Abstract
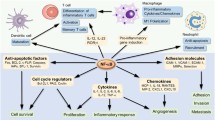
The nuclear factor (NF)-κB family of transcription factors are ubiquitous and pleiotropic molecules that regulate the expression of more than 150 genes involved in a broad range of processes including inflammation, immunity, cell proliferation, differentiation, and survival. The chronic activation or dysregulation of NF-κB signaling is the central cause of pathogenesis in many disease conditions and, therefore, NF-κB is a major focus of therapeutic intervention. Because of this, understanding the relationship between NF-κB and the induction of various downstream signaling molecules is imperative. In this review, we provide an updated synopsis of the role of NF-κB in DNA repair and in various ailments including cardiovascular diseases, HIV infection, asthma, herpes simplex virus infection, chronic obstructive pulmonary disease, and cancer. Furthermore, we also discuss the specific targets for selective inhibitors and future therapeutic strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In multicellular organisms, the precise control of gene expression is a key for initiating and maintaining cell differentiation. In eukaryotes, transcription factors, which are sequence-specific DNA-binding proteins that control the passage of genetic information from DNA to RNA, are central to this regulation. Nuclear factor (NF)-κB is a transcription factor ubiquitous in distribution that controls the expression of genes involved in cell cycle, immune responses, cell and tissue differentiation, and DNA repair. Regulator of more than 150 genes, NF-κB is induced by a multitude of stimulants including bacterial and viral antigens, T and B cells, mitogens, cytokines, oxidized low-density lipoprotein and free radicals like superoxide O2 −, and nitric oxide. First identified through its binding to the DNA sequence in the regulatory region of immunoglobulin κ light chain gene in B cells, the most important role NF-κB plays in the biological system is in regulating immune responses (Baltimore 2009; Ma et al. 2011). Since first identified, five mammalian NF-κB family members have been discovered: NF-κB 1 (p50/p105), NF-κB 2 (p52/p100), RelA (p65), RelB, and c-Rel. NF-κB family members share a highly conserved 300-amino acid Rel homology domain, responsible for their dimerization and binding to DNA and IκB (inhibitor of NF-κB). Transcriptional regulation by NF-κB occurs only after two members form a dimer, with the most abundant activated form consisting of a p50 subunit and a p65 subunit, which is found in almost all cell types; however, the existence and relevance of all possible dimer combinations have not been clearly demonstrated. In contrast to p50/65, other NF-κB dimers (p65/p65, p65/c-Rel, p65/p52, c-Rel/c-Rel, p52/c-Rel, p50/c-Rel, p50/p50, RelB/p50, and RelB/p52) have been found to exist, with some only in specific subset of cells (Hayden and Ghosh 2004). Of these, only p65, RelB, and c-Rel contain c-terminal transactivation domains, which are binding sites for other transcription co-regulators that may be co-activators or co-repressors. Not all Rel dimer combinations are transcriptionally active. In fact, studies have shown that DNA-bound p50 and p52 homo- and heterodimers repress κB-dependent transcription, possibly by preventing the binding of transcriptionally active NF-κB dimers to κB sites, or through recruitment of deacetylases to promoter regions (Zhong et al. 2002).
Due to the presence of ankyrin repeats at their C-terminus, the p105 and p100 precursors can inhibit nuclear localization and activation of NF-κB dimers that they associate with, and can thus be classified as IκB proteins. Binding of IκB prevents the translocation of NF-κB to the nucleus. Upstream activation signals induce the phosphorylation of IκB by IκB kinase (IKK), which triggers the degradation of IκB through the ubiquitin system allowing free NF-κB to translocate to the nucleus and activate transcription. Thus, regulatory mechanisms associated with the activation of NF-κB include nuclear localization and export signals, phosphorylation, and proteolytic processing.
The IKK complex is composed of an IKK-α/IKK-β heterodimer associated with the regulatory subunit IKK-γ (NEMO) (Biswas et al. 2004; Lemmers et al. 2007). Activation of the IKK complex leads to the phosphorylation and subsequent degradation of IκB proteins (IκBα, IκBβ and IκBε) (Staal et al. 2011). IκBβ binds with NF-κB-DNA complexes and regulates the activity of transcription factor. Whereas, the expression and function of IκBε has been reported to limit in cells of hematopoetic lineage suggesting unique roles of transcription factor in response to variety of stimulus in different cells.
Both canonical and non-canonical pathways lead to the activation of NF-κB, with the common regulatory step in both being the activation of an IKK complex. In the canonical signaling pathway, binding of ligand to a surface receptor leads to the recruitment and activation of an IKK complex (Gilmore 2006). The IKK complex then phosphorylates IκB leading to its proteasomal degradation and NF-κB translocates to the nucleus to activate target genes. In the non-canonical pathway, ligand binding to the receptor leads to the activation of NF-κB-inducing kinase (NIK), which functions with IKKα leading to phosphorylation-dependent ubiquitination and processing of p100 and formation of the p52/RelB active heterodimers (Hayden and Ghosh 2008).
Dysregulation or alteration of NF-κB signaling is the underlying cause of various ailments. Consequently, understanding the relationship between NF-κB and the induction of various downstream signaling molecules is imperative. Generally, NF-κB acts as a switch or a sensor that turns on genes responsible for the inflammatory response to various stimuli including infectious agents or free radicals; however, NF-κB can inadvertently become a driving force of pathogenic change in the event of its regulatory malfunction (Grilli and Memo 1999). Activators of NF-κB include free fatty acids, reactive oxygen species (ROS), and pro-inflammatory cytokines as well as viral proteins that inhibit apoptosis such as infected cell protein (ICP)4 and ICP27. Exposure to lipopolysaccharide (LPS), a component of the outer membrane of Gram-negative bacteria and of cigarette smoke, has been shown to increase the activation of NF-κB in a wide variety of model systems. Furthermore, epidermal growth factor (EGF) and the DNA damage sensor poly-(ADP-ribose) polymerase-1 (PARP1) also regulate NF-κB activation. Based on the involvement of transcription factor NF-κB in diverse pathways and disease states, it is essential to understand its selectivity, activity, and the transcriptional potential. In this review, we will highlight the role of NF-κB in various ailments including cardiovascular disease, HIV infection, asthma, herpes simplex virus infection, chronic obstructive pulmonary disease (COPD), and cancer (Table 1).
NF-κB and Cardiovascular Diseases
NF-κB expression correlates with numerous cardiovascular diseases including atherosclerosis, myocardial ischemia, heart failure, and cardiac hypertrophy. Moreover, the activity of NF-κB can either be beneficial or detrimental to the cardiovascular system. For example, the inhibition of NF-κB by its inhibitor BAY 11-7085 during the developmental stage of the chicken heart results in cardiac outflow defects, suggesting a crucial function of NF-κB in development of the heart (Hernandez-Gutierrez et al. 2006). BAY 11-7085 is an IKK inhibitor that prevents phosphorylation of IKK, thereby inhibiting the interaction between IKK and IκB and subsequently translocation of NF-κB to the nucleus where it exerts its biological activity (Fig. 1a). On the other hand, increased expression of NF-κB has been found in the atheromatous area of the atherosclerotic lesion whereas NF-κB activation was undetectable in vessels lacking atherosclerosis. Atherosclerosis is characterized by the deposition of lipids in arterial walls, which leads to myocardial infarction (Hollander 1976). In addition, leukocyte recruitment and adhesion within the endothelial layer of blood vessels is an important step and the hallmark of atherosclerosis. The expression of adhesion molecules, which is regulated by NF-κB in response to CD40, interleukin (IL)-1, and tumor necrosis factor (TNF)-α in endothelial cells, allows leukocytes to stick to the artery wall. In experimental models of atherosclerosis, the inhibition of pro-inflammatory cytokines like TNF-α leads to diminished expression of adhesion proteins, intercellular adhesion molecule (ICAM)-1 and vascular cell adhesion molecule (VCAM)-1, resulting in reduced disease progression (Bourdillon et al. 2000; Branen et al. 2004; Collins et al. 2000; Nakashima et al. 1998) (Fig. 1b). Moreover, NF-κB pathways are activated in most cells involved in the different stages of lesion development (de Winther et al. 2005). Activation of the NF-κB pathway by TNF-α plays critical role in the progression of heart failure (HF). Further, the elevation of TNF-α levels is linked with myocyte hypertrophy, remodeling of the extracellular matrix (ECM) with increased fibrosis and apoptosis (Hutchinson et al. 2010). Pentoxifylline, a xanthine-derived agent, is a phosphodiesterase inhibitor that downregulates TNF-α synthesis at the transcriptional level. Further, it protects the myocardium from apoptosis and thus inhibits the progression of HF. The TNF-α converting enzyme (TACE) is required for processing of pro-TNF-α into its mature form, and anti-TNF-α treatment strategies including metalloproteinase inhibitors and aprotinin have been shown to decrease TNF-α processing by inhibiting TACE (Balakumar and Singh 2006). TNF-α activates p38 MAPK pathway, which leads to the progression of matrix remodeling and inflammation. Experimental evidence shows that p38 inhibitors like SB203580 and FR167653 reduce apoptosis, fibrosis, hypertrophy, LV dilatation and increased ejection fraction and contractility (Hutchinson et al. 2010). Furthermore, increased expression of TNF-α was found to increase the gelatinolytic activity of the metalloproteinase that causes fibrosis and remodeling through direct digestion of matrix components (Li et al. 2000). Therefore, including TNF-α, targeting of metalloproteinase is an important therapeutic approach to mitigate cardiovascular disease. In this regard, batimastat, ilomastat, marimastat, and prinomastat have been developed for HF. The existing clinical studies seem to support the idea that treatment with anti-TNF-α may have a favorable effect on endothelial dysfunction and atherosclerotic processes in patients with inflammatory arthropathies; however, TNF-α is required for physiological processes (Li et al. 2000). Therefore, drug design will require selective inhibition of this inflammatory mediator so that its function is not reduced below required levels. To this end, the selective inhibition of tumor necrosis factor receptor (TNFR)-1 with specific antibodies, antagonists, or siRNAs provides a promising opportunity for treatment of the proinflammatory, proatherogenic effects of TNF-α, keeping intact the TNFR2-mediated cardio-protective immunomodulatory responses.
NF-κB and cardiovascular diseases. a BAY11-7085 irreversibly inhibits TNF-α-stimulated IκBα-phosphorylation and the administration of cholecalciferol markedly attenuated the advancement of cardiac hypertrophy via downregulation of the TNF-α/NF-κB/p65 signaling pathway. b The expression of adhesion molecules, which is regulated by NF-κB in response to CD40, IL-1, and TNF-α in endothelial cells, allows leukocytes to stick to the artery wall in atherosclerosis. MCPIP protects heart from inflammatory responses by obstructing IκB kinase complex activity and by inhibiting NF-κB activation. c The stimulation of TLRs leads to downstream signaling events and activation of NF-κB. TLR4 and NF-κB regulate the expression of many inflammatory cytokines involved in the inflammatory response to myocardial injury
The zinc-finger protein monocyte chemotactic protein (MCP)-1-induced protein (MCPIP) is expressed primarily in monocytes, and is known to protect the heart from inflammatory pathologies in septic myocardial inflammation and dysfunction. In vivo and in vitro studies have shown an NF-κB inhibitory activity of MCPIP. In fact, MCPIP transgenic mice showed a distinct reduction in myocardial inflammatory cytokines TNF-α, IL-1β, IL-6, decreased caspase-3/7 activities, inducible nitric oxide synthase (iNOS) expression and peroxynitrite formation, and less apoptotic cell death following LPS administration as compared to wild-type mice. Decreased cardiac NF-κB activation was also reported in LPS-treated MCPIP transgenic mice compared to wild-type mice. Moreover, enforced expression of MCPIP resulted in decreased phosphorylation of IκB kinase (IKKα/β), and as a result in reduced NF-κB p65 translocation into the nucleus and the subsequent attenuation of inflammation (Niu et al. 2011).
