Abstract
Myeloid-derived suppressor cells (MDSCs) are a heterogeneous population of cells involved in immune regulation. This population subdivides into granulocytic MDSCs and monocytic MDSCs, which regulate immune responses via the production of various molecules including reactive oxygen species, nitric oxide, arginase-1, interleukin-10, and transforming growth factor-β. Most studies of MDSCs focused on their role in tumors. MDSCs protect tumor cells from immune responses, and thus the frequency of MDSCs associates with poor prognosis. Many recent studies reported an important role for MDSCs in inflammatory diseases via the regulation of immune cells. In addition, the utilization of MDSCs by infectious pathogens suggests an immune evasion mechanism. Thus, MDSCs are important immune regulators in inflammatory diseases, as well as in tumors. This review focuses on the role of MDSCs in the regulation of inflammation in non-tumor settings.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Since myeloid-derived suppressor cells (MDSCs) were first described in 1987 in a mouse model of lung cancer (Young et al. 1987), they have been reported in almost all types of murine tumor models and cancer patients. In recent years, MDSCs have been implicated in a variety of inflammatory immune responses, such as viral infections (Goh et al. 2013), non-neoplastic inflammation (Enioutina et al. 2011), and autoimmunity (Cripps and Gorham 2011). MDSCs elicit strong immunosuppressive effects by inhibiting T cell-mediated inflammation and may therefore be a therapeutic target for autoimmunity and transplantation. MDSCs are a heterogeneous population of cells consisting of myeloid lineage progenitors and immature myeloid cells. In healthy individuals, these progenitors rapidly differentiate into mature and functional granulocytes, macrophages, and dendritic cells (DCs). In pathological conditions, such as cancer, certain autoimmune diseases, various infectious diseases, and bone marrow transplantation (BMT), MDSCs accumulate and are activated, which suppresses innate and adaptive immune responses (Youn and Gabrilovich 2010).
Most knowledge of MDSCs stems from cancer studies, and this has already been addressed in several reviews (Jiang et al. 2014; Markowitz et al. 2013; Ostrand-Rosenberg et al. 2012). Interest in the roles of MDSCs in inflammatory diseases dramatically increased recently, and this new information needs to be reviewed. This review describes recent studies of MDSCs in non-neoplastic inflammatory conditions, such as autoimmunity, infection, and transplantation, paying particular attention to the distributions of subpopulations of MDSCs in each condition. This review also discusses the mechanisms underlying the expansion and immunosuppressive functions of MDSCs as well as the therapeutic benefits for inflammatory diseases and transplantation.
MDSCs
Phenotypes
MDSCs are broadly classified into two subgroups with granulocytic and monocytic phenotypes. As the name suggests, monocytic MDSCs (Mo-MDSCs) have a similar appearance to that of monocytes in that they have a single, large, spherical nucleus, while granulocytic MDSCs (Gr-MDSCs) have multi-lobed nuclei resembling those of polymorphonuclear (PMN) cells (Ostrand-Rosenberg 2010). These two types of MDSCs are distinguished by the expression of surface markers and their distinct mechanisms of immunosuppression. In mice, Gr-MDSCs are CD11b+Ly-6G+Ly6Clow MDSCs, while Mo-MDSCs are CD11b+Ly-6G−Ly6Chigh MDSCs (Youn and Gabrilovich 2010). In contrast to murine MDSCs, which are defined by their expression of Gr-1 and CD11b, human MDSCs are inadequately characterized owing to the lack of uniform markers. CD34+ MDSCs were first reported in patients with head and neck cancer in 1995 (Pak et al. 1995); however, the lack of clearly defined cell surface markers for human MDSCs makes the study of such cells particularly challenging. Nevertheless, a well-organized review of various subtypes of human MDSCs was published (Wang et al. 2012). The surface markers of human Mo-MDSCs and Gr-MDSCs are CD33+CD11b+HLA-DRlow/−CD14±CD15low/− and CD33+CD11b+HLA-DRlow/−CD14−CD15+CD66b+, respectively (Gantt et al. 2014). Gr-MDSCs appear to be predominant in cancer. However, the relative importance of Mo-MDSCs and Gr-MDSCs in other chronic diseases, including viral infections and inflammatory diseases, is unclear (Gantt et al. 2014).
