Abstract
Objective
To investigate the influence of a combined therapy consisting of dexamethasone and osteoprotegerin (OPG) on bone alterations and disease activity in antigen-induced arthritis (AIA) in the rat.
Methods
AIA rats received dexamethasone (0.25 mg kg−1 day−1, i.p.), OPG (2.5 mg kg−1 day−1, i.p.), or a combination of both at regular intervals for 21 consecutive days. At the end of the treatment, bone structure was analyzed by histomorphometry. Primary spongiosa was measured using linear scanning.
Results
AIA led to significant periarticular and axial bone loss. Dexamethasone monotherapy substantially suppressed joint swelling without inhibiting bone loss of the secondary spongiosa, whereas OPG monotherapy showed no anti-inflammatory effect. Despite reduction of bone resorption, OPG did not inhibit AIA-induced bone loss. In contrast, the combination of dexamethasone and OPG not only produced an anti-inflammatory effect, but also resulted in inhibition of periarticular and axial bone loss. OPG increased trabecular number of the primary spongiosa whilst combination therapy led to an increase in both trabecular number and trabecular width.
Conclusion
The principle of combining a glucocorticoid together with inhibition of the receptor activator of NF-kappaB ligand (RANKL) may be an effective bone-saving therapy in rheumatoid arthritis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Rheumatoid arthritis (RA) is characterized by chronic joint inflammation, progressive destruction of periarticular bone and periarticular and systemic osteoporosis [1–3]. Glucocorticoids, highly effective in suppressing this inflammation and thereby proffering an increase in mobility and quality of life, have been shown to inhibit joint destruction (radiological progression) in early RA in various randomized placebo-controlled trials [4–7]. Conversely, glucocorticoids are also known to contribute to osteoporosis and fractures in RA [2, 8, 9].
The osteoclastogenesis regulating system consisting of the receptor activator of NF-kappaB ligand (RANKL), its receptor RANK, and osteoprotegerin (OPG), the RANKL decoy receptor, is critically involved in both inflammation- and glucocorticoid-induced bone loss in RA [10–16]. Inhibition of RANKL, which is essential for osteoclastogenesis and osteoclastic activation by its decoy receptor OPG has been shown to protect bone from arthritis-induced alterations and inhibit bone loss in various animal models of RA [17–22]. Furthermore, the monoclonal anti-RANKL antibody denosumab has been shown to retard radiological progression in RA [23]. Glucocorticoids induce the expression of RANKL and suppress that of OPG [10, 13, 16, 24]. Therefore, to modulate the influence of glucocorticoid therapy on arthritis-induced bone alterations, an additional therapy that interacts with the RANKL-RANK-OPG system is of great interest. Against this background of complex interactions of glucocorticoids with the RANKL-RANK-OPG system, the question of whether an additional therapy decreasing the RANKL/OPG ratio could block potentially unfavourable effects of glucocorticoids on bone, but still preserve, or even amplify, the bone protective effects of glucocorticoids in chronic joint inflammation needs to be explored.
This study compared the influence of a combination therapy consisting of a glucocorticoid and OPG with the respective monotherapies on arthritis-induced bone alterations and inflammatory disease activity in antigen-induced arthritis (AIA) of the rat—an animal model sharing essential features with RA such as synovial hyperplasia, inflammatory infiltration, cartilage destruction and substantial periarticular and moderate axial bone loss [22, 25–28].
Materials and methods
Arthritis induction
Eight-week-old female Lewis rats (LEW/Crl; Charles River, Sulzfeld, Germany) maintained under standardized conditions were subjected to a 12 h/12 h light/darkness cycle and fed with pellet food (Altromin, No 1326, Lage, Germany) and water ad libitum. The animals were immunized subcutaneously with 0.5 mg methylated bovine serum albumin (mBSA, Sigma, Deisenhofen, Germany) in 0.5 ml saline and emulsified in 0.5 ml complete Freund’s adjuvant (Sigma), containing 2 mg/ml heat-killed Mycobacterium tuberculosis strain H37RA (Difco, Detroit, MI) 14 and 21 days before AIA induction.
Arthritis was elicited by injecting 0.5 mg mBSA in 50 μl sterile phosphate-buffered saline (PBS) into the right knee joint cavity. The same volume of PBS was injected into the left knee as an intra-individual control. Ethical guidelines for experimental investigations in animals were conformed to [29]. All procedures complied with the regulations of the Thuringian Commission for Animal Protection.
Drug administration
Rats with AIA were divided into four groups to receive intraperitoneal injections after AIA induction according to the following regimens:
-
Group 1: PBS (200 μl) after 3 h and on days 1, 3, 6, 8, 11, 14 and 17 (n = 8)
-
Group 2: Dexamethasone (Merck Pharma, Darmstadt, Germany; 0.25 mg/kg in 200 μl PBS) after 3 h and on days 1, 2, 3, 5, 8, 11, 14 and 17 (n = 10)
-
Group 3: Recombinant OPG fusion protein (Fc-OPG; 2.5 mg/kg in 200 μl PBS) after 3 h and on days 1, 3, 6, 8, 11, 14 and 17 (n = 7)
-
Group 4: Dexamethasone (0.25 mg/kg in 200 μl) after 3 h and on days 1, 2, 3, 5, 8, 11, 14 and 17 + OPG (2.5 mg/kg in 200 μl PBS) after 3 h and on days 1, 3, 6, 8, 11, 14 and 17 (combination therapy; n = 7)
Fc-OPG, a chimeric molecule comprising amino acids 22–194 of human OPG, fused at the N-terminus to the C-terminus of human IgG1 [3] was kindly provided by Amgen (Thousand Oaks, CA).