Many compounds show a protective role in cardiovascular diseases by inhibiting or downregulating NF-κB activation. Astragaloside (AsIV), small molecular saponin derived from the medicinal plant Astragalus membranaceus, has been shown to produce anti-inflammatory and anti-apoptotic effects in myocardial ischemia–reperfusion (MI/R) injury model in rats (Lu et al. 2015). AsIV downregulates the expression of TLR4 and NF-κB leading to reduced apoptosis of cardiomyocytes and decreased MI/R injury-induced inflammatory cytokines (TNF-α, IL-6), subsequently attenuating the injury in rats. Cholecalciferol (Vit-D3) exerts cardioprotective effects through the regulation of mRNA levels of pro-inflammatory cytokines, which may play an important role in the development and advancement of left ventricle (LV) hypertrophy (Fig. 1c). Further, IκBα mRNA was increased in tissues from LV of rats treated with Vit-D3 compared to controls. However, identification of the mechanisms underlying the protective role of this compound will require further investigation (Al-Rasheed et al. 2015).
The role of oxidative stress in the pathogenesis of cardiovascular diseases has also been described extensively (Dhalla et al. 2000). During the onset and progression of hypertension, stroke, and atherosclerosis, the activity of NADPH oxidase increases leading to the production of ROS (Ceconi et al. 2003) (Fig. 1c). Amongst the various stimuli of NF-κB, ROS play a powerful role. The NADPH oxidase complex is considered to be the major source of superoxide while other sources of ROS production include xanthine–xanthine oxidase, cyclooxygenase, NOS, and oxidative phosphorylation. Xanthine oxidase converts hypoxanthine to xanthine and xanthine to urate. Urate acts as a potent antioxidant to attenuate adverse effect of ROS on host tissue. On the other hand, it also upregulates virulence genes including mftR (major facilitator transport regulator) and mftP (major facilitator transport protein) (Gupta and Grove 2014). Furthermore, NADPH oxidase is highly expressed in many cardiovascular cells including smooth muscle cells, pericytes, fibroblasts, and cardiac myocytes (Lassegue et al. 2012; Panday et al. 2015a). Reports suggest NADPH oxidase/ROS-mediated activation of NF-κB under various experimental conditions (Bonizzi et al. 1999; Brar et al. 2002; Clark and Valente 2004). Induction of NF-κB has been shown in K562 human erythroleukemia cells after transfection with gp91phox (an essential component of NADPH oxidase) construct (Clark and Valente 2004), and siRNA-mediated silencing of p22phox (an important subunit of NADPH oxidase) significantly attributes to the overexpression of the NF-κB inhibitor IκBα in human airway smooth muscle cells (Brar et al. 2002). This strongly indicates a relationship between NADPH oxidase and NF-κB activation and their role in cardiovascular diseases.
Interestingly, microRNAs (miRs) which are small non-coding RNAs, potentially regulate genes related to various physiological processes and the pathophysiology of diseases including cardiovascular diseases such as atherosclerosis and hypertension (Jansen et al. 2015; Ma et al. 2011; Madrigal-Matute et al. 2013; Wahid et al. 2010). MiRs-181, 21, 155 and 301a are known to control the expression of NF-κB (Jansen et al. 2015). In addition, MiR-181 has been shown to modulate endothelial VCAM-1 and E-selectin expression in an NF-κB-dependent way while other miRs are associated in the complex control of NF-κB activation. Moreover, substantial evidence has shown that miRs play an important role in modulating the vascular aging processes in humans and animal models. Expansion upon this research is needed to further explore the functions of miRs in age-associated vascular pathologies (Jansen et al. 2015; Ma et al. 2011).
Although the role of NF-κB has been extensively studied in cardiovascular disease, there is still the need for further investigations. Identification of the molecular mechanisms by which NF-κB inhibitors exert their effects and the identification of their specific targets are important areas for future investigations. For example, determination of the molecular mechanisms associated with specific ROS intermediates that mediate activation of NF-κB and further downstream events, as well as identification of the mechanisms underlying the induction of cell adhesion molecules in response to NF-κB activation will be extremely beneficial in designing improved therapeutic strategies.
NF-κB and HIV
Human immunodeficiency virus (HIV) causes acquired immunodeficiency syndromes (AIDS) in which the immune system is greatly compromised leading to the loss of the ability to fight infections. HIV infects essential immune cells including CD4 T cells, macrophages, and dendritic cells resulting in massive cell loss due to apoptosis. Viral proteins processed by the HIV protease, including gp120 and Tat, augment cell death. Apoptosis is induced in HIV-infected cells through both extrinsic and intrinsic pathways including procaspase-8 and 9, downstream regulation of Bcl-2, mitochondrial cytochrome c release, shortening of telomeres, integration of HIV-1 genes, and the activation of NF-κB.
The NF-κB pathway has been identified as a possible target for permanent HIV-1 suppression. Hsp90 is a heat-shock protein that localizes to viral promoter DNA and is required for HIV-1 transcription and reactivation from latency (Zhong et al. 2014) under conditions of hyperthermia. Moreover, it has been reported that Hsp90 and co-chaperone Cdc37 bind to the IKK complex keeping this complex functional (Anderson et al. 2014). Thus, the reactivation of HIV-1 from latency is modulated by the NF-κB pathway through the connection of T cell activation with HIV-1 replication. The Hsp90 inhibitor AUY922 has been evaluated in clinical trials as a potential cancer chemotherapeutic; however, its clinical effectiveness in HIV-1 suppression still remains to be tested (Dahabieh et al. 2015).
Long terminal repeats (LTRs) serve as control centers for viral gene expression (Karn and Stoltzfus 2012). Followed by partially being transcribed into RNA, LTRs are reverse transcribed into complementary DNA, thereafter mediating integration of retroviral DNA into the host genome through LTR-specific integrase. Once the provirus has been integrated, the LTR on the 5′ end serves as the promoter for the entire retroviral genome. LTRs consist of functional regions including the TAR (transactivation response element), core, enhancer, and modulatory elements. The TAR region binds the viral transactivator Tat and the core region contains the initiator and TATA box as well as three Sp-1 binding sites. The enhancer region consists of two adjacent NF-κB-binding sites (–109 and –79), which play indispensable roles in mediating HIV-1 gene expression (Dahiya et al. 2014b). In addition, NFAT1 negatively regulates the LTR by competing with NF-κB for its binding site, whereas NFAT2 positively regulates the HIV-1 LTR through the NF-κB-binding sites (Dahiya et al. 2014a) (Fig. 2a). Thus, viral LTRs are regulated by the host factor NF-κB upon its activation in virus-infected cells resulting in increased expression from the HIV-1 LTR. HIV infection is associated with a decline in the numbers of CD4+ T lymphocytes. Procaspase-8, which activates NF-κB, also plays an indispensable role in inducing apoptosis of HIV-infected CD4+ T cells. The HIV protease cleaves BCL-2 and procaspase-8, with cytotoxic effects produced by other HIV proteins (Weng et al. 2014). The cleavage of procaspase-8 performed by HIV-1 protease gives rise to a unique 41 kDa protein fragment known as Casp8p41. Under homeostatic conditions the anti-apoptotic Bcl-2 family members maintain mitochondrial integrity by preventing the pro-apoptotic, multidomain Bcl-2 family members, Bax and Bak, from causing mitochondrial damage. Bcl-2 homology 3 proteins are activated by pro-caspase-8. Consequently, the inhibition of Bax and Bak is relieved, leading to their oligomerization and formation of a channel through which cytochrome c is released into the cytosol (Brubaker et al. 2015). Cytochrome c then associates with Apaf-1 and ATP, forming a platform for recruitment and activation of procaspase-9, a complex also known as the apoptosome. Active caspase-9 cleaves and activates the downstream caspases that are crucial for the execution of apoptotic cell death. Thus, procaspase-8 is responsible for NF-κB-mediated HIV LTR expression and the apoptosis of HIV-infected CD4 T cells (Fig. 2b).
NF-κB and HIV. a LTRs consist of functional regions including the TAR, core, enhancer, and modulatory elements. The TAR region binds the viral transactivator Tat and the core region contains the initiator and TATA box as well as three Sp-1-binding sites. The enhancer region consists of two adjacent NF-κB-binding sites (–109 and –79), which play indispensable roles in mediating HIV-1 gene expression. b HIV infection is associated with a decline in the numbers of CD4+ T lymphocytes. Procaspase-8 which activates NF-κB also plays an indispensable role in inducing apoptosis of HIV-infected CD4 T cells. c A common ailment in HIV-infected patients is HIV-associated neurocognitive disorder (HAND), also known as HIV dementia, which occurs when HIV infects nerve cells
HIV-1 primarily resides in both macrophages and microglia and thus a common ailment in HIV-infected patients is HIV-associated neurocognitive disorder (HAND), also known as HIV dementia, which occurs when HIV infects nerve cells (McArthur and Brew 2010). The ability to cross the blood–brain barrier is a critical element for successful infection of the central nervous system (CNS) by HIV, which is achieved by HIV mainly through the successful migration of monocytes and lymphocytes. Once HIV-1-infected macrophages have found safe harbor in the brain, they secrete chemokines such as stromal-derived factor (SDF)-1, RANTES or MIP-1 α/β, thereby inducing recruitment of additional monocytic cells from the periphery into the CNS. HIV-1 interacts with chemokine receptors CXCR4 and CCR5 in conjunction with CD4 to infect both microglia and macrophages (Ambegaokar and Kolson 2014). Natural ligands of CXCR4 and CCR5, the α-chemokine SDF-1 and the β-chemokines MIP-1 α/β or RANTES, interfere with HIV/gp120 binding and signaling and pro-inflammatory events (Ambegaokar and Kolson 2014) (Fig. 2c). Release of pro-inflammatory cytokines (such as IL-1β, IL-6, TNF-α) and chemokines leads to activation of microglia, a reduction in synaptic/dendritic density, and selective neuronal loss. Together, these events cause neurological complications characterized by impairment of the nerves involved in attention, memory, language and speech, symptoms which are seen in more than half of all adults suffering from AIDS (Doyle et al. 2013; Heaton et al. 2011). In addition, this neurotoxicity involves the production of neurotoxic factors including quinolinic acid, TNF, platelet-activating factor, and arachidonic acid metabolites in association with macrophage infection. Many experts have proposed that a common end pathway of toxicity is through excitation of N-methyl-d-aspartate (NMDA)-subtype glutamate receptors, which has the potential for inducing apoptosis. Support for this model includes experiments that show decreased glutamate secretion by infected macrophages is partially protective in an in vitro model of macrophage-mediated neurotoxicity. Moreover, toxicity associated with viral proteins is dependent on expression of glutamate receptors and the areas that are most affected are associated with an increased concentration of NMDA receptors (Guo et al. 2014).
Astrocytes represent a major population of non-neuronal cells in the brain and play a critical role in the neuropathogenesis of HAND (Spudich and González-Scarano 2012). These cells express the HIV co-receptors CXCR4 and CCR5 in addition to other chemokine receptors, but lack CD4 (Brown et al. 2014). Therefore, astrocyte reactivity may be influenced by the natural chemokine receptor ligands and thus stimulated by CD4-independent effects of HIV/gp120. Further, astrocytes are activated by and are responsible for overexpression of the inflammatory cytokine IL-6, which is associated with many pathological conditions (Shah et al. 2011). HIV gp120 is responsible for virus entry into cells where its expression increases the phosphorylation of endogenous IκBα and subsequent translocation of NF-κB into the nucleus leading to overexpression of IL-6 and IL-8 (Shah et al. 2011). Moreover, treatment of astrocytes from mice with gp120 also leads to increased production of pro-inflammatory cytokines including IL-1β and TNF-α (Hoffmann and Baltimore 2006; Kedzierska and Crowe 2001; Mogensen and Paludan 2001).