Immunosuppressive Mechanisms
MDSCs suppress immune responses through various mediators, including arginase-1, inducible nitric oxide synthase (iNOS), and reactive oxygen species (ROS), and through the manipulation of immune cell functions (Youn and Gabrilovich 2010). These mediators function independently of, or cooperatively with, each other and play a pivotal role in suppressing host immune responses. This section describes the immunosuppressive mechanism of MDSCs.
Arginase-1 and iNOS
Control of the l-arginine level by arginase-1 and iNOS is an important mechanism underlying MDSC-mediated immune suppression. Arginase-1 and iNOS metabolize l-arginine. Arginine starvation inhibits T cell proliferation via decreasing CD3ζ-chain expression (Rodriguez et al. 2004) and preventing the expression of cell cycle regulators such as cyclin D3 and Cdk4 (Rodriguez et al. 2007). Gantt et al. (2014) reported that MDSCs might contribute to feto-maternal tolerance and ineffective immune responses to various infections and vaccines during early postnatal life. Arginase-mediated suppression of maternal T cell responses significantly increases during human pregnancy (Kropf et al. 2007). Of the MDSC subpopulations, Gr-MDSCs, which produce a large amount of arginase in humans (Popovic et al. 2007), are present in large numbers in pregnant women and cord blood, and their number decreases rapidly during infancy (Gervassi et al. 2014; Rieber et al. 2013).
During its metabolism by iNOS, l-arginine is converted into citrulline and nitric oxide (NO), and the produced NO suppresses T cell function via inhibiting Janus kinase and signal transducer and activator of transcription (STAT) 3 signaling (Bingisser et al. 1998), reducing major histocompatibility complex (MHC) class II expression, and inducing T cell apoptosis (Harari and Liao 2004). The detailed mechanisms underlying these effects were previously reviewed (Gabrilovich and Nagaraj 2009).
ROS and Peroxynitrite
ROS, particularly hydrogen peroxide (H2O2), can act on immature myeloid cells to reduce their ability to differentiate into macrophages and DCs (Kusmartsev and Gabrilovich 2003). Murine Gr-MDSCs are generally thought to exert their immunosuppressive effects via ROS, usually generated by NADPH oxidase, and Mo-MDSCs produce NO via iNOS (Dietlin et al. 2007). It is expected that murine Mo-MDSCs use iNOS and arginase-1, while Gr-MDSCs use ROS and arginase-1. Unlike their mouse counterparts, human Mo-MDSCs and Gr-MDSCs share the same mechanism of suppression because they both suppress T cell proliferation and interferon (IFN)-γ production via ROS generated by NADPH oxidase (Corzo et al. 2009). The generated ROS can modify proteins directly or in combination with NO to generate peroxynitrite (Nagaraj et al. 2007). Peroxynitrite is another important mediator of MDSC-mediated suppression of T cell function (Gabrilovich and Nagaraj 2009; Lu et al. 2011). NO induces the free radical peroxynitrite, the most powerful oxidant in the human body. Peroxynitrite induces nitration and nitrosylation of the amino acids cysteine, methionine, tryptophan, and tyrosine (Vickers et al. 1999). Peroxynitrite drives apoptosis of antigen-specific T cells by nitrotyrosylating key proteins, thereby preventing tyrosine phosphorylation of proteins necessary for T cell activation (Brito et al. 1999). Production of peroxynitrite by MDSCs via their direct contact with T cells results in the nitration of T cell receptor (TCR) and CD8 (Nagaraj et al. 2007). As a result, structural changes in the TCR-CD3 complex reduce the physical interaction between CD8 and TCR. Thereafter, TCRs lose the ability to recognize specific antigens, and T cell signaling is disturbed (Nagaraj et al. 2010). In addition, high levels of peroxynitrite are associated with the progression of many types of cancer (Ekmekcioglu et al. 2000; Kinnula et al. 2004; Nakamura et al. 2006), and this effect is linked with T cell unresponsiveness.