The choice of drug concentrations of OPG and dexamethasone was based on previous investigations in adjuvant arthritis [30] and AIA of the rat [22, 28]. It has been shown that daily subcutaneous injection of 2.5 mg/kg OPG preserved or enhanced bone mineral density and prevented bone erosion in adjuvant arthritis of the rat [30] and that the i.p. administration of 3 mg/kg OPG every 2–3 days inhibited bone loss in AIA of the rat [22]. The i.p. administration of 0.25 mg kg−1 day−1 of dexamethasone led to a highly significant reduction of joint swelling (inflammatory activity) and destruction in AIA of the rat [28]. Additionally, eight healthy animals without AIA and no immunization were used as healthy controls.
Assessment of arthritis
Arthritis was monitored by measuring the mediolateral joint diameter using a vernier caliper [25]. Swelling was expressed as the difference in millimeters between the right arthritic and the left reference joint.
Preparation of bones for histomorphometric analysis
All 32 arthritic and 8 healthy rats were sacrificed on day 21 after AIA induction. Both the right tibia head of the arthritic knee joint and the third lumbar vertebra were removed and used for histomorphometric analysis. After preparation, the bones were fixed in acetone for 24 h and embedded with the help of the embedding system Technovit 9100 NEU (Haraeus-Kulzer, Wehrheim, Germany) for mineralized tissue. In principal, the system is based on chemical polymerization employing a catalytic system consisting of peroxide and amine without oxygen. A Polycut S-Special Microtome was used to cut 5 μm thick sections (Jung/Leica, Heidelberg, Germany). Trichrome Masson/Goldner staining was performed to differentiate mineralized bone and osteoid [31]. In addition, Giemsa staining was performed to allow distinction of cellular components.
Histomorphometric analysis of bone structure
Bone structure analysis involved an examination of the primary and secondary spongiosa of the right tibia head as well as the third lumbar vertebra. Structural homogeneity of bone architecture is an important aspect relating to the method used for investigating bone structure. Primary spongiosa of growing rats is characterized by partial resorption of calcified longitudinal cartilage septa by osteoclasts followed by blood vessel invasion and formation of woven bone on the basis of these septa by osteoblasts [32, 33]. The growing process results in an inhomogeneity of the bone structure of primary spongiosa. The bone architecture of the primary spongiosa during physiological growing conditions is characterized by a continuous increase of trabecular width and a decrease of trabecular number with increasing distance from the growth plate. Therefore, histomorphometry is not suitable for the analysis of bone structure of primary spongiosa. In contrast, secondary spongiosa consists of lamellar bone without cartilage cores, which represents homogeneous bone tissue and can thus be investigated using histomorphometry.
Histomorphometric measurement of the bone structure of secondary spongiosa was performed commencing at a distance of 1.25 mm from the growth plate. In this zone, the trabecular bone volume, trabecular width and histomorphometric parameters of bone formation and bone resorption were evaluated by standard histomorphometry [31, 34, 35] (see Table 1 for details of parameters evaluated).
Additional analysis of the bone structure of the primary spongiosa was performed on periarticular bone within a distance of 1.25 mm from the growth plate. The primary spongiosa, identified by means of morphological criteria (presence of cartilage cores) and by its distance from the growth plate [33, 36] was subsequently analyzed employing linear scanning [36, 37] (Table 2).
In a digital image of the tibial head generated by a camera, a line was drawn by a cursor at the first calcified structures below the growth plate. The length of the line was measured. This line was successively advanced from the first calcified structures towards the distal end in equal steps of 15 μm through the 975 μm of the proximal tibial metaphysis. Automatic scrolling and measuring procedures were accomplished by a software application (Zeiss, Jena, Germany). At every step, the following parameters were measured automatically: (1) the number of trabeculae encountered by the measuring line; and (2) the total length of the measuring line covering calcified structures. These parameters were analyzed as a function of distance from the first calcified structures below the growth plate.
In various previous studies in rat AIA with parallel investigation of inflammatory disease activity, cartilage destruction and bone loss including healthy controls and investigation of the contralateral (non-arthritic) tibia head, spontaneous bone loss and cartilage destruction in healthy animals or at the knee joint not affected by arthritis were not observed [25–27].
Statistical analysis
Data were presented as means ± standard deviation. The data were analysed statistically using the SPSS for Windows Statistical Programme [38]. Data were subjected to the non-parametric Kruskall-Wallis-analysis and, subsequently to the non-parametric Mann–Whitney U test. In the primary spongiosa, the different parameters were compared at identical distances from the growth plate. Differences of P < 0.05 were considered significant.
Results
Influence of dexamethasone, OPG, and the combination of dexamethasone plus OPG on clinical disease activity
Dexamethasone monotherapy resulted in a significant reduction of joint swelling in comparison to untreated AIA from day 8 up to the end of the study (Fig. 1, Table 3). At all time points, joint swelling was numerically lower in animals receiving combination therapy with dexamethasone and OPG; however, the differences in comparison to vehicle-treated AIA reached significance only on days 17 and 20. In contrast to therapy regimens containing dexamethasone, OPG therapy showed no anti-inflammatory effects.