NF-κB of host has binding sites on LTR and has a role in HIV gene expression. To design potential therapeutic, it is important to study the structural basis of this interaction and to find out the subunit of NF-κB that directly interacts with the enhancer region of LTR. Furthermore, LTR region has the binding sites for the transcriptional activator protein, it may be interesting to search for a repressor binding site and use this approach as therapeutic potential. Moreover, it may be interesting to investigate the role of epigenetic modifications on NF-κB binding to the enhancer region of LTR. Since the activation of NF-κB is related to several pathological conditions, use of inhibitors which function on upstream kinases like SC-514, a specific inhibitor of the IKK-2 pathway, or BAY11-7082, which blocks NF-κB activation by inhibiting TNF-α induced phosphorylation of IKK (IKKβ) or downstream effectors, represent attractive therapeutic targets. Use of NF-κB-specific siRNA can also be utilized as alternate approach for the treatment of HIV-infected patients (Gangwani et al. 2013; Shah and Kumar 2010).
NF-κB and Asthma
Asthma is responsible for a significant number of mortalities worldwide. Characterized by recurring episodes of wheezing, chest pain, shortness of breath, and coughing in affected individuals, asthma is a result of the infiltration of eosinophils, mast cells, and macrophages into narrow airways resulting in airway obstruction. NF-κB is one of the major players underlying the progression of this disease. In fact, mouse studies have revealed that in the absence of the p50 or c-Rel subunits of NF-κB, mice do not develop hypersensitive airway inflammation (Janssen-Heininger et al. 2009). In response to histamine release by mast cells, NF-κB is activated and mediates upregulation of various pro-inflammatory cytokines (including TNF-α, IL-1β, IL-4, IL-5, MCP-1, GM-CSF), and adhesion molecules including VCAM-1 and ICAM, contributing to the chronic inflammation and subsequent obstruction of airways (Fig. 3) (Li and Verma 2002; Rico-Rosillo and Vega-Robledo 2011). Furthermore, activation of iNOS and cyclooxygenase-2 by NF-κB is a hallmark of asthma. Moreover, NF-κB acts synergistically with the transcriptional factor activator protein-1 (AP-1) to induce maximal expression of genes responsible for the pathogenesis of asthma (Roth and Black 2006). Thus, NF-κB is an attractive and important therapeutic target for treatment of asthma. To this end, various plant-derived compounds including quercetins, epigallocatechins, resveratrol, and lignans which belong to various classes of polyphenols have been studied as potential inhibitors of NF-κB (Nam 2006). Intraperitoneal administration of andrographolide, a derivative compound of the medicinal plant Andrographis paniculata, resulted in reduced infiltration of neutrophils, eosinophils, and macrophages as well as decreased IL-4, IL-5, and IL-13 in the bronchoalveolar lavage fluid of an allergic asthma mouse model (Rico-Rosillo and Vega-Robledo 2011). Moreover, andrographolide was shown to suppress the translocation of NF-κB to the nucleus resulting in the reduction of inflammation and cellular infiltration (Li et al. 2009). A recent report suggests the use of a novel cinnamate derived from Piper longum [ethyl 3′, 4′, 5′-trimethoxythionocinnamate (ETMTC)] as a treatment for allergic asthma (Kumar et al. 2013). The effect of ETMTC was assessed in an ovalbumin-sensitized and challenged murine model where it reduced airway inflammation and hypersensitivity. This amelioration of disease symptoms was found to be associated with reduced activation of NF-κB, expression of Th2 cytokines, and cell adhesion molecules. ETMTC, a potent inhibitor of cell adhesion molecules, functions by inhibiting TNF-α-induced nuclear translocation and activation of NF-κB due to its ability to block IκB kinase activity. Thus, ETMTC is considered as potential therapeutic for treatment of asthma (Fig. 3).
NF-κB and asthma. In response to histamine release by mast cells, NF-κB is activated and mediates upregulation of various pro-inflammatory cytokines (including TNF-α, IL-1β, IL-4, IL-5, MCP-1, GM-CSF, and adhesion molecules including VCAM-1, and ICAM) contributing to the chronic inflammation and subsequent obstruction of airways
The effect of dehydroxymethylepoxy quinomycin (DHMEQ), an NF-κB inhibitor, was studied in BALB/c mice challenged intraperitoneally with ovalbumin. DHMEQ is a unique inhibitor of NF-κB which acts at the level of the nuclear translocation, it inhibits the translocation of NF-κB to the nucleus without effecting IκB. DHMEQ directly binds to the canonical and non-canonical NF-κB components to inhibit their DNA binding. The inhibitory effect of DHMEQ is more potent on the NF-κB1/RelA heterodimers than on the NF-κB (p50) homodimer. Indicators of asthma, including airway hyper-responsiveness, eosinophilic airway inflammation, and levels of Th2 cytokines in bronchoalveolar lavage fluid, were mitigated by treatment with DHMEQ (Shimizu et al. 2012) (Fig. 3). Similarly, aspirin and salicylate affect NF-κB signaling resulting in the suppression of TNF-α-induced mRNA synthesis and surface expression of VCAM-1 and ICAM-1 on endothelial cells thereby preventing leukocyte recruitment and transendothelial migration (Pierce et al. 1996; Yamamoto and Gaynor 2001). Both aspirin and sodium salicylate inhibit ATP binding to IKKβ and thus prevent its degradation and subsequent activation of the NF-κB pathway (Yin et al. 1998). Furthermore, bronchial biopsies from asthma patients treated with budesonide (a glucocorticoid steroid) exhibit a reduction in binding affinity between NF-κB and DNA (Wilson et al. 2001). Targeting IKKβ using TPCA-1 (2-[(aminocarbonyl)amino]-5-[4-fluorophenyl]-3-thiophenecarboxamide, a small molecular weight inhibitor) resulted in reproducible anti-inflammatory activity in response to a wide range of stimuli in human airway smooth muscle cells and a rat model of asthma (Birrell et al. 2005). Yet, another study used the proteasome inhibitor MG-132 to target IκB-mediated activation of NF-κB and assessed its effect on human airway smooth muscle cells (ASMs; the principle immunomodulatory cells in asthma). Short-term pretreatment of ASM cells with MG-132 resulted in a reduction of TNF-α-induced IL-6 due to inhibition of IκBα degradation and thus activation of NF-κB (Moutzouris et al. 2010) (Fig. 3). Therefore, the proteasome is also a potential therapeutic target for blocking NF-κB-mediated inflammatory pathways in asthmatics. The anti-inflammatory actions of glucocorticoids (GCs) play an important role in treatment of asthma and involves the inhibition of NF-κB-induced gene transcription. Ligand-bound GC receptors (GRs) bind to suppress the transcription of NF-κB responsive genes. Thus, the therapeutic efficacy of GCs is largely contributed by GC inhibition on the transcription factor NF-κB (Nelson et al. 2003).
With the advances made in bioinformatics and computational modelling, the analysis of disease models has become more focused. A recent study utilized an asthma model to design an interactive network comprised of genes that are differentially expressed in asthmatics. This facilitated identification of factors whose perturbations could lead to disease symptoms and also provided validation of their functional and pathophysiological roles. One of such factors identified through this analysis was GAB1 (GRB-associated binder-1; an adaptor protein which serves as immediate substrates for tyrosine kinases and a link between receptors and downstream events) which was shown to activate NF-κB signaling leading to increased production of pro-inflammatory cytokines (Sharma et al. 2015). These findings are enticing, as this approach may lead to identification of novel drug targets, which are desperately needed considering the failure of glucocorticoid therapy in asthmatics due to glucocorticoid resistance.
Furthermore, macrolide antibiotics, which show bacteriostatic activity and affect inflammatory responses, have been shown to inhibit NF-κB activation resulting in diminished CXCL8 release and decreased neutrophilic inflammation (Barnes 2015). Additionally, these compounds may expand phagocytosis and efferocytosis, which is impeded in patients with COPD and extreme asthma (Mammen and Sethi 2012). In patients with serious neutrophilic asthma, a course of azithromycin was shown to fundamentally reduce sputum neutrophil counts and CXCL8 production. This finding suggests that it may be worthwhile to utilize a restorative trial of macrolide antibiotics in patients with extreme asthma who have transcendent neutrophilic aggravation (Barnes 2015).
Since traditional therapies have not proved beneficial for asthma treatment, recent studies using specific tyrosine kinase inhibitors are also yielding interesting results (Wong and Leong 2004). Furthermore, characterization of epigenetic changes associated with transcription factors like NF-κB can open doors for the prevention strategies and development of novel drugs. Determining the temporal regulation of epigenetic signatures associated with the disease progress can play critical role in designing future therapeutic strategies.
NF-κB and Infection with Herpes Simplex Virus Type 1
Herpes simplex virus (HSV)-1 is a member of Herpesviridae family responsible for the presentation of blisters on skin or mucous membranes of the mouth and/or genitals. Infection with HSV-1 induces both NF-κB and activation of the DNA double-strand break responses (DSB). While DSB are required for viral replication, the absence of NF-κB induction results in significantly reduced viral yields (Taddeo et al. 2004). The initial phase of HSV infection induces apoptosis of infected dendritic cells; however, subsequent protein synthesis in infected cells blocks this process by inhibiting caspases 3, 8, and 9 (Bosnjak et al. 2005; Goodkin et al. 2003).
HSV genome contains NF-κB-binding sites within the promoters of ICP0 and VP 16 Icp0 and vp16. In infected cells, NF-κB p60/p50 heterodimers are primarily activated and are required for efficient HSV replication. HSV has been shown to regulate NF-κB signaling in a number of ways. NF-κB activation occurs in two steps during viral infection, with the initial activation occurring following virus attachment and subsequent viral entry into cells, and delayed activation occurring following expression of apoptosis-preventing viral proteins such as ICP4 and ICP27 (Hargett et al. 2006). Activation of IKK and degradation of IκBα is observed during the early stage of HSV-1 infection, which induces the translocation of NF-κB from the cytoplasm to the nucleus (Fig. 4). This translocation is thought to prevent the stress-induced apoptosis associated with viral gene expression (Taddeo et al. 2004). Recent study showed that HSV glycoproteins gH/gL and gB are ligands for TLR2, although gH/gL is sufficient to initiate a signaling cascade leading to activation of NF-κB (Leoni et al. 2012). Additionally, the tegument protein UL37 of HSV-1 is able to directly activate NF-κB signaling through the TRAF6 adaptor protein in TLR2 independent manner. The second wave of NF-κB activation is dependent upon IκB turnover and is sustained for the length of infection. This wave occurs at later time point and is dependent on viral gene products. Moreover, the timing of NF-κB translocation into the nucleus during HSV-1 infection coincides with the inhibition of apoptosis (Egan et al. 2013; Leoni et al. 2012; Liu et al. 2013). Activation of NF-κB in HSV-1-infected dendritic cells is also dependent on the activation of protein kinase R which leads to phosphorylation of eIF-2α, a translation initiation factor that causes the inhibition of protein synthesis (Deng et al. 2004; Hargett et al. 2006) (Fig. 4). Therefore, activated NF-κB has been identified to have two roles; the first is to block apoptosis during the initial phase of infection, and the second is to enhance HSV-1 viral replication by inducing NF-κB-dependent cellular proteins (Goodkin et al. 2003).
NF-κB and infection with herpes simplex virus type 1 (HSV-1). Activation of IKK and degradation of IκBα is observed during the early stage of HSV-1 infection, which induces the translocation of NF-κB from the cytoplasm to the nucleus. The second wave of NF-κB activation is dependent on IκB turnover and is sustained for the length of infection. Activation of NF-κB in HSV-1-infected dendritic cells is also dependent on the activation of protein kinase R (PKR) which leads to phosphorylation of eIF-2α, a translation initiation factor that causes the inhibition of protein synthesis
In terms of therapeutic implications, several attempts have been made to attenuate HSV infection. Commonly used treatment against HSV infections includes nucleoside analogues such as acyclovir and pencyclovir and certain highly bioavailable prodrugs such as valacyclovir and famciclovir (Wutzler 1997). The major drawback of these drugs is the promotion of viral mutations that leads to the development of drug-resistant HSVs (Piret and Boivin 2011). Thus, because of this need, some Chinese medicines showing anti-viral activities have come into the picture. For example, N-docosanol, a long-chained alcohol, has anti-HSV activity and was approved by the Food and Drug Administration (FDA) as a topical treatment for herpes simplex labialis (Marcelletti 2002). More importantly, Houttuynia cordata (H. cordata), which is a traditional natural Chinese herbal medicine of the Saururaceae family, exerts inhibitory effects against HSV by targeting NF-κB (Li and Zhao 2015). H. cordata water extracts (HCWEs) affected multiple stages of HSV infection, such as viral binding and penetration and viral gene expression by targeting host NF-κB, and HSV replication (Hung et al. 2015). Moreover, HCWEs attenuate NF-κB-mediated ICP0 transcription. ICP0 is an HSV immediate-early gene, which can bind to several cellular proteins as well as viral promoters resulting in increased expression of viral E and L genes (Cai and Schaffer 1992). Further analysis of compounds in HCWEs revealed that quercetin and isoquercitrin inhibit NF-κB activation and also exert an inhibitory effect on viral entry (Hung et al. 2015).