Induction of Regulatory T Cells
MDSCs can induce forkhead box P3+ T cells in vivo. In mice bearing 1D8 ovarian tumors, MDSCs mediate the expansion of regulatory T (Treg) cells through CD152, also known as cytotoxic lymphocyte antigen 4, expressed on Treg cells (Yang et al. 2006). In addition to the direct interaction between MDSCs and Treg cells, MDSCs also can induce the expansion of Treg cells through the production of cytokines. Huang et al. (2006) reported that the induction of Treg cells by MDSCs required the activation of tumor-specific T cells and the presence of IFN-γ and interleukin (IL)-10, but was independent of NO production. In a mouse model of lymphoma, MDSCs induced Treg cell expansion via a mechanism that involved arginase-1 and the capture, processing, and presentation of tumor-associated antigens, but which was independent of transforming growth factor (TGF)-β (Serafini et al. 2008). By contrast, Movahedi et al. (2008) showed that the Treg cell population was maintained at a high level and was not related to the kinetics of MDSC population expansion during tumor growth. Thus, they could not find a significant correlation between the frequencies of Treg cells and MDSCs in their system (Movahedi et al. 2008). Furthermore, Dugast et al. (2008) showed that MDSCs co-express CD80 and CD86 and that such cells slightly inhibit Treg cell expansion in a rat kidney allograft tolerance model, although this inhibition is not as effective as MDSC inhibition of effector T cells. Thus, MDSCs from different experimental systems and disease conditions can have different effects on Treg expansion. This is possibly due to the heterogeneity of MDSC populations.
Regulation of T cell Activation
MDSCs can take up soluble antigens and process and present these to T cells. Down-regulation of the TCRζ chain is critical for the inhibition of normal T cell function, including their proliferation and IFN-γ production (Baniyash 2004). The blockade of MDSC–T cell interactions using a specific anti-MHC-I antibody abrogates MDSC-mediated inhibition of T cell responses in vitro and in vivo (Gabrilovich et al. 2001; Kusmartsev et al. 2005). MDSCs constitutively express ADAM17 at their surface and cleave l-selectin from T cells. As a result, T cells cannot move to tumor-draining lymph nodes, where they normally detect tumor antigens, and therefore cannot be activated (Ostrand-Rosenberg 2010). Kim et al. (2011) suggested a potential mechanism for IL-10- and IFN-γ-dependent regulation of CD8+ T cell function by MDSCs via the interaction between programmed cell death-1 and its ligand.
MDSCs can impair tumor immunity not only by suppressing T cell activation but also by interacting with macrophages to increase IL-10 production and decrease IL-12 production, thereby promoting a tumor-promoting type 2 response (Sinha et al. 2007). Delano et al. (2007) demonstrated that expansion of MDSCs in vivo contributed to the induced Th2 polarization of antibody responses after sepsis. Turnquist et al. reported that IL-33 administration greatly increases the population of splenic MDSCs in normal and transplanted mice and suggested that IL-33 prolongs cardiac allograft survival by promoting Th2 responses (Turnquist et al. 2011).
Th17 cells are a distinct subset of CD4+ T cells, play an important role in host defense against specific pathogens, and potently induce autoimmunity and tissue inflammation (Dong 2008). Similar to other T cells, there are controversial findings regarding the effects of MDSCs on Th17 cells. Wang et al. (2015) reported that Th17 cell differentiation was suppressed when CD4+ T cells were co-cultured with MDSCs in vitro and following adoptive transfer of MDSCs into collagen-induced arthritis (CIA) mice. By contrast, in the experimental autoimmune encephalomyelitis (EAE) disease model, MDSCs promote Th17 cell differentiation (Yi et al. 2012). These differing effects of MDSCs on Th17 cells can be explained by the fact that Mo-MDSCs were adoptively transferred into the EAE disease model in the study by Yi et al. (2012), whereas Gr-MDSCs were adoptively transferred into the CIA disease model in the study by Wang et al. (2015). Different subsets of MDSCs, such as Gr-MDSCs and Mo-MDSCs, may inhibit inflammation via different mechanisms. Furthermore, the inflammatory responses differ between EAE and CIA. However, evidence supporting these hypotheses is lacking, and each subtype of MDSCs must be further characterized.