Inflammatory disease activity measured by knee joint swelling depicted as difference between right and left joint (mm) during the course of antigen-induced arthritis (AIA). Dexamethasone monotherapy (DEX) resulted in a significant reduction in joint swelling from day 8 in comparison to vehicle-treated AIA (PBS) and from day 6 up to the end of the study (P < 0.05 to < 0.01) compared with AIA treated with osteoprotegerin (OPG) monotherapy. Joint swelling was also lower in animals receiving combination therapy with dexamethasone and OPG in comparison to vehicle-treated AIA, and compared to AIA treated with OPG monotherapy, but the differences reached significance only on days 17 and 20 and between day 14 and 20, respectively (P < 0.05 to < 0.01). In contrast to therapy regimens containing dexamethasone, OPG therapy showed no anti-inflammatory effect
Influence of AIA on periarticular and axial bone (secondary spongiosa)
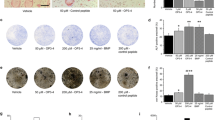
Vehicle-treated AIA resulted in a significant decrease of trabecular bone volume in both periarticular and axial bone in comparison to healthy controls (Figs. 2a,b; 3a; 4a). This periarticular and systemic bone loss was associated with an increase in bone turnover as reflected by a highly significant increase in the resorption surface with osteoclasts at periarticular bone and a numerical increase of this parameter at the lumbar vertebra as well as by a significant increase in the osteoid surface covered with osteoblasts at both sites (Figs. 3b–d, 4b–d).
Assessment of bone histology, primary and secondary spongiosa of the right tibia head (representative Masson/Goldner stained sections). In comparison to healthy controls (a), vehicle-treated AIA resulted in significant bone loss in both primary and secondary spongiosa (b). OPG monotherapy inhibited bone loss of the primary spongiosa without protecting effect on secondary spongiosa (c). Combination therapy of dexamethasone plus OPG was effective in prevention of bone loss at both primary and secondary spongiosa (d)
Trabecular bone volume and cellular bone turnover parameters (secondary spongiosa of the right tibia head: arthritic joint) in AIA of the rat. Influence of dexamethasone monotherapy (AIA + DEX), OPG monotherapy (AIA + OPG) and combination therapy with dexamethasone and OPG (AIA + DEX + OPG). In comparison to healthy controls, vehicle-treated AIA (AIA + PBS) resulted in a highly significant decrease in periarticular trabecular bone volume associated with a significant increase in both resorption surface with osteoclasts and the osteoid-covered surface with osteoblasts (a, b, d). Dexamethasone monotherapy had no effect on arthritis-induced bone alterations. OPG monotherapy reduced resorption surface with osteoclasts significantly in comparison to vehicle-treated AIA without inhibiting arthritis-associated bone loss (a, b). Only combination therapy with dexamethasone and OPG resulted in a partial but significant inhibition of AIA-induced bone loss associated with a significant reduction in resorption surface with osteoclasts (a, b). **P < 0.01; *P < 0.05 vs healthy controls, ++ P < 0.01; + P < 0.05 vs AIA + PBS, °P > 0.05 vs AIA + OPG)
Trabecular bone volume and cellular bone turnover parameters (secondary spongiosa of the third lumbar vertebra) in AIA of the rat. Influence of dexamethasone monotherapy (AIA + DEX), OPG monotherapy (AIA + OPG) and combination therapy with dexamethasone and OPG (AIA + DEX + OPG). In comparison to healthy controls, vehicle-treated AIA (AIA + PBS) resulted in a highly significant decrease in trabecular bone volume associated with a numerical increase in resorption surface with osteoclasts and a significant increase in osteoid-covered surface with osteoblasts (a, b, d). Neither dexamethasone and OPG monotherapy had any significant effect on arthritis-induced bone alterations. However, combination therapy with dexamethasone and OPG resulted in a complete inhibition of AIA-induced bone loss associated with a reduction of cellular bone turnover (a, b). Resorption surface with osteoclasts was significantly lower and trabecular bone volume was significantly higher in the combination therapy group in comparison to AIA treated with OPG monotherapy. (**P < 0.01; *P < 0.05 vs healthy controls, ++P < 0.01 vs AIA + PBS, °P > 0.05 vs AIA + OPG)
Influence of dexamethasone, OPG, and the combination of dexamethasone plus OPG on arthritis-induced bone alterations of the periarticular bone (secondary spongiosa)
Monotherapy with OPG resulted in a significant decrease in the resorption surface with osteoclasts in comparison to vehicle-treated AIA, and reduced this parameter to the levels of healthy animals (Fig. 3b). However, this suppressive effect on osteoclastic bone resorption was not associated with a prevention of periarticular bone loss (Figs. 2, 3a). Although the resorption surface with osteoclasts in dexamethasone-treated rats was not significantly different to healthy controls, and was lower numerically than in vehicle-treated AIA (Fig. 3b), AIA-induced bone loss was not prevented by dexamethasone (Fig. 3a).