Heparanse (HPSE) is an endo-β-d-glucuronidase that breaks down heparin sulfate (HS) polymers in the extracellular matrix and contributes to tissue redesign. According to studies conducted by Hadigal group on a human corneal epithelial cell line, the host heparan sulfate-degrading enzyme, HPSE, is upregulated through NF-κB and translocates to the cell surface upon HSV-1 disease for the evacuation of HS and to facilitate viral infection (Hadigal et al. 2015). Moreover, a significant change in HPSE release occurred in vivo during infection of murine corneas and the in vivo knockdown of HPSE hinders shedding. By and large this suggests that HPSE acts as a molecular switch for turning cells from a virus-permissive, attachment mode to a virus-deterring detachment mode. Since many human viruses use HS as an attachment receptor, the HPSE–HS interplay might outline a common mechanism for virus release. Recent studies have suggested that the overexpression of HPSE plays a role in various pathways including angiogenesis, tumor metastasis, and inflammation (Tiwari et al. 2015). HPSE disrupts epithelial and endothelial membranes producing expanded vascular porousness with leukocyte extravasation and releases HS-bound cytokines and growth factors, which are normally sequestered in the ECM, making them accessible for angiogenesis. In light of these recent discoveries, heparanase might be an extraordinary therapeutic target as heparanase inhibitors may diminish viral discharge and control the host inflammatory reaction. Therefore, targeting various dimeric forms of NF-κB at different stages of infection can be useful to design better therapeutic strategies.
NF-κB and COPD
COPD is a progressive pulmonary disease characterized by chronic, abnormal inflammation of the airways, which can be caused by several factors such as bacterial infection, exposure to smoke, and/or exposure to dust and allergens. COPD involves oxidative stress, programmed cell death, and impaired lung cell repair, which combined can lead to emphysema. NF-κB is known to play a key role in the pathogenesis of COPD through the induction of several of the inflammatory mediators that cause this disease (Kniss et al. 2001). Recent study demonstrated an increase in the number of macrophages and CD8+ and CD4+ T cells expressing NF-κB, STAT-4, and interferon γ in lung bronchial biopsies from patients with mild COPD and from healthy smokers (Di Stefano et al. 2009). In addition, exposure of the cytomembrane to cigarette smoke (CS) or LPS increases the expression of TLR4, phosphorylation of IKKα/β, activation of NF-κB, and degradation of IκBα (Meng et al. 2013). The increase in TLR4 expression is related to increase in NF-κB translocation into the nucleus, which causes pronounced IL-8 secretion, inflammatory cell infiltration, emphysema, and release of Mucin 5AC (MUC5AC) (Fig. 5a). MUC5AC is responsible for the mucus hypersecretion in the pulmonary tracts that is linked to COPD. In fact, CXCL8 has also been found to play a pivotal role in inducing expression of MUC5AC in COPD (Gilowska 2014). Similarly, three of the proteins released by neutrophils—S100A8, S100A9 and S100A12—have been recently identified to play a role in the increased production of MUC5AC in chronic inflammatory diseases like COPD and cystic fibrosis. These are being considered as potential biomarkers for these diseases and may be important for diagnostic purposes (Kang et al. 2015).
NF-κB and chronic obstructive pulmonary disease. a Exposure of the cytomembrane to cigarette smoke or LPS increases the expression of TLR4, phosphorylation of IKKα/β, activation of NF-κB activation, and degradation of IκBα degradation. b Another mechanism which can regulate NF-κB pathway and affect inflammation is through the activity of histone deacetylases and exposure to cigarette smoke abrogates HDAC2 activity due to increased expression of proteostasis mediators like VCP. In fact, the degradation of IκBα via VCP proteasomal degradation leads to overexpression of NF-κB
Another mechanism which can regulate NF-κB pathway and affect inflammation is through the activity of histone deacetylases. Exposure to cigarette smoke abrogates histone deacetylase 2 (HDAC2) activity due to increased expression of proteostasis mediators like valosin-containing protein (VCP). VCP is a major retrograde translocation factor that pulls out the proteins from estrogen receptor membrane that are destined for proteosomal degradation. Since VCP is upregulated while protein turnover is low in COPD, this may be an anticipated mechanism to induce protein aggregation that triggers chronic obstructive stress, inflammation and apoptosis leading to the pathogenesis of severe emphysema. VCP acts as an ubiquitin segregase that remodels protein complexes by extracting polyubiquitinated proteins for recycling or proteasomal degradation. Expression of proteostasis mediators in the presence of CS and LPS is one of the factors that correlate with the severity of COPD and presentation of emphysema, and a direct correlation has also been reported between higher expression of VCP and the severity of emphysema (Min et al. 2011). In addition, VCP induces pronounced expression of inflammatory stress mediators like NF-κB (Vij 2008) and increased expression of VCP is related to chronic inflammatory diseases like cystic fibrosis. VCP is important due to its role in protein extraction from the estrogen receptors (ER) and ubiquitin–proteasome-mediated protein degradation by endoplasmic reticulum-associated protein degradation. In fact, VCP-mediated proteasomal degradation of IκBα leads to increased activation of NF-κB. VCP also plays a role in proteasomal degradation of HDAC2 and Nrf2, which leads to corticosteroid resistance and oxidative stress, respectively, during emphysema (Ito et al. 2006) (Fig. 5b). An improved understanding of the proteostasis regulatory networks in the lung will open multiple new areas for the discovery of drugs and public health strategies to reduce the burden of lung disease. Ivacaftor has been suggested to protect against cigarette smoke-induced COPD by promoting airway hydration and mucociliary clearance. Therapies targeting histone acetyltransferases, particularly HDAC2, likely operate through proteostatic mechanisms (Balch et al. 2014). Even crude manipulations of proteostasis with bortezomib, an inhibitor of the 26S proteasome, have proven remarkably effective for the treatment of multiple myeloma and fibrosis in lung and skin (Mutlu et al. 2012). Proteostasis research in the lung could pave the way for rapid development of novel treatments for common lung diseases including COPD, idiopathic pulmonary fibrosis (IPF), acute lung injury (ALI) and others for which there are few or no specific therapies.
An approach to ameliorate NF-κB-dependent disease symptoms is to inhibit the activation of NF-κB. This inhibition can be achieved via interaction of peroxisome proliferator-activated receptor-α with the p65 and c-Jun subunits of NF-κB and AP-1, respectively, thereby regulating their transactivation and suppressing the expression of cytokines like IL-6 (Chen et al. 2014; Delerive et al. 1999). The expression of a mitochondrial sirutin, SIRT4, is markedly down-regulated in CS-treated human pulmonary endothelial cells. SIRT4 is a negative regulator of NF-κB activation via IL-1β and thus its overexpression protects endothelial cells against the CS-mediated oxidative stress response (Chen et al. 2014). These results suggest that the NF-κB signaling pathway and its mediators can serve as a therapeutic target for treatment of COPD. However, contradictory results regarding NF-κB signaling and COPD have emerged recently, forcing the re-evaluation of previous hypotheses. In yet another study, the role of NF-κB in CS-induced inflammation was studied using C57BL/6 mice with no increase observed in the DNA association of NF-κB either on 3 or 14 days of exposure (Rastrick et al. 2013). Furthermore, there was no significant change in neutrophil counts in bronchoalveolar lavage fluid from IKK-gene knock-out mice as compared to the wild-type mice. These observations suggest that CS-induced inflammation is actually independent of the NF-κB pathway. However, because the inflammatory response downstream of NF-κB signaling is quite complex, it is possible that multiple factors are actually involved which still need careful evaluation.
Interestingly, recent studies have shown that administration of antioxidants seems to be a rational adjunct for treatment of COPD. For example, administration of N-acetylcysteine (NAC) proved powerful in reducing oxidative biomarkers in peripheral blood and exhaled breath condensate and in lessening the rate of COPD intensifications (Zhang et al. 2015). Antioxidant protection in the tissues, specifically in the respiratory tract, is mainly provided by the glutathione, a redox-cycler thiol present in the epithelial lining fluid. However, antioxidant enzymes like superoxide dismutase and aldehyde dehydrogenase also tend to balance the oxidant and inflammatory reactions of cigarette smoke. Clinical studies have shown a dose-dependent effect of NAC in COPD patients (Cazzola et al. 2015; Sanguinetti 2015). Attributable to antioxidant properties, NAC is able to maintain redox-dependent cell signaling and transcription, and in particular, the regulation of NF-κB, p38 MAPK and other pro-inflammatory genes. This positive impact of NAC might be imparted by low NAC doses over prolonged periods of administration; however, it can be more quickly achieved when higher NAC daily doses are given, which likely exerts greater control over the activation of an inflammatory factor like NF-κB (Zhang et al. 2015).
NF-κB in DNA Damage and Cancer
To maintain genomic stability, cells deal with various DNA lesions including DNA double-strand break (DSB). DSBs are caused by a variety of agents, including ionizing radiation, chemical agents, ultraviolet light, and by-products of the cell’s own metabolism such as ROS. Studies reveal that every cell sustains a constant level of approximately 50,000 lesions. Among these, DSBs are notably lethal. If DNA becomes damaged and is not repaired properly, the resulting mutation significantly contributes to tumorigenesis and aging-related syndrome. Cells initiate DSB repair in the context of chromatin that involves eviction of histone and non-histone protein from the break site and affects the transcription of gene located at break site (Panday et al. 2015b; Panday and Grove 2016; Tsukuda et al. 2005). However, in response to severe DNA damage, cells choose the option of apoptosis to avoid tumor formation. Several reports show a connection between the NF-κB pathway and DNA damage response (Guo et al. 2013; Janssens and Tschopp 2006). An earlier report shows that DNA damage sensor PARP1 assembles nucleoplasmic signalosome involving ataxia telangiectasia mutated (ATM) resulting in phosphorylation and sumoylation of IKKγ and thereby IKK activation (Fig. 6a) (Stilmann et al. 2009). Another report shows that TNF-α-mediated interaction between NF-κB and the homologous recombination (HR) protein leads to the increased NF-κB transcriptional activity in breast cancer-associated gene 1 (BRCA1). This increase in transcriptional activity results in almost a threefold increase in DSB repair efficiency (Benezra et al. 2003; Volcic et al. 2012). In most of the eukaryotes, including in mammalian cells, DSBs can be repaired either by non-homologous end joining (NHEJ), or by HR protein. Using K562 cells, it has been shown that NF-κB/p65 stimulation upregulates HR protein BRCA2 and ATM, and NHEJ protein ku70 (Volcic et al. 2012).
