation by sodium lauryl sulphate. Exp Dermatol. 2007;16:1032–40.
Ulfgren AK, Klareskog L, Lindberg M. An immunohistochemical analysis of cytokine expression in allergic and irritant contact dermatitis. Acta Derm Venereol. 2000;80:167–70.
Eberhard Y, Ortiz S, Ruiz LA, Kuznitzky R, Serra HM. Up-regulation of the chemokine CCL21 in the skin of subjects exposed to irritants. BMC Immunol. 2004;5:7–15.
Loffler H, Aramaki J, Effendy I. Response to thermal stimuli in skin pretreated with sodium lauryl sulfate. Acta Derm Venereol. 2001;81:395–7.
Pedersen JL, Kehlet H. Hyperalgesia in a human model of acute inflammatory pain: a methodological study. Pain. 1998;74:139–51.
Rolke R, Magerl W, Campbell KA, Schalber C, Caspari S, Birklein F, et al. Quantitative sensory testing: a comprehensive protocol for clinical trials. Eur J Pain. 2006;10:77–88.
Sandkuhler J. Models and mechanisms of hyperalgesia and allodynia. Physiol Rev. 2009;89:707–58.
Hoffmann RT, Schmelz M. Time course of UVA- and UVB-induced inflammation and hyperalgesia in human skin. Eur J Pain. 1999;3:131–9.
Sycha T, Gustorff B, Lehr S, Tanew A, Eichler HG, Schmetterer L. A simple pain model for the evaluation of analgesic effects of NSAIDs in healthy subjects. Br J Clin Pharmacol. 2003;56:165–72.
Kilo S, Forster C, Geisslinger G, Brune K, Handwerker HO. Inflammatory models of cutaneous hyperalgesia are sensitive to effects of ibuprofen in man. Pain. 1995;62:187–93.
Chassaing C, Schmidt J, Eschalier A, Cardot JM, Dubray C. Hyperalgesia induced by cutaneous freeze injury for testing analgesics in healthy volunteers. Br J Clin Pharmacol. 2006;61:389–97.
Rukwied R, Mayer A, Kluschina O, Obreja O, Schley M, Schmelz M. NGF induces non-inflammatory localized and lasting mechanical and thermal hypersensitivity in human skin. Pain. 2010;148:407–13.
Fullerton A, Rode B, Serup J. Skin irritation typing and grading based on laser Doppler perfusion imaging. Skin Res Technol. 2002;8:23–31.
Gloor M, Senger B, Langenauer M, Fluhr JW. On the course of the irritant reaction after irritation with sodium lauryl sulphate. Skin Res Technol. 2004;10:144–8.
Fairweather I, McGlone F, Reilly D, Rukwied R. Controlled dermal cell damage as human in vivo model for localised pain and inflammation. Inflamm Res. 2004;53:118–23.
Koltzenburg M, Torebjork HE, Wahren LK. Nociceptor modulated central sensitization causes mechanical hyperalgesia in acute chemogenic and chronic neuropathic pain. Brain. 1994;117(Pt 3):579–91.
Bishop T, Ballard A, Holmes H, Young AR, McMahon SB. Ultraviolet-B induced inflammation of human skin: Characterisation and comparison with traditional models of hyperalgesia. Eur J Pain. 2009;13:524–32.
Wallengren J, Hakanson R. Effects of capsaicin, bradykinin and prostaglandin E2 in the human skin. Br J Dermatol. 1992;126:111–7.
Schwarz T, Luger TA. Effect of UV irradiation on epidermal cell cytokine production. J Photochem Photobiol B. 1989;4:1–13.
Willis CM, Stephens CJ, Wilkinson JD. Differential patterns of epidermal leukocyte infiltration in patch test reactions to structurally unrelated chemical irritants. J Invest Dermatol. 1993;101:364–70.
Scheynius A, Fischer T, Forsum U, Klareskog L. Phenotypic characterization in situ of inflammatory cells in allergic and irritant contact dermatitis in man. Clin Exp Immunol. 1984;55:81–90.
Avnstorp C, Ralfkiaer E, Jorgensen J, Wantzin GL. Sequential immunophenotypic study of lymphoid infiltrate in allergic and irritant reactions. Contact Derm. 1987;16:239–45.
Urbanski A, Schwarz T, Neuner P, Krutmann J, Kirnbauer R, Kock A, et al. Ultraviolet light induces increased circulating interleukin-6 in humans. J Invest Dermatol. 1990;94:808–11.
Scholzen TE, Brzoska T, Kalden DH, O’Reilly F, Armstrong CA, Luger TA, et al. Effect of ultraviolet light on the release of neuropeptides and neuroendocrine hormones in the skin: mediators of photodermatitis and cutaneous inflammation. J Investig Dermatol Symp Proc. 1999;4:55–60.
Yoshizumi M, Nakamura T, Kato M, Ishioka T, Kozawa K, Wakamatsu K, et al. Release of cytokines/chemokines and cell death in UVB-irradiated human keratinocytes, HaCaT. Cell Biol Int. 2008;32:1405–11.
Angst MS, Clark JD, Carvalho B, Tingle M, Schmelz M, Yeomans DC. Cytokine profile in human skin in response to experimental inflammation, noxious stimulation, and administration of a COX-inhibitor: a microdialysis study. Pain. 2008;139:15–27.
Summer GJ, Romero-Sandoval EA, Bogen O, Dina OA, Khasar SG, Levine JD. Proinflammatory cytokines mediating burn-injury pain. Pain. 2008;135:98–107.
Opree A, Kress M. Involvement of the proinflammatory cytokines tumor necrosis factor-alpha, IL-1 beta, and IL-6 but not IL-8 in the development of heat hyperalgesia: effects on heat-evoked calcitonin gene-related peptide release from rat skin. J Neurosci. 2000;20:6289–93.
Gustorff B, Anzenhofer S, Sycha T, Lehr S, Kress HG. The sunburn pain model: the stability of primary and secondary hyperalgesia over 10 hours in a crossover setting. Anesth Analg. 2004;98:173–7. (table).
Acknowledgments
The study was supported by research grants from the Rosa and Asta Jensen Foundation and the Viborg Hospital Research Foundation.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: G. Geisslinger.
Rights and permissions
About this article
Cite this article
Petersen, L.J., Lyngholm, A.M. & Arendt-Nielsen, L. A novel model of inflammatory pain in human skin involving topical application of sodium lauryl sulfate. Inflamm. Res. 59, 775–781 (2010). https://doi.org/10.1007/s00011-010-0189-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-010-0189-1