In contrast to monotherapy with dexamethasone or OPG, the combination of both substances led to a partial prevention of periarticular bone loss, as indicated by a significantly higher trabecular bone volume in this group in comparison to vehicle-treated arthritic animals as well as animals treated with dexamethasone monotherapy (Figs. 2, 3a). This preventive effect was associated with a significant decrease in the resorption surface with osteoclasts in comparison to untreated AIA (Fig. 3b). In contrast to animals treated with OPG monotherapy, animals receiving combination therapy demonstrated a significantly higher osteoid surface covered with osteoblasts than healthy animals.
Influence of dexamethasone, OPG, and the combination of dexamethasone plus OPG on arthritis-induced bone alterations of axial bone (secondary spongiosa)
Neither dexamethasone nor OPG monotherapy prevented vertebral bone loss (Fig. 4). In contrast, the combination of dexamethasone plus OPG resulted in a complete prevention of AIA-induced bone loss at the axial bone (Fig. 4a). Moreover, the resorption surface with osteoclasts was lowest in rats receiving a combination of dexamethasone plus OPG; however, due to the high standard deviation the statistical differences were significant only when compared to animals treated with OPG monotherapy (Fig. 4b).
Influence of AIA on primary spongiosa
In comparison to healthy controls, vehicle-treated AIA resulted in a significant decrease in trabecular number, beginning at a distance of 645 μm up to a distance of 910 μm from the growth plate, as well as in a significant decrease in trabecular width between 30 and 60 μm and between 675 and 705 μm from the growth plate (Figs. 2, 5, 6). Accordingly, mineralized tissue was significantly lower in vehicle-treated AIA in comparison to healthy controls between 585 and 870 μm from the growth plate.
Number of trabeculae of the proximal zone of the tibial metaphysis (primary spongiosa) measured by linear scanning at day 21 of AIA. Vehicle-treated AIA resulted in a significant decrease in trabecular number beginning at a distance of 645 μm up to a distance of 910 μm from the growth plate (a; P < 0.05 to < 0.01). OPG monotherapy was associated with a significant increase in trabecular number in comparison to both vehicle-treated AIA (PBS) and healthy controls commencing at a distance of 165 μm (a) and also compared to AIA rats receiving dexamethasone monotherapy where the distance commenced at 120 μm from the growth plate (c; P < 0.01 to < 0.001). Combination therapy with dexamethasone plus OPG led to a significant increase in trabecular number compared to vehicle-treated AIA and animals receiving dexamethasone monotherapy from a distance of 315 μm (b, c), and compared to healthy rats from a distance of 495 μm from the growth plate (b; P < 0.05 to < 0.001). Trabecular number was not influenced by dexamethasone monotherapy (d)
Mineralized tissue of the proximal zone of the tibial metaphysis (primary spongiosa) measured by linear scanning at day 21 of AIA. Vehicle-treated AIA (PBS) was associated with a significant decrease in mineralized tissue in comparison to healthy controls at distances between 585 and 870 μm from the growth plate (d; P < 0.05 – < 0.01). Monotherapy with dexamethasone did not inhibit AIA-induced bone loss (d). Both OPG monotherapy and combination therapy with dexamethasone plus OPG prevented AIA- associated bone loss of the primary spongiosa (a, b). Thus, animals with OPG monotherapy had a significantly higher mineralized tissue content in comparison to vehicle-treated AIA at distances between 225 and 750 μm and between 810 and 975 μm from the growth plate (a; P < 0.005 to < 0.001), in comparison to healthy controls at a distance between 270 and 645 μm from the growth plate (a; P < 0.05 to < 0.01) and compared with rats receiving dexamethasone monotherapy beginning at a distance of 270 μm from the growth plate (c; P < 0.05 to < 0.01) Mineralized tissue in animals with combination therapy with dexamethasone plus OPG was not only significantly higher in comparison to vehicle-treated AIA (commencing at a distance of 45 μm from growth plate; P < 0.05 to < 0.001; b) to healthy controls (at a distance between 120 and 780 μm; P < 0.05 to < 0.001; b) and to rats with dexamethasone monotherapy (starting at a distance of 105 μm; P < 0.05 to < 0.001; c) but also compared to rats with OPG monotherapy (at a distance between 15 and 600 μm from the growth plate; P < 0.05 to < 0.01; c). Between 30 and 120 μm from the growth plate, mineralized tissue was lower in rats treated with OPG monotherapy in comparison to healthy rats (P < 0.05 to < 0.01; a)
Influence of dexamethasone, OPG, and the combination of dexamethasone plus OPG on arthritis-induced bone alterations of primary spongiosa
In animals receiving OPG monotherapy, trabecular number was significantly higher starting at a distance of 165 μm from the growth plate in comparison to vehicle-treated AIA and healthy controls, and also compared to rats receiving dexamethasone monotherapy commencing at a distance of 120 μm from the growth plate (Fig. 5a, c). In contrast to the increase in trabecular number, trabecular width was significantly lower in the OPG group in comparison to healthy controls at distances between 30–210 μm and 585–765 μm from the growth plate, respectively. In comparison to rats receiving dexamethasone monotherapy, trabecular width was lower in the OPG group commencing at distances of 30–510 μm from the growth plate. Due to the increase in trabecular number and despite a relative reduction in trabecular width, animals with OPG monotherapy had a significantly higher mineralized tissue content in comparison to vehicle-treated AIA at distances between 225 and 750 μm and between 810 and 975 μm from the growth plate. Moreover, mineralized tissue content was significantly higher in AIA rats with OPG monotherapy compared with AIA rats receiving dexamethasone monotherapy beginning at a distance of 270 μm from the growth plate and in comparison to healthy controls at distances between 270 and 645 μm from the growth plate (Fig. 6a, c). Interestingly, the effects of OPG monotherapy on parameters measured by linear scanning beginning at distances very close to the growth plate are in part different to the effect on these parameters at distances further away from the growth plate. For example, in OPG-treated AIA rats, trabecular number was significantly lower in comparison to healthy animals commencing at a distance of 15–45 μm from the growth plate (Fig. 5a) and mineralized tissue mass was significantly lower compared to healthy controls at a distance of 30–120 μm from the growth plate (Fig. 6a).