NF-κB pathway and DNA damage response. a DNA damage sensor PARP1 assembles nucleoplasmic signalosome involving ATM resulting in phosphorylation and sumoylation of NEMO and thereby NF-κB activation. b The canonical NF-κB activation that contributes to the initiation and progression of breast cancer mediated by pro-inflammatory cytokines and NEMO that leads to the degradation of IκBα and the translocation of p50/p65 heterodimer into the nucleus. An alternative pathway is the partial degradation of the inhibitory molecule p100 into p52 through NIK leading to the nuclear translocation of p52/RelB dimers. c NF-κB acts as biphasic regulator in ovarian cancer, as it plays a role as a tumor suppressor and induces apoptosis but it can be reprogrammed and act as oncogene in chemoresistant ovarian cancer cells. d NF-κB in myeloid cells induces lung and pancreatic cancer through the secretion of pro-inflammatory cytokines. Factors associated with angiogenesis like vascular endothelial growth factor (VEGF), TNF-α, IL-8, IL-6, MCP-1 and matrix metalloproteinases (MMPs) are also activated by NF-κB in tumor cells
Some studies connect signal transduction mechanisms associated with DNA damage in the nucleus with the activation of NF-κB in the cytoplasm (Janssens and Tschopp 2006; McCool and Miyamoto 2012). The NF-κB essential modulator (NEMO), also known as IKKγ, is a regulatory subunit of IKK and plays an indispensable role in the activation of NF-κB in response to DNA-damaging agents. In this regard, NEMO-deficient fibroblast-derived cell line 5R with impaired NF-κB activation when stably reconstituted with wild-type NEMO showed restored NF-κB activation demonstrating the role in NF-κB in cells challenged with DNA-damaging agents (Courtois et al. 1997; Rooney et al. 1990; Yamaoka et al. 1998). Structural analysis of NEMO reveals that NEMO consists of a C-terminal zinc-finger domain and its deletion attenuates NF-κB activation following treatment with the DNA-damaging agents including aphidicolin or hydroxyurea (Israel 2010; McCool and Miyamoto 2012). In addition, zinc-finger domain is important for the post-translational modifications (SUMOylation, phosphorylation, ubiquitination) of NEMO after DNA damage, and the impairment in post-translational modification leads to the attenuation of NF-κB activation (Mabb et al. 2006; Stilmann et al. 2009). In 2012, Volcic et al. (2012) reported that NF-κB interacts with CtIP–BRCA1 complexes and promotes BRCA1 stabilization, and thereby contributes to homologous recombination (HR) (Fig. 6a). Furthermore, this group also showed induced expression of replication protein A and Rad51 foci upon NF-κB activation (Fig. 6a). This indicates HR stimulation through DSB resection by the interacting CtIP–BRCA1 complex and Rad51 filament formation (Volcic et al. 2012). Together, these studies suggest a significant role of NF-κB in DNA repair; therefore, provide an intriguing explanation for NF-κB-mediated resistance to chemo- and radiation therapies, along with sensitization to therapeutic intervention of NF-κB. Since DNA damage and repair have been directly linked with the generation of cancer, the role of NF-κB in cancer is not unrecognized.
Cancer is a hyperproliferative disorder resulting from tumor initiation and promotion leading to tumor metastasis. Various types of solid tumors including breasts, ovarian, colon, thyroid, bladder, pancreatic and prostate carcinomas as well as melanomas are characterized by persistent activation of NF-κB transcription factors (Cherry et al. 2015; Omur and Baran 2014; Setia et al. 2014). The oncogenic function of NF-κB has been well documented in various types of cancers, and is due to its target genes involved in anti-apoptosis, cell cycle progression, and angiogenesis (Karin 2006; Luo et al. 2004; Wang et al. 1996). We will focus on the role of NF-κB in most common forms of cancer in this review (Table 2).
Breast cancer is the second most common cancer, after lung cancer, and most common cancer among women. A common hallmark in most breast cancer tumors is the constitutive activation of NF-κB. NF-κB activation correlates with the conversion of breast cancer cells to hormone independent growth, a characteristic of more aggressive and metastatic tumors (Chen et al. 2013). Progression of tumor from hormone dependent to hormone independent increases the risk of metastasis and decreases the treatment options. The canonical NF-κB activation that contributes to the initiation and progression of breast cancer mediated by pro-inflammatory cytokines (TNF-α or IL-1β) (Hayden and Ghosh 2008) (Fig. 6b). Apart from activating by classical and alternative pathway, NF-κB is also activated by epidermal growth factor (EGF) especially in estrogen receptor (ER)-negative breast cancer cells (Biswas et al. 2000) (Fig. 6b). EGF causes phosphorylation of IκBα at Tyr42, and its ubiquitination in IKK-independent pathway unlike TNF-α, EGF induced NF-κB activation in a dosage-dependent manner but EGF-induced NF-κB activation showed slower kinetics and lower optimal activation levels suggesting differences in the activation mechanisms regulated by TNF-α and EGF (Sethi et al. 2007).
Contribution of classical pathway in the progression of breast cancer is substantiated due to a report which suggested the role of peptide inhibitor for IκB-kinase in breast cancer SKBr3 cells, which blocked heregulin-mediated activation of NF-κB and induced apoptosis in proliferating cells (Biswas et al. 2004). Heregulin is a soluble secreted growth factor that involves in cell proliferation. MCF-7 human breast cell line overexpressing NF-κB protein p65 shows increased epithelial–mesenchymal transition, a process by which cells lose their polarity and gain migratory and invasive properties. Thus, in ER-negative breast cancers, NF-κB acts as potential therapeutic target.
Role of alternative pathway in the development of breast cancer is supported by the studies demonstrating overexpression of the NF-κB/p52 and increased p52/RelB activity in breast cancer cells (MDA-MB-435 cells) (Cogswell et al. 2000; Dejardin et al. 1995). In mouse transgenic model on B6D2 background, overexpressing p100/p52 in mammary epithelium using β-lactoglobulin milk protein promotor showed impaired normal ductal development (Connelly et al. 2007). Furthermore, while the breast cancer cells showed elevated levels of p100/p52, the normal human epithelial cells were observed to have very low expression (Connelly et al. 2007). The abnormally elevated levels of p100/p52 causes the thickening of primary ducts, loss of epithelial cell organization and small areas of hyperplastic growth (Shostak and Chariot 2011). However, it is intriguing that alternative NF-κB signaling pathway does not result in elevated nuclear levels of p65. These findings indicate the independent functioning of two pathways.
Like breast cancer, ovarian cancer is also a major cause of cancer related deaths from gynecological malignancies, and is also derived from epithelial origin. Ovarian cancer has been linked to inflammatory processes, such as repeated ovulation, endometriosis and pelvic infections (Cramer and Welch 1983; Mandai et al. 2009). NF-κB acts as biphasic regulator in ovarian cancer, as it plays a role as a tumor suppressor and induces apoptosis but it can be reprogrammed and act as oncogene in chemoresistant ovarian cancer cells including SKOV3, HEY (Yang et al. 2011) (Fig. 6c). The tumor suppressor function of NF-κB is mediated by down-regulating protein phosphatase 1α, which leads to the preservation of phosphorylation on MEK1/2 and ERK1/2 and the inhibition apoptotic pathway (Yang et al. 2011) (Fig. 6c). The critical issue in ovarian cancer treatment is the resistance to platinum-based chemotherapy (treatment with cisplatin and related molecules), and NF-κB acts as a key regulator for developing this resistance and thus attributes to the aggressive recurrent ovarian cancer (Yang et al. 2011). Furthermore, NIK-dependent non-canonical pathway of NF-κB provides strength to the pathogenesis of ovarian cancer. Silencing of NIK caused significant decrease in p52 and the phosphorylated forms of IKKα in ovarian cancer. Delayed ovarian tumor formation was also observed when nude mice were subcutaneously xenografted with NIK-depleted tumorigenic ovarian cell line RMG-I, (Cherry et al. 2015). Moreover, co-expression of transcriptional factors p65 and RelB with IKKα is reported in patients having newly diagnosed advanced ovarian cancer that indicates the role of NF-κB in the pathogenesis of ovarian cancer (Annunziata et al. 2010). Since NF-κB has biphasic role, NF-κB targeting agents must be cautiously used in relation to breast and ovarian cancer.
While discussing about the role of NF-κB in cancer, its role in thyroid cancer cannot be left aside as it is the most common endocrine malignancy and accounts for approximately 1 % of all newly diagnosed cancer cases (Parker et al. 1997). The first evidence of NF-κB role in thyroid cancer was reported as an aberrant NF-κB activity in the nuclei of different thyroid cancer-derived cell lines including TPC-1, WRO, NPA, ARO, FRO, NIM 1 and B-CPAP (Visconti et al. 1997). Besides that, tissue specimen from primary human anaplastic thyroid carcinomas also showed high basal activity of NF-κB (Mitsiades et al. 2006; Pacifico et al. 2004). Inhibition of programmed cell death allows cancer cells to survive beyond normal life span and promote tumor by angiogenesis and invasiveness is also a hallmark of thyroid cancer cells. The BRAF gene, which is a member of rapidly accelerated fibrosarcoma family, a serine/threonine protein kinase (proto-oncogene) acts as a bridge between the RAS and MAPK pathway and regulates apoptosis and cell growth. Raf-1-mediated activation of NF-κB regulates the genes responsible for anti-apoptotic behavior and invasiveness of thyroid cancer cells (Palona et al. 2006). Furthermore, DNA-binding assay depicts the elevated affinity of NF-κB to the DNA in thyroid cancer cells (Palona et al. 2006).
Prevalence of both, pancreatic and lung cancer is very high in United States and also worldwide. Pancreatic cancer is well characterized by its aggressive and fast metastatic nature which attributes to the high mortality with an overall five year survival rate of 5 % (Jemal et al. 2008; Li et al. 2004). Furthermore, due to its poor prognosis, it is the fifth leading cause of cancer deaths in United States (Nitecki et al. 1995). Deregulated NF-κB signal transduction pathway has been reported in the pancreatic cancer. Human pancreatic adenocarcinoma and human pancreatic cancer cell lines show constitutively activated RelA (Wang et al. 1999). It is reported that the alternative pathway characterized by the overexpression of NIK is constitutively activated in pancreatic cancer cells (Nishina et al. 2009; Wharry et al. 2009). The role of alternative pathway in pancreatic cancer is further supported by siRNA-mediated silencing of NIK expression that leads to the suppression of rapidly dividing pancreatic cancer cells line including PK-1, PK-59 (Nishina et al. 2009). Subcellular fractionation studies performed using pancreatic cancer cell lines, PANC-1, PK-1, and KP-1 N, showed significant accumulation of p52 and RelB in nuclear fractions which indicates processing of p100 to p52, thereby strengthening the argument about the involvement of constitutively activated alternative pathway in the pancreatic cancer (Nishina et al. 2009). In pancreatic cancer, the upregulation of miR-301a is well reported. miR-301a negatively regulates NF-κB-repressing factor (NKRF) resulting in NF-κB activation in pancreatic cancer (Lee et al. 2007; Lu et al. 2011). NF-κB in turn further promotes miR-301a transcription resulting in a positive feedback loop (Lu et al. 2011). Since NF-κB activation is regulated in several ways and its dysregulation leads to the pancreatic cancer; there is urgent need to develop strategy to control the dysregulated NF-κB pathway and ameliorate pancreatic cancer. NIK/miR-301a/NKRF regulates NF-κB activation at different levels and can be targeted for intervention to combat the dysregulated mechanisms.
In case of lung cancer, pertaining to cancer cell resistance to chemotherapy and radiotherapy, the role of NF-κB is well known (Chen et al. 2011). NF-κB in myeloid cells induces lung cancer through the secretion of pro-inflammatory cytokines like CCL2, CCL3, IL-6 and TNF-α (Chen et al. 2011) (Fig. 6d). A significant reduction in the levels of these cytokines and infiltration of immune cells was observed in lung cancer cells in response to blocking of NF-κB by proteasome inhibitor MG-132. Furthermore, reduction in tumor size was also observed in response to blocking of NF-κB (Chen et al. 2011; Takahashi et al. 2010; Wong et al. 2010) Factors associated with angiogenesis like VEGF, TNF-α, IL-8, IL-6, MCP-1, and matrix metalloproteinases are also activated by NF-κB in lung tumor cells (Chen et al. 2011; Grivennikov et al. 2010; Wang and Lin 2008) (Fig. 6d).