In contrast to OPG monotherapy, dexamethasone monotherapy had no influence on the parameters of the primary spongiosa measured by linear scanning (Figs. 5d, 6d).
Animals receiving the combination therapy of dexamethasone plus OPG showed a significantly higher trabecular number starting at a distance of 315 μm from the growth plate in comparison to both vehicle-treated AIA and rats receiving dexamethasone monotherapy, and compared to healthy rats at a distance commencing 495 μm from growth plate, respectively (Fig. 5b, c). Regarding the trabecular width, combination therapy with dexamethasone plus OPG resulted in a significant increase of this parameter compared with vehicle-treated AIA (at a distance between 180 and 465 μm from the growth plate), healthy controls (at a distance between 315 and 480 μm from the growth plate), and OPG monotherapy (at a distance between 60 and 630 μm from the growth plate). Due to the increase in both trabecular number and trabecular width, the mineralized tissue content in rats receiving combination therapy with dexamethasone plus OPG was significantly higher in most distances from the growth plate in comparison with all other groups (Figs. 2d; 6b, c).
Discussion
The present study clearly demonstrates the inhibition of periarticular and axial bone loss of the secondary spongiosa by means of a combination therapy consisting of dexamethasone plus OPG, whereas monotherapy with both of these substances at identical concentrations was ineffective in reducing bone loss.
In this regard, it is important to discuss the finding that dexamethasone monotherapy had no significant influence on AIA-induced bone alterations of the secondary spongiosa of both periarticular and axial bone despite effective suppression of inflammation. The main reason for the ineffectiveness of dexamethasone monotherapy on AIA-induced bone alterations may be due to glucocorticoid-induced suppression of OPG expression by both direct and indirect cytokine-mediated influences.
With respect to the increased bone resorption in AIA, effective prevention of bone loss is dependent on the equilibrium between RANKL and its decoy receptor OPG. Against the background of no inflammation, glucocorticoids have been shown to stimulate the expression of RANKL and inhibit OPG, resulting in an increase of bone resorption thereby contributing to glucocorticoid-induced osteoporosis [10, 13, 16, 24]. In the case of inflammation, as is present in AIA, glucocorticoids may indirectly inhibit RANKL expression by suppressing proinflammatory cytokines that induce RANKL, such as TNF-α, IL-1 and IL-6 [39], resulting in a protective effect on inflammation-induced bone alterations. However, the proinflammatory cytokines that are suppressed by glucocorticoids induce not only RANKL but also OPG [40–45]. Thus, glucocorticoid inhibition of these cytokines in the inflammatory setting may also result in a decrease of OPG. Although the expression of RANKL and OPG was not determined in the present study, it can be presumed that dexamethasone monotherapy induced a relative deficiency of OPG by means of direct and indirect cytokine-mediated effects and, in so doing, counteracted its own potential protective effect on AIA-induced bone alterations. Thus, the addition of OPG in the combination therapy, in contrast to dexamethasone monotherapy, may counter the effect of the glucocorticoid-induced relative OPG deficiency and lead to effective prevention of AIA-induced bone loss.
The possibility that dexamethasone may inhibit alternative RANKL-independent pathways of osteoclastogenesis and osteoclastic activation, potentiating OPG effects in the combination therapy group is unlikely, since RANKL is the crucial cytokine involved in osteoclastic activation [3, 17]. Furthermore, in particular with regard to axial bone loss, it is likely that suppression of inflammation by dexamethasone in combination with OPG may prevent immobilization of the animals and protect bone from the consequences of immobility. The ineffectiveness of OPG monotherapy in inhibiting periarticular and systemic bone loss of the secondary spongiosa despite a significant reduction of osteoclast resorption surface observed in this study is not in accordance with previous findings and calls for an explanation. OPG monotherapy has been shown to inhibit arthritis-induced bone loss in collagen-induced arthritis (CIA) of rats and mice, arthritis in human TNF-transgenic mice und adjuvant arthritis in the rat [17–21]. In previous investigations, we were able to demonstrate that a higher OPG dose of 3 mg/kg, applied ten times over a period of 23 days, inhibited periarticular bone loss in AIA [22]. The cumulative dose in this previous investigation amounted to 30 mg/kg in 23 days of arthritis compared with 20 mg/kg in 21 days of AIA in the present study. Therefore, it is probable that the OPG monotherapy dose used in the present study is to low to allow effective inhibition of osteoclastic bone resorption. However, because the decrease in resorption surface for osteoclasts is comparable in both studies (approximately 80% in the previous and around 60% in the present study), it is probable that the OPG dose of 2.5 mg/kg, though not high enough to inhibit AIA-induced bone resorption during the acute phase of AIA typified by high inflammatory activity, is sufficient to reduce bone resorption in the chronic phase of arthritis characterized by lower inflammatory activity. Trabecular bone volume is the result of bone turnover throughout the duration of arthritis; however, a decrease in the resorption surface for osteoclasts reflects the inhibition of osteoclastic differentiation, a cellular event that can occur in a relatively short time period of 12–24 h following treatment with OPG-Fc-fusion protein [16]. Therefore, it can be argued that inhibition of osteoclastogenesis by OPG in the chronic phase of arthritis and at the end of the study, occurs too late to inhibit bone loss. This assumption is in accordance with the observation of Stolina et al. [46], who demonstrated a rapid increase in local concentrations of RANKL protein followed by a slow decrease in the joints of CIA and adjuvant arthritis in rats.
The finding that OPG monotherapy is ineffective in reducing joint swelling is not surprising and is in compliance with previous investigations in AIA of the rat [22], CIA of mice [19] and rats [18], arthritis of human TNF-transgenic mice [20, 21] and in rat adjuvant arthritis [17].
Our data show that bone loss during AIA is substantially lower in primary spongiosa in comparison to secondary spongiosa as indicated by a reduction of mineralized tissue mass of the primary spongiosa of the order of around 20–25% compared with a reduction of periarticular bone volume of the secondary spongiosa of more than 50%. Both a continuous apposition of newly formed bone as well as differential hormonal mechanisms of regulating bone modeling and remodeling may contribute to a reduced sensitivity of primary spongiosa in comparison to secondary spongiosa with regard to inflammation-induced bone loss [47]. Thus, the OPG dosage, which was not sufficient to prevent AIA-induced bone loss of the secondary spongiosa, led to a complete inhibition of AIA-induced bone loss of the primary spongiosa by means of effecting the AIA-induced reduction of trabecular number. However, the higher trabecular number in AIA rats subjected to OPG monotherapy, which is due to the inhibition of the physiological reduction of trabecular number with increasing distance from growth plate in comparison to healthy rats, indicates an interference of OPG with the physiological remodeling process via inhibition of osteoclastic bone resorption in the primary spongiosa. The decrease in trabecular number following OPG therapy in the area closer to the growth plate may be a consequence of OPG interfering with bone growth—a process partly dependent on RANKL owing to an indirect inhibition of bone formation mediated either by the coupling process or due to RANKL inhibition [3, 16, 21]. Combination therapy with dexamethasone plus OPG induced an increase not only in trabecular number, but also in trabecular width as a result of the inhibition of both inflammation-induced bone loss by the glucocorticoid and the resorption of newly formed trabeculae by OPG.
In summary, the results of the present study show that the combination of glucocorticoids with RANKL inhibition through osteoprotegerin in doses that are ineffective when applied as monotherapy, results in an effective inhibition of periarticular and systemic bone loss in AIA. The reason for this protective effect of the combination therapy on bone may be the substitution of OPG deficiency that results from a glucocorticoid-induced OPG loss, thus restoring the equilibrium between inhibition of both inflammation and bone resorption, and the suppression of ongoing bone resorption despite suppression of inflammation. Therefore, the principle of a combination therapy consisting of glucocorticoids with RANKL inhibition may provide an effective means of saving bone in rheumatoid arthritis.
References
Gough AK, Lilley J, Eyre S, Holder RL, Emery P. Generalised bone loss in patients with early rheumatoid arthritis. Lancet. 1994;344:23–7.
Lodder MC, Haugeberg G, Lems WF, Uhlig T, Orstavik RE, Kostense PJ, et al. Oslo-Truro-Amsterdam (OSTRA) Collaborative Study. Radiographic damage associated with low bone mineral density and vertebral deformities in rheumatoid arthritis: the Oslo-Truro-Amsterdam (OSTRA) collaborative study. Arthritis Rheum. 2003;49:209–15.
Schett G, Redlich K, Hayer S, Zwerina J, Bolon B, Dunstan C, et al. Osteoprotegerin protects against generalized bone loss in tumor necrosis factor-transgenic mice. Arthritis Rheum. 2003;48:2042–51.
Kirwan JR. The effect of glucocorticoids on joint destruction in rheumatoid arthritis. The Arthritis and Rheumatism Council Low-Dose Glucocorticoid Study Group. N Engl J Med. 1995;333:142–6.
Van Everdingen AA, Jacobs JW, Siewertsz van Reesema DR, Bijlsma JW. Low-dose prednisone therapy for patients with early active rheumatoid arthritis: clinical efficacy, disease-modifying properties, and side effects: a randomized, double-blind, placebo-controlled clinical trial. Ann Intern Med. 2002;136:1–12.
Wassenberg S, Rau R, Steinfeld P, Zeidler H. Very low-dose prednisolone in early rheumatoid arthritis retards radiographic progression over two years: a multicenter, double-blind, placebo-controlled trial. Arthritis Rheum. 2005;52:3371–80.
Svensson B, Boonen A, Albertsson K, van der Heijde D, Keller C, Hafström I. Low-dose prednisolone in addition to the initial disease-modifying antirheumatic drug in patients with early active rheumatoid arthritis reduces joint destruction and increases the remission rate: a two-year randomized trial. Arthritis Rheum. 2005;52:3360–70.