Since NF-κB promotes angiogenesis and is involved in cancer progression, it acts as a major target for the therapeutic purposes. Various pharmacological substances like thalidomide, celecoxib, gemcitabine, cisplatin, doxorubicin, genistein, bortezomib, sulfasalazine and others either alone or in combination with radiotherapy or other pharmacological agents have already entered clinical studies and show promising results to treat various types of cancer (Hada and Mizutari 2004; Heinemann et al. 2000; Jimeno et al. 2006; Lo et al. 2010; Loehrer et al. 2011; Pavese et al. 2010; Wang et al. 2012). The use of the proteasome inhibitor PS-341 as a systemic inhibitor of NF-κB in conjunction with CPT-11 treatment (topoisomerase 1 inhibitor) also helped in significantly improving chemotherapeutic outcome through enhanced apoptosis (Cusack et al. 2001). Other molecules that have been shown to inhibit NF-κB include the non-steroidal anti-inflammatory drug sulindac, cyclopentenone prostaglandins, arsenic trioxide, a variety of antioxidants, and natural products such as parthenolide. On the other side, it is well known that NF-κB plays an indispensable role in both innate and adaptive immunity, so its inhibition may compromise the immunity. It remains to be investigated in detail, whether NF-κB inhibition will attenuate or promote the associated pathogenesis during the treatment of cancer (Vallabhapurapu and Karin 2009).
Conclusions
The transcription factor NF-κB is the most common and abundant of all transcription factors. Regulated by diverse stimuli including genotoxic, inflammatory, and oxidative stresses, NF-κB is also associated with cellular processes including the response to DNA damage; however, aberrant activation of NF-κB is attributed to various ailments including asthma, cancer, chronic obstructive pulmonary disease, and cardiovascular disease. Because of its abundance in cells and its importance in various diseases, NF-κB is a focus of therapeutic intervention. Currently, most research regarding this vital transcription factor is done using cell cultures and animal models. Hence, future studies will be required to define the clinical importance of this factor in the mentioned ailments in humans. While NF-κB regulates many genes, the regulation of NF-κB itself is under epigenetic control, specifically through modifications of NF-κB promoter. Thus, the role of histone trimethylation within the NF-κB promoter and deacetylation of histones or NF-κB by HDACs resulting in regulation of NF-κB activity are important areas of study and will provide additional insights from the mechanistic point of view. In addition, it is important to understand the role of the various dimeric forms of NF-κB in different conditions as well as the numerous ligands that activate or suppress their expression in response to a diverse range of stimuli. Therapeutically, the major difficulty with long-term inhibition of the NF-κB family of transcription factors is that they also play important roles in the maintenance of cellular homeostasis, survival, and host defense. Therefore, current research is focused on the generation of unique approaches to inhibit specific downstream targets of NF-κB responsible for specific outcomes in various pathological conditions. With regard to clinically relevant molecules, a recent report (Tornatore et al. 2014) showed that DTP3, a tripeptide with similar anticancer potency but greater cancer cell specificity (>100-fold) than the clinical standard, bortezomib, blocked the NF-κB-regulated anti-apoptotic factor GADD45β and the JNK kinase MKK7 leading to selective killing of myeloma cells. These findings underscore the potential utility of targeting the NF-κB pathway as a novel approach to generate improved therapeutic strategies for various pathological conditions.
References
Al-Rasheed NM, Al-Rasheed NM, Bassiouni YA et al (2015) Vitamin D attenuates pro-inflammatory TNF-alpha cytokine expression by inhibiting NF-small ka, CyrillicB/p65 signaling in hypertrophied rat hearts. J Physiol Biochem 71:289–299
Ambegaokar SS, Kolson DL (2014) Heme oxygenase-1 dysregulation in the brain: implications for HIV-associated neurocognitive disorders. Curr HIV Res 12:174
Anderson I, Low JS, Weston S et al (2014) Heat shock protein 90 controls HIV-1 reactivation from latency. Proc Natl Acad Sci USA 111:E1528–E1537
Annunziata CM, Stavnes HT, Kleinberg L et al (2010) Nuclear factor kappaB transcription factors are coexpressed and convey a poor outcome in ovarian cancer. Cancer 116:3276–3284
Balakumar P, Singh M (2006) Anti-tumour necrosis factor-alpha therapy in heart failure: future directions. Basic Clin Pharmacol Toxicol 99:391–397
Balch WE, Sznajder JI, Budinger S et al (2014) Misfolded protein structure and proteostasis in lung diseases. Am J Respir Crit Care Med 189:96–103
Baltimore D (2009) Discovering NF-kappaB. Cold Spring Harb Perspect Biol 1:a000026
Barnes PJ (2015) Therapeutic approaches to asthma-chronic obstructive pulmonary disease overlap syndromes. J Allergy Clin Immunol 136:531–545
Benezra M, Chevallier N, Morrison DJ et al (2003) BRCA1 augments transcription by the NF-kappaB transcription factor by binding to the Rel domain of the p65/RelA subunit. J Biol Chem 278:26333–26341
Birrell MA, Hardaker E, Wong S et al (2005) Ikappa-B kinase-2 inhibitor blocks inflammation in human airway smooth muscle and a rat model of asthma. Am J Respir Crit Care Med 172:962–971
Biswas DK, Cruz AP, Gansberger E et al (2000) Epidermal growth factor-induced nuclear factor κB activation: a major pathway of cell-cycle progression in estrogen-receptor negative breast cancer cells. Proc Natl Acad Sci USA 97:8542–8547
Biswas DK, Shi Q, Baily S et al (2004) NF-kappa B activation in human breast cancer specimens and its role in cell proliferation and apoptosis. Proc Natl Acad Sci USA 101:10137–10142
Bonizzi G, Piette J, Schoonbroodt S et al (1999) Reactive oxygen intermediate-dependent NF-kappaB activation by interleukin-1beta requires 5-lipoxygenase or NADPH oxidase activity. Mol Cell Biol 19:1950–1960
Bosnjak L, Miranda-Saksena M, Koelle DM et al (2005) Herpes simplex virus infection of human dendritic cells induces apoptosis and allows cross-presentation via uninfected dendritic cells. J Immunol 174:2220–2227
Bourdillon MC, Poston RN, Covacho C et al (2000) ICAM-1 deficiency reduces atherosclerotic lesions in double-knockout mice (ApoE(−/−)/ICAM-1(−/−)) fed a fat or a chow diet. Arterioscler Thromb Vasc Biol 20:2630–2635
Branen L, Hovgaard L, Nitulescu M et al (2004) Inhibition of tumor necrosis factor-alpha reduces atherosclerosis in apolipoprotein E knockout mice. Arterioscler Thromb Vasc Biol 24:2137–2142
Brar SS, Kennedy TP, Sturrock AB et al (2002) NADPH oxidase promotes NF-kappaB activation and proliferation in human airway smooth muscle. Am J Physiol Lung Cell Mol Physiol 282:L782–L795
Brown LA, Scarola J, Smith AJ et al (2014) The role of tau protein in HIV-associated neurocognitive disorders. Mol Neurodegener 9:40
Brubaker SW, Bonham KS, Zanoni I et al (2015) Innate immune pattern recognition: a cell biological perspective. Annu Rev Immunol 33:257–290
Cai W, Schaffer PA (1992) Herpes simplex virus type 1 ICP0 regulates expression of immediate-early, early, and late genes in productively infected cells. J Virol 66:2904–2915
Cazzola M, Calzetta L, Page C et al (2015) Influence of N-acetylcysteine on chronic bronchitis or COPD exacerbations: a meta-analysis. Eur Respir Rev 24:451–461
Ceconi C, Boraso A, Cargnoni A et al (2003) Oxidative stress in cardiovascular disease: myth or fact? Arch Biochem Biophys 420:217–221
Chen W, Li Z, Bai L et al (2011) NF-kappaB in lung cancer, a carcinogenesis mediator and a prevention and therapy target. Front Biosci 16:1172–1185
Chen YJ, Yeh MH, Yu MC et al (2013) Lapatinib–induced NF-kappaB activation sensitizes triple-negative breast cancer cells to proteasome inhibitors. Breast Cancer Res 15:R108
Chen Y, Wang H, Luo G et al (2014) SIRT4 inhibits cigarette smoke extracts-induced mononuclear cell adhesion to human pulmonary microvascular endothelial cells via regulating NF-κB activity. Toxicol Lett 226:320–327
Cherry EM, Lee DW, Jung JU et al (2015) Tumor necrosis factor-like weak inducer of apoptosis (TWEAK) promotes glioma cell invasion through induction of NF-κB-inducing kinase (NIK) and noncanonical NF-κB signaling. Mol Cancer 14:9
Clark RA, Valente AJ (2004) Nuclear factor kappa B activation by NADPH oxidases. Mech Ageing Dev 125:799–810
Cogswell PC, Guttridge DC, Funkhouser WK et al (2000) Selective activation of NF-kappa B subunits in human breast cancer: potential roles for NF-kappa B2/p52 and for Bcl-3. Oncogene 19:1123–1131
Collins RG, Velji R, Guevara NV et al (2000) P-Selectin or intercellular adhesion molecule (ICAM)-1 deficiency substantially protects against atherosclerosis in apolipoprotein E-deficient mice. J Exp Med 191:189–194
Connelly L, Robinson-Benion C, Chont M et al (2007) A transgenic model reveals important roles for the NF-κB alternative pathway (p100/p52) in mammary development and links to tumorigenesis. J Biol Chem 282:10028–10035
Courtois G, Whiteside ST, Sibley CH et al (1997) Characterization of a mutant cell line that does not activate NF-kappaB in response to multiple stimuli. Mol Cell Biol 17:1441–1449
Cramer DW, Welch WR (1983) Determinants of ovarian cancer risk. II. Inferences regarding pathogenesis. J Natl Cancer Inst 71:717–721
Cusack JC Jr, Liu R, Houston M et al (2001) Enhanced chemosensitivity to CPT-11 with proteasome inhibitor PS-341: implications for systemic nuclear factor-kappaB inhibition. Cancer Res 61:3535–3540
Dahabieh MS, Battivelli E, Verdin E (2015) Understanding HIV latency: the road to an HIV cure. Annu Rev Med 66:407–421
Dahiya S, Liu Y, Nonnemacher MR et al (2014a) CCAAT enhancer binding protein and nuclear factor of activated T cells regulate HIV-1 LTR via a novel conserved downstream site in cells of the monocyte-macrophage lineage. PLoS One 9:e88116
Dahiya S, Liu Y, Williams J et al (2014b) Role of downstream elements in transcriptional regulation of the HIV-1 promoter. J Hum Virol Retrovirol 1:00006
de Winther MP, Kanters E, Kraal G et al (2005) Nuclear factor kappaB signaling in atherogenesis. Arterioscler Thromb Vasc Biol 25:904–914
Dejardin E, Bonizzi G, Bellahcene A et al (1995) Highly-expressed p100/p52 (NFKB2) sequesters other NF-kappa B-related proteins in the cytoplasm of human breast cancer cells. Oncogene 11:1835–1841
Delerive P, De Bosscher K, Besnard S et al (1999) Peroxisome proliferator-activated receptor alpha negatively regulates the vascular inflammatory gene response by negative cross-talk with transcription factors NF-kappaB and AP-1. J Biol Chem 274:32048–32054
Deng J, Lu PD, Zhang Y et al (2004) Translational repression mediates activation of nuclear factor kappa B by phosphorylated translation initiation factor 2. Mol Cell Biol 24:10161–10168
Dhalla NS, Temsah RM, Netticadan T (2000) Role of oxidative stress in cardiovascular diseases. J Hypertens 18:655–673
Di Stefano A, Caramori G, Gnemmi I et al (2009) T helper type 17-related cytokine expression is increased in the bronchial mucosa of stable chronic obstructive pulmonary disease patients. Clin Exp Immunol 157:316–324
Doyle KL, Loft S, Morgan EE et al (2013) Prospective memory in HIV-associated neurocognitive disorders (HAND): the neuropsychological dynamics of time monitoring. J Clin Exp Neuropsychol 35:359–372
Edwards MR, Bartlett NW, Clarke D et al (2009) Targeting the NF-kappaB pathway in asthma and chronic obstructive pulmonary disease. Pharmacol Ther 121:1–13
Egan KP, Wu S, Wigdahl B et al (2013) Immunological control of herpes simplex virus infections. J Neurovirol 19:328–345
Gangwani MR, Noel RJ Jr, Shah A et al (2013) Human immunodeficiency virus type 1 viral protein R (Vpr) induces CCL5 expression in astrocytes via PI3 K and MAPK signaling pathways. J Neuroinflammation 10:136
Gilmore TD (2006) Introduction to NF-kappaB: players, pathways, perspectives. Oncogene 25:6680–6684
Gilowska I (2014) CXCL8 (interleukin 8)—the key inflammatory mediator in chronic obstructive pulmonary disease? Postepy Hig Med Dosw (Online) 68:842–850
Goodkin ML, Ting AT, Blaho JA (2003) NF-kappaB is required for apoptosis prevention during herpes simplex virus type 1 infection. J Virol 77:7261–7280
Grilli M, Memo M (1999) Possible role of NF-kappaB and p53 in the glutamate-induced pro-apoptotic neuronal pathway. Cell Death Differ 6:22–27
Grivennikov SI, Greten FR, Karin M (2010) Immunity, inflammation, and cancer. Cell 140:883–899
Guo F, Li J, Du W et al (2013) mTOR regulates DNA damage response through NF-kappaB-mediated FANCD2 pathway in hematopoietic cells. Leukemia 27:2040–2046
Guo H, Gao J, Taxman DJ et al (2014) HIV-1 infection induces interleukin-1β production via TLR8 protein-dependent and NLRP3 inflammasome mechanisms in human monocytes. J Biol Chem 289:21716–21726
Gupta A, Grove A (2014) Ligand-binding pocket bridges DNA-binding and dimerization domains of the urate-responsive MarR homologue MftR from Burkholderia thailandensis. Biochemistry 53:4368–4380
Hada M, Mizutari K (2004) A case of advanced pancreatic cancer with remarkable response to thalidomide, celecoxib and gemcitabine. Cancer Chemother 31:959–961
Hadigal SR, Agelidis AM, Karasneh GA et al (2015) Heparanase is a host enzyme required for herpes simplex virus-1 release from cells. Nat Commun 6:6985
Hargett D, Rice S, Bachenheimer SL (2006) Herpes simplex virus type 1 ICP27-dependent activation of NF-kappaB. J Virol 80:10565–10578
Hayden MS, Ghosh S (2004) Signaling to NF-kappaB. Genes Dev 18:2195–2224
Hayden MS, Ghosh S (2008) Shared principles in NF-kappaB signaling. Cell 132:344–362
Heaton RK, Franklin DR, Ellis RJ et al (2011) HIV-associated neurocognitive disorders before and during the era of combination antiretroviral therapy: differences in rates, nature, and predictors. J Neurovirol 17:3–16
Heinemann V, Wilke H, Mergenthaler HG et al (2000) Gemcitabine and cisplatin in the treatment of advanced or metastatic pancreatic cancer. Ann Oncol 11:1399–1403
Hernandez-Gutierrez S, Garcia-Pelaez I, Zentella-Dehesa A et al (2006) NF-kappaB signaling blockade by Bay 11-7085 during early cardiac morphogenesis induces alterations of the outflow tract in chicken heart. Apoptosis 11:1101–1109
Hoffmann A, Baltimore D (2006) Circuitry of nuclear factor kappaB signaling. Immunol Rev 210:171–186
Hollander W (1976) Role of hypertension in atherosclerosis and cardiovascular disease. Am J Cardiol 38:786–800
Hung PY, Ho BC, Lee SY et al (2015) Houttuynia cordata targets the beginning stage of herpes simplex virus infection. PLoS One 10:e0115475
Hutchinson KR, Stewart JA Jr, Lucchesi PA (2010) Extracellular matrix remodeling during the progression of volume overload-induced heart failure. J Mol Cell Cardiol 48:564–569
Israel A (2010) The IKK complex, a central regulator of NF-kappaB activation. Cold Spring Harb Perspect Biol 2:a000158
Ito K, Yamamura S, Essilfie-Quaye S et al (2006) Histone deacetylase 2-mediated deacetylation of the glucocorticoid receptor enables NF-kappaB suppression. J Exp Med 203:7–13
Jansen F, Yang X, Nickenig G et al (2015) Role, function and therapeutic potential of microRNAs in vascular aging. Curr Vasc Pharmacol 13:324–330
Janssen-Heininger YM, Poynter ME, Aesif SW et al (2009) Nuclear factor kappaB, airway epithelium, and asthma: avenues for redox control. Proc Am Thorac Soc 6:249–255
Janssens S, Tschopp J (2006) Signals from within: the DNA-damage-induced NF-kappaB response. Cell Death Differ 13:773–784
Jemal A, Siegel R, Ward E et al (2008) Cancer statistics, 2008. CA Cancer J Clin 58:71–96
Jimeno A, Amador ML, Kulesza P et al (2006) Assessment of celecoxib pharmacodynamics in pancreatic cancer. Mol Cancer Ther 5:3240–3247
Joyce D, Albanese C, Steer J et al (2001) NF-kappaB and cell-cycle regulation: the cyclin connection. Cytokine Growth Factor Rev 12:73–90
Kang JH, Hwang SM, Chung IY (2015) S100A8, S100A9 and S100A12 activate airway epithelial cells to produce MUC5AC via extracellular signal-regulated kinase and nuclear factor-κB pathways. Immunology 144:79–90
Karin M (2006) Nuclear factor-kappaB in cancer development and progression. Nature 441:431–436
Karin M (2009) NF-kappaB as a critical link between inflammation and cancer. Cold Spring Harb Perspect Biol 1:a000141
Karn J, Stoltzfus CM (2012) Transcriptional and posttranscriptional regulation of HIV-1 gene expression. Cold Spring Harb Perspect Med 2:a006916
Kedzierska K, Crowe SM (2001) Cytokines and HIV-1: interactions and clinical implications. Antivir Chem Chemother 12:133–150
Kniss DA, Rovin B, Fertel RH et al (2001) Blockade NF-kappaB activation prohibits TNF-alpha-induced cyclooxygenase-2 gene expression in ED27 trophoblast-like cells. Placenta 22:80–89
Kumar S, Mabalirajan U, Rehman R et al (2013) A novel cinnamate derivative attenuates asthma features and reduces bronchial epithelial injury in mouse model. Int Immunopharmacol 15:150–159
Lassegue B, San Martin A, Griendling KK (2012) Biochemistry, physiology, and pathophysiology of NADPH oxidases in the cardiovascular system. Circ Res 110:1364–1390
Lee EJ, Gusev Y, Jiang J et al (2007) Expression profiling identifies microRNA signature in pancreatic cancer. Int J Cancer 120:1046–1054
Lemmers B, Salmena L, Bidere N et al (2007) Essential role for caspase-8 in Toll-like receptors and NFkappaB signaling. J Biol Chem 282:7416–7423
Leoni V, Gianni T, Salvioli S et al (2012) Herpes simplex virus glycoproteins gH/gL and gB bind Toll-like receptor 2, and soluble gH/gL is sufficient to activate NF-κB. J Virol 86:6555–6562
Li Q, Verma IM (2002) NF-kappaB regulation in the immune system. Nat Rev Immunol 2:725–734
Li J, Zhao F (2015) Anti-inflammatory functions of Thunb. and its compounds: a perspective on its potential role in rheumatoid arthritis. Exp Ther Med 10:3–6
Li YY, Feng YQ, Kadokami T et al (2000) Myocardial extracellular matrix remodeling in transgenic mice overexpressing tumor necrosis factor alpha can be modulated by anti-tumor necrosis factor alpha therapy. Proc Natl Acad Sci USA 97:12746–12751
Li D, Xie K, Wolff R et al (2004) Pancreatic cancer. Lancet 363:1049–1057
Li J, Luo L, Wang X et al (2009) Inhibition of NF-kappaB expression and allergen-induced airway inflammation in a mouse allergic asthma model by andrographolide. Cell Mol Immunol 6:381–385
Liu H, Chen K, Feng W et al (2013) TLR4-MyD88/Mal-NF-κB axis is involved in infection of HSV-2 in human cervical epithelial cells. PLoS One 8:e80327
Lo M, Ling V, Low C et al (2010) Potential use of the anti-inflammatory drug, sulfasalazine, for targeted therapy of pancreatic cancer. Curr Oncol 17:9
Loehrer PJ, Feng Y, Cardenes H et al (2011) Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. J Clin Oncol 29:4105–4112
Lu Z, Li Y, Takwi A et al (2011) miR-301a as an NF-kappaB activator in pancreatic cancer cells. EMBO J 30:57–67
Lu M, Tang F, Zhang J et al (2015) Astragaloside IV attenuates injury caused by myocardial ischemia/reperfusion in rats via regulation of toll-like receptor 4/nuclear factor-kappaB signaling pathway. Phytother Res 29:599–606
Luo JL, Maeda S, Hsu LC et al (2004) Inhibition of NF-kappaB in cancer cells converts inflammation- induced tumor growth mediated by TNFalpha to TRAIL-mediated tumor regression. Cancer Cell 6:297–305
Ma X, Becker Buscaglia LE, Barker JR et al (2011) MicroRNAs in NF-kappaB signaling. J Mol Cell Biol 3:159–166
Mabb AM, Wuerzberger-Davis SM, Miyamoto S (2006) PIASy mediates NEMO sumoylation and NF-kappaB activation in response to genotoxic stress. Nat Cell Biol 8:986–993
Madrigal-Matute J, Rotllan N, Aranda JF et al (2013) MicroRNAs and atherosclerosis. Curr Atheroscler Rep 15:322
Mammen MJ, Sethi S (2012) Macrolide therapy for the prevention of acute exacerbations in chronic obstructive pulmonary disease. Pol Arch Med Wewn 122:54–59
Mandai M, Yamaguchi K, Matsumura N et al (2009) Ovarian cancer in endometriosis: molecular biology, pathology, and clinical management. Int J Clin Oncol 14:383–391
Marcelletti JF (2002) Synergistic inhibition of herpesvirus replication by docosanol and antiviral nucleoside analogs. Antiviral Res 56:153–166
McArthur JC, Brew BJ (2010) HIV-associated neurocognitive disorders: is there a hidden epidemic? AIDS 24:1367–1370
McCool KW, Miyamoto S (2012) DNA damage-dependent NF-kappaB activation: NEMO turns nuclear signaling inside out. Immunol Rev 246:311–326
Meng Y, Yu CH, Li T et al (2013) Expression and significance of Toll-like receptor-4 in rats lung established by passive smoking or associated with intratracheal instillation of lipopolysaccharide. Zhonghua Yi Xue Za Zhi 93:2230–2234
Min T, Bodas M, Mazur S et al (2011) Critical role of proteostasis-imbalance in pathogenesis of COPD and severe emphysema. J Mol Med 89:577–593
Mitsiades CS, McMillin D, Kotoula V et al (2006) Antitumor effects of the proteasome inhibitor bortezomib in medullary and anaplastic thyroid carcinoma cells in vitro. J Clin Endocrinol Metab 91:4013–4021
Mogensen TH, Paludan SR (2001) Molecular pathways in virus-induced cytokine production. Microbiol Mol Biol Rev 65:131–150
Moutzouris JP, Che W, Ramsay EE et al (2010) Proteasomal inhibition upregulates the endogenous MAPK deactivator MKP-1 in human airway smooth muscle: mechanism of action and effect on cytokine secretion. Biochim Biophys Acta 1803:416–423
Mutlu GM, Budinger GR, Wu M et al (2012) Proteasomal inhibition after injury prevents fibrosis by modulating TGF-β1 signalling. Thorax 67:139–146