Haugeberg G, Orstavik RE, Uhlig T, Falch JA, Halse JI, Kvien TK. Clinical decision rules in rheumatoid arthritis: do they identify patients at high risk for osteoporosis? Testing clinical criteria in a population based cohort of patients with rheumatoid arthritis recruited from the Oslo Rheumatoid Arthritis Register. Ann Rheum Dis. 2002;61:1085–9.
Oelzner P, Schwabe A, Lehmann G, Eidner T, Franke S, Wolf G, et al. Significance of risk factors for osteoporosis is dependent on gender and menopause in rheumatoid arthritis. Rheumatol Int. 2008;28:1143–50.
Vidal NO, Brändström H, Jonsson KB, Ohlsson C. Osteoprotegerin mRNA is expressed in primary human osteoblast-like cells: down-regulation by glucocorticoids. J Endocrinol. 1998;159:191–5.
Gravallese EM, Manning C, Tsay A, Naito A, Pan C, Amento E, et al. Synovial tissue in rheumatoid arthritis is a source of osteoclast differentiation factor. Arthritis Rheum. 2000;43:250–8.
Takayanagi H, Iizuka H, Juji T, Nakagawa T, Yamamoto A, Miyazaki T, et al. Involvement of receptor activator of nuclear factor kappaB ligand/osteoclast differentiation factor in osteoclastogenesis from synoviocytes in rheumatoid arthritis. Arthritis Rheum. 2000;43:259–69.
Mancini L, Paul-Clark MJ, Rosignoli G, Hannon R, Martin JE, Macintyre I, et al. Calcitonin and prednisolone display antagonistic actions on bone and have synergistic effects in experimental arthritis. Am J Pathol. 2007;170:1018–27.
Haynes D, Crotti T, Weedon H, Slavotinek J, Au V, Coleman M, et al. Modulation of RANKL and osteoprotegerin expression in synovial tissue from patients with rheumatoid arthritis in response to disease-modifying antirheumatic drug treatment and correlation with radiologic outcome. Arthritis Rheum. 2008;59:911–20.
Ainola M, Mandelin J, Liljeström M, Konttinen YT, Salo J. Imbalanced expression of RANKL and osteoprotegerin mRNA in pannus tissue of rheumatoid arthritis. Clin Exp Rheumatol. 2008;26:240–6.
Kearns AE, Khosla S, Kostenuik PJ. Receptor activator of nuclear factor kappaB ligand and osteoprotegerin regulation of bone remodeling in health and disease. Endocr Rev. 2008;29:155–92.
Kong YY, Feige U, Sarosi I, Bolon B, Tafuri A, Morony S, et al. Activated T cells regulate bone loss and joint destruction in adjuvant arthritis through osteoprotegerin ligand. Nature. 1999;402:304–9.
Romas E, Sims NA, Hards DK, Lindsay M, Quinn JW, Ryan PF, et al. Osteoprotegerin reduces osteoclast numbers and prevents bone erosion in collagen-induced arthritis. Am J Pathol. 2002;161:1419–27.
Saidenberg-Kermanac’h N, Corrado A, Lemeiter D, de Vernejoul MC, Boissier MC, Cohen-Solal ME. TNF-alpha antibodies and osteoprotegerin decrease systemic bone loss associated with inflammation through distinct mechanisms in collagen-induced arthritis. Bone. 2004;35:1200–7.
Redlich K, Görtz B, Hayer S, Zwerina J, Doerr N, Kostenuik P, et al. Repair of local bone erosions and reversal of systemic bone loss upon therapy with anti-tumor necrosis factor in combination with osteoprotegerin or parathyroid hormone in tumor necrosis factor-mediated arthritis. Am J Pathol. 2004;164:543–55.
Zwerina J, Hayer S, Tohidast-Akrad M, Bergmeister H, Redlich K, Feige U, et al. Single and combined inhibition of tumor necrosis factor, interleukin-1, and RANKL pathways in tumor necrosis factor-induced arthritis: effects on synovial inflammation, bone erosion, and cartilage destruction. Arthritis Rheum. 2004;50:277–90.
Neumann T, Oelzner P, Petrow PK, Thoss K, Hein G, Stein G, et al. Osteoprotegerin reduces the loss of periarticular bone mass in primary and secondary spongiosa but does not influence inflammation in rat antigen-induced arthritis. Inflamm Res. 2006;55:32–9.
Cohen SB, Dore RK, Lane NE, Ory PA, Peterfy CG, Sharp JT, et al. Denosumab treatment effects on structural damage, bone mineral density, and bone turnover in rheumatoid arthritis: a twelve-month, multicenter, randomized, double-blind, placebo-controlled, phase II clinical trial. Arthritis Rheum. 2008;58:1299–309.
Kondo T, Kitazawa R, Yamaguchi A, Kitazawa S. Dexamethasone promotes osteoclastogenesis by inhibiting osteoprotegerin through multiple levels. J Cell Biochem. 2008;103:335–45.
Bräuer R, Kette H, Henzgen S, Thoss K. Influence of cyclosporin A on cytokine levels in synovial fluid and serum of rats with antigen-induced arthritis. Agents Actions. 1994;41:96–8.
Thoss K, Henzgen S, Petrow PK, Katenkamp D, Bräuer R. Immunomodulation of rat antigen-induced arthritis by leflunomide alone and in combination with cyclosporine A. Inflamm Res. 1996;45:103–7.
Oelzner P, Bräuer R, Henzgen S, Thoss K, Wünsche B, Hersmann G, et al. Periarticular bone alterations in chronic antigen-induced arthritis: Free and liposome-encapsulated clodronate prevent loss of bone mass in the secondary spongiosa. Clin Immunol. 1999;90:79–88.
Oelzner P, Kunze A, Henzgen S, Thoss K, Hein G, Stein G, et al. High-dose clodronate therapy prevents joint destruction in chronic antigen-induced arthritis of the rat but inhibits bone formation at the axial skeleton. Inflamm Res. 2000;49:424–33.
Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–10.
Campagnuolo G, Bolon B, Feige U. Kinetics of bone protection by recombinant osteoprotegerin therapy in Lewis rats with adjuvant arthritis. Arthritis Rheum. 2002;46:1926–36.
Delling G. Endocrine bone diseases. Stuttgart: Fischer; 1975. p. 3–33.
Baron R. Anatomy and ultrastructure of bone. In: Favus MJ, editor. Primer on the metabolic bone diseases and disorders of mineral metabolism. New York: Raven; 1993. p. 3–9.
Murakami H, Nakamura T, Tsurukami H, Abe M, Barbier A, Suzuki K. Effects of tiludronate on bone mass, structure and turnover at the epiphyseal, primary and secondary spongiosa in the proximal tibia of growing rats after sciatic neurectomy. J Bone Miner Res. 1994;9:1355–64.
Malluche HH, Faugere MC. Atlas of mineralized bone histology. Basel: Karger, 1986.
Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, et al. Bone histomorphometry: Standardization of nomenclature, symbols and units. Report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res. 1987;2:595–610.
Ma YF, Jee WS, Ke HZ, Lin BY, Liang XG, Li M, et al. Human parathyroid hormone (1–38) restores cancellous bone to the immobilized, osteopenic proximal tibial metaphysis in rats. J Bone Miner Res. 1995;10:496–505.
Erben RG, Kohn B, Rambeck WA, Zucker H. Histomorphometric analysis of the rat proximal tibial metaphysis by “linear scanning”. Scanning Microsc. 1990;4:625–38.
Einspruch EL. An introductory guide to SPSS for windows. London: Sage; 2005.
Makrygiannakis D, af Klint E, Catrina SB, Botusan IR, Klareskog E, Klareskog L, et al. Intraarticular corticosteroids decrease synovial RANKL expression in inflammatory arthritis. Arthritis Rheum. 2006;54:1463–72.
Brandstrom H, Jonsson KB, Vidal O, Ljunghall S, Ohlsson C, Ljunggren O. Tumor necrosis factor-alpha and -beta upregulate the levels of osteoprotegerin mRNA in human osteosarcoma MG-63 cells. Biochem Biophys Res Commun. 1998;248:454–7.
Hofbauer LC, Lacey DL, Dunstan CR, Spelsberg TC, Riggs BL, Khosla S. Interleukin-1beta and tumor necrosis factor-alpha, but not interleukin-6, stimulate osteoprotegerin ligand gene expression in human osteoblastic cells. Bone. 1999;25:255–9.
Yano K, Nakagawa N, Yasuda H, Tsuda E, Higashio K. Synovial cells from a patient with rheumatoid arthritis produce osteoclastogenesis inhibitory factor/osteoprotegerin: reciprocal regulation of the production by inflammatory cytokines and basic fibroblast growth factor. J Bone Miner Metab. 2001;19:365–72.
Kubota A, Hasegawa K, Suguro T, Koshihara Y. Tumor necrosis factor-alpha promotes the expression of osteoprotegerin in rheumatoid synovial fibroblasts. J Rheumatol. 2004;31:426–35.
Granet C, Maslinski W, Miossec P. Increased AP-1 and NF-kappaB activation and recruitment with the combination of the proinflammatory cytokines IL-1beta, tumor necrosis factor alpha and IL-17 in rheumatoid synoviocytes. Arthritis Res Ther. 2004;6:R190–8.
Tunyogi-Csapo M, Kis-Toth K, Radacs M, Farkas B, Jacobs JJ, Finnegan A, et al. Cytokine-controlled RANKL and osteoprotegerin expression by human and mouse synovial fibroblasts: Fibroblast-mediated pathologic bone resorption. Arthritis Rheum. 2008;58:2397–408.
Stolina M, Adamu S, Ominsky M, Dwyer D, Asuncion F, Geng Z, et al. RANKL is a marker and mediator of local and systemic bone loss in two rat models of inflammatory arthritis. J Bone Miner Res. 2005;20:1756–65.
Loveridge N, Farquharson C, Palmer R, Lobley GE, Flint DJ. Growth hormone and longitudinal bone growth in vivo: short-term effect of a growth hormone antiserum. J Endocrinol. 1995;146:55–62.
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: J. Di Battista.
Rights and permissions
About this article
Cite this article
Oelzner, P., Fleissner-Richter, S., Bräuer, R. et al. Combination therapy with dexamethasone and osteoprotegerin protects against arthritis-induced bone alterations in antigen-induced arthritis of the rat. Inflamm. Res. 59, 731–741 (2010). https://doi.org/10.1007/s00011-010-0184-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00011-010-0184-6