Nakashima Y, Raines EW, Plump AS et al (1998) Upregulation of VCAM-1 and ICAM-1 at atherosclerosis-prone sites on the endothelium in the ApoE-deficient mouse. Arterioscler Thromb Vasc Biol 18:842–851
Nam NH (2006) Naturally occurring NF-kappaB inhibitors. Mini Rev Med Chem 6:945–951
Namba H, Saenko V, Yamashita S (2007) Nuclear factor-κB in thyroid carcinogenesis and progression: a novel therapeutic target for advanced thyroid cancer. Arq Bras Endocrinol Metab 51:843–851
Nelson G, Wilde GJ, Spiller DG et al (2003) NF-kappaB signalling is inhibited by glucocorticoid receptor and STAT6 via distinct mechanisms. J Cell Sci 116(Pt 12):2495–2503
Nishina T, Yamaguchi N, Gohda J et al (2009) NIK is involved in constitutive activation of the alternative NF-κB pathway and proliferation of pancreatic cancer cells. Biochem Biophys Res Commun 388:96–101
Nitecki SS, Sarr MG, Colby TV et al (1995) Long-term survival after resection for ductal adenocarcinoma of the pancreas. Is it really improving? Ann Surg 221:59
Niu J, Wang K, Graham S et al (2011) MCP-1-induced protein attenuates endotoxin-induced myocardial dysfunction by suppressing cardiac NF-small ka, CyrillicB activation via inhibition of Ismall ka, Cyrillic B kinase activation. J Mol Cell Cardiol 51:177–186
Omur O, Baran Y (2014) An update on molecular biology of thyroid cancers. Crit Rev Oncol Hematol 90:233–252
Osorio FG, Lopez-Otin C, Freije JM (2012) NF-κB in premature aging. Aging 4:726–727
Pacifico F, Mauro C, Barone C et al (2004) Oncogenic and anti-apoptotic activity of NF-κB in human thyroid carcinomas. J Biol Chem 279:54610–54619
Palona I, Namba H, Mitsutake N et al (2006) BRAFV600E promotes invasiveness of thyroid cancer cells through nuclear factor κB activation. Endocrinology 147:5699–5707
Panday A, Grove A (2016) The high mobility group protein HMO1 functions as a linker histone in yeast. Epigenetics Chromatin 9:13
Panday A, Sahoo MK, Osorio D et al (2015a) NADPH oxidases: an overview from structure to innate immunity-associated pathologies. Cell Mol Immunol 12:5–23
Panday A, Xiao L, Grove A (2015b) Yeast high mobility group protein HMO1 stabilizes chromatin and is evicted during repair of DNA double strand breaks. Nucleic Acids Res 43:5759–5770
Parker SL, Tong T, Bolden S et al (1997) Cancer statistics, 1997. CA Cancer J Clin 47:5–27
Pavese JM, Farmer RL, Bergan RC (2010) Inhibition of cancer cell invasion and metastasis by genistein. Cancer Metastasis Rev 29:465–482
Pierce JW, Read MA, Ding H et al (1996) Salicylates inhibit I kappa B-alpha phosphorylation, endothelial-leukocyte adhesion molecule expression, and neutrophil transmigration. J Immunol 156:3961–3969
Piret J, Boivin G (2011) Resistance of herpes simplex viruses to nucleoside analogues: mechanisms, prevalence, and management. Antimicrob Agents Chemother 55:459–472
Rastrick J, Stevenson CS, Eltom S et al (2013) Cigarette smoke induced airway inflammation is independent of NF-κB signalling. PLoS One 8:e54128
Rico-Rosillo G, Vega-Robledo GB (2011) The involvement of NF-?B Transcription factor in asthma. Rev Alerg Mex 58:107–111
Rooney JW, Emery DW, Sibley CH (1990) 1.3E2, a variant of the B lymphoma 70Z/3, defective in activation of NF-kappa B and OTF-2. Immunogenetics 31:73–78
Roth M, Black JL (2006) Transcription factors in asthma: are transcription factors a new target for asthma therapy? Curr Drug Targets 7:589–595
Sanguinetti CM (2015) N-acetylcysteine in COPD: why, how, and when? Multidiscip Respir Med 11:8
Sethi G, Ahn K, Chaturvedi M et al (2007) Epidermal growth factor (EGF) activates nuclear factor-κB through IκBα kinase-independent but EGF receptor-kinase dependent tyrosine 42 phosphorylation of IκBα. Oncogene 26:7324–7332
Setia S, Nehru B, Sanyal SN (2014) Activation of NF-κB: bridging the gap between inflammation and cancer in colitis-mediated colon carcinogenesis. Biomed Pharmacother 68:119–128
Shah A, Kumar A (2010) HIV-1 gp120-mediated increases in IL-8 production in astrocytes are mediated through the NF-kappaB pathway and can be silenced by gp120-specific siRNA. J Neuroinflamm 7:96
Shah A, Verma AS, Patel KH et al (2011) HIV-1 gp120 induces expression of IL-6 through a nuclear factor-kappa B-dependent mechanism: suppression by gp120 specific small interfering RNA. PLoS One 6:e21261
Sharma A, Menche J, Huang C et al (2015) A disease module in the interactome explains disease heterogeneity, drug response and captures novel pathways and genes. Hum Mol Genet 24:3005–3020
Shimizu K, Konno S, Ozaki M et al (2012) Dehydroxymethylepoxyquinomicin (DHMEQ), a novel NF-kappaB inhibitor, inhibits allergic inflammation and airway remodelling in murine models of asthma. Clin Exp Allergy 42:1273–1281
Shostak K, Chariot KS (2011) NF-κB, stem cells and breast cancer: the links get stronger. Breast Cancer Res 13:214
Spudich S, González-Scarano F (2012) HIV-1-related central nervous system disease: current issues in pathogenesis, diagnosis, and treatment. Cold Spring Harbor Perspect Med 2:a007120
Staal J, Bekaert T, Beyaert R (2011) Regulation of NF-kappaB signaling by caspases and MALT1 paracaspase. Cell Res 21:40–54
Stilmann M, Hinz M, Arslan SC et al (2009) A nuclear poly(ADP-ribose)-dependent signalosome confers DNA damage-induced IkappaB kinase activation. Mol Cell 36:365–378
Tabruyn SP, Griffioen AW (2007) A new role for NF-[kappa]B in angiogenesis inhibition. Cell Death Differ 14:1393–1397
Taddeo B, Zhang W, Lakeman F et al (2004) Cells lacking NF-kappaB or in which NF-kappaB is not activated vary with respect to ability to sustain herpes simplex virus 1 replication and are not susceptible to apoptosis induced by a replication-incompetent mutant virus. J Virol 78:11615–11621
Takahashi H, Ogata H, Nishigaki R et al (2010) Tobacco smoke promotes lung tumorigenesis by triggering IKKβ-and JNK1-dependent inflammation. Cancer Cell 17:89–97
Tiwari V, Tarbutton MS, Shukla D (2015) Diversity of heparan sulfate and HSV entry: basic understanding and treatment strategies. Molecules 20:2707–2727
Tornatore L, Sandomenico A, Raimondo D et al (2014) Cancer-selective targeting of the NF-kappaB survival pathway with GADD45beta/MKK7 inhibitors. Cancer Cell 26:495–508
Tsukuda T, Fleming AB, Nickoloff JA et al (2005) Chromatin remodelling at a DNA double-strand break site in Saccharomyces cerevisiae. Nature 438:379–383
Vallabhapurapu S, Karin M (2009) Regulation and function of NF-kappaB transcription factors in the immune system. Annu Rev Immunol 27:693–733
Vij N (2008) AAA ATPase p97/VCP: cellular functions, disease and therapeutic potential. J Cell Mol Med 12:2511–2518
Visconti R, Cerutti J, Battista S et al (1997) Expression of the neoplastic phenotype by human thyroid carcinoma cell lines requires NFkB p65 protein expression. Oncogene 15:1987–1994
Volcic M, Karl S, Baumann B et al (2012) NF-kappaB regulates DNA double-strand break repair in conjunction with BRCA1-CtIP complexes. Nucleic Acids Res 40:181–195
Wahid F, Shehzad A, Khan T et al (2010) MicroRNAs: synthesis, mechanism, function, and recent clinical trials. Biochim Biophys Acta 1803:1231–1243
Wang X, Lin Y (2008) Tumor necrosis factor and cancer, buddies or foes&quest. Acta Pharmacol Sinica 29:1275–1288
Wang C-Y, Mayo MW, Baldwin AS (1996) TNF-and cancer therapy-induced apoptosis: potentiation by inhibition of NF-κB. Science 274:784–787
Wang W, Abbruzzese JL, Evans DB et al (1999) The nuclear factor-κB RelA transcription factor is constitutively activated in human pancreatic adenocarcinoma cells. Clin Cancer Res 5:119–127
Wang H, Cao Q, Dudek AZ (2012) Phase II study of panobinostat and bortezomib in patients with pancreatic cancer progressing on gemcitabine-based therapy. Anticancer Res 32:1027–1031
Weng D, Marty-Roix R, Ganesan S et al (2014) Caspase-8 and RIP kinases regulate bacteria-induced innate immune responses and cell death. Proc Natl Acad Sci 111:7391–7396
Wharry CE, Haines KM, Carroll RG et al (2009) Constitutive noncanonical NFκB signaling in pancreatic cancer cells. Cancer Biol Ther 8:1567–1576
Wilson SJ, Wallin A, Della-Cioppa G et al (2001) Effects of budesonide and formoterol on NF-kappaB, adhesion molecules, and cytokines in asthma. Am J Respir Crit Care Med 164:1047–1052
Wong WS, Leong KP (2004) Tyrosine kinase inhibitors: a new approach for asthma. Biochim Biophys Acta 1697:53–69
Wong K, Jacks T, Dranoff G (2010) NF-kappaB fans the flames of lung carcinogenesis. Cancer Prev Res 3:403–405
Wutzler P (1997) Antiviral therapy of herpes simplex and varicella-zoster virus infections. Intervirology 40:343–356
Yamamoto Y, Gaynor RB (2001) Therapeutic potential of inhibition of the NF-kappaB pathway in the treatment of inflammation and cancer. J Clin Invest 107:135–142
Yamaoka S, Courtois G, Bessia C et al (1998) Complementation cloning of NEMO, a component of the IkappaB kinase complex essential for NF-kappaB activation. Cell 93:1231–1240
Yang G, Xiao X, Rosen DG et al (2011) The biphasic role of NF-κB in progression and chemoresistance of ovarian cancer. Clin Cancer Res 17:2181–2194
Yin MJ, Yamamoto Y, Gaynor RB (1998) The anti-inflammatory agents aspirin and salicylate inhibit the activity of I(kappa)B kinase-beta. Nature 396:77–80
Zelarayan L, Renger A, Noack C et al (2009) NF-κB activation is required for adaptive cardiac hypertrophy. Cardiovasc Res 84:416–424
Zhang JQ, Zhang JQ, Fang LZ et al (2015) Effect of oral N-acetylcysteine on COPD patients with microsatellite polymorphism in the heme oxygenase-1 gene promoter. Drug Des Devel Ther 9:6379–6387
Zhong H, May MJ, Jimi E et al (2002) The phosphorylation status of nuclear NF-kappa B determines its association with CBP/p300 or HDAC-1. Mol Cell 9:625–636
Zhong M, Zheng K, Chen M et al (2014) Heat-shock protein 90 promotes nuclear transport of herpes simplex virus 1 capsid protein by interacting with acetylated tubulin. PLoS One 9:e99425
Acknowledgments
We would like to thank Tania Hicks, Gagandeep Kaur and Dhirendra P. Singh from Environmental Toxicology PhD Program, Southern University and A&M College, Baton Rouge, Louisiana 70813 for critical reading of the manuscript. This work was supported by Young Clinical Scientist Award from the Flight Attendant Medical Research Institute (FAMRI- 123253_YCSA_Faculty); NIH R15 (7 R15 ES023151 02); Southern University System Foundation Grant (COSC0016) and Louisiana Biomedical Research Network (LBRN) Startup Funds 2P20GM103424-14 (Subaward No. 100011) to SB.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Panday, A., Inda, M.E., Bagam, P. et al. Transcription Factor NF-κB: An Update on Intervention Strategies. Arch. Immunol. Ther. Exp. 64, 463–483 (2016). https://doi.org/10.1007/s00005-016-0405-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00005-016-0405-y