Regulation of Natural Killer Cells and DCs
Natural killer (NK) cells are involved in the first line of immune defense and play critical roles in antitumor immunity. MDSCs suppress NK cell function (Li et al. 2009; Suzuki et al. 2005); therefore, it is important to understand NK cell-MDSC interactions. Li et al. (2009) showed that down-regulation of NK cell function inversely correlated with a marked increase in the population of MDSCs in the liver and spleen of orthotopic liver cancer-bearing mice. MDSCs inhibit cytotoxicity, NKG2D expression, and IFN-γ production of NK cells in vitro and in vivo via membrane-bound TGF-β1 (Li et al. 2009). In another study, the absence of invariant natural killer T (NKT) cells in mice during influenza A virus (IAV) infection resulted in the expansion of MDSCs, leading to a high IAV titer and increased mortality (De Santo et al. 2008). However, there are conflicting findings regarding the effect of MDSCs on NK cell function in murine tumor models. Ko et al. (2009) suggested that murine NKT cells facilitate the conversion of immunosuppressive MDSCs into immunogenic antigen-presenting cells (APCs), eliciting antitumor immunity and providing the basis for alternative cell-based vaccines, which indicates that MDSCs can be converted into inflammation-inducing cells under certain conditions. Nausch et al. (2008) reported an unexpected activating role of MDSCs on NK cells, which is possibly regulated through STAT1 (Liu et al. 2007). To understand the effects of MDSCs on NK cells and to apply this to the treatment of various diseases, further work is required in humans. Only one study examined NK cell-MDSC crosstalk in humans. Hoechst et al. (2009) showed that NK cell function was impaired in patients with hepatocellular carcinoma (HCC). In an in vitro study, MDSCs impaired NK cell function, and depletion of MDSCs from peripheral blood mononuclear cells (PBMCs) improved NK cell cytotoxic activity. The increased population of MDSCs in HCC patients might be one reason for their impaired NK cell function. Interestingly, arginase-1, iNOS, and ROS do not mediate the suppression of NK cells; rather, blockade of NKp30 can partially reverse the inhibitory function of human MDSCs on NK cells in vitro (Hoechst et al. 2009).
DCs are key initiators and regulators of immune responses. Interestingly, DCs and MDSCs have opposing roles in the immune system (Torres-Aguilar et al. 2010). Whereas activation of endogenous DC function suppresses cancer progression, suppression of DC function is beneficial in mouse models of autoimmunity and transplantation (Pulendran et al. 2010). In contrast to DCs, MDSCs can contribute to the expansion of cancer cells and help to control autoimmune phenomena and transplantation rejection (Gabrilovich and Nagaraj 2009). The establishment of a positive feedback loop between prostaglandin E2 (PGE2) and cyclooxygenase 2 in differentiating monocytes is necessary and sufficient for the differentiation of DCs toward Mo-MDSCs (Obermajer et al. 2011). In addition, recent data showed that MDSCs can differentiate into regulatory DCs in lung cancer (Zhong et al. 2014). Most studies reported MDSC–NK cell or MDSC–DC interactions in cancer conditions, and the mechanism underlying these interactions in inflammatory disease conditions needs to be confirmed. It is suggested that human NK cells can be divided into tolerant NK cells, cytotoxic NK cells, and regulatory NK cells based on their function (Fu et al. 2014). Thus, the effects of MDSCs on specific NK cell populations also need to be tested.
MDSCs in Autoimmunity
MDSC expansion is associated with autoimmunity and inflammation. Given the immunosuppressive function of MDSCs, it is not surprising that recent studies identified an important role for MDSCs in inflammatory diseases. In in vitro experiments, MDSCs can inhibit T cells, typically through the increased production of NO and induction of T cell apoptosis. However, in vivo data are inconclusive. For example, endogenous MDSCs appear to be ineffective at mitigating autoimmune diseases and may even exacerbate such diseases (King et al. 2009). By contrast, exogenous MDSCs can limit autoimmunity in mouse models of inflammatory bowel disease (IBD) (Westendorf et al. 2006), alopecia areata (Marhaba et al. 2007), and type 1 diabetes (Yin et al. 2010). This section describes the latest studies concerning MDSC functions in autoimmunity, both in humans and mouse disease model systems, and discusses their therapeutic benefits (Table 1).
MDSCs in Autoimmune Hepatitis
Autoimmune hepatitis (AIH) is a liver-specific autoimmune disease in which T cells expressing IFN-γ accumulate in liver portal tracts and liver parenchyma and cause hepatocellular damage and liver necrosis (Krawitt 2006). Cripps et al. (2010) showed that Th1 cell accumulation in BALB/c Tgfb1 −/− mouse liver is accompanied by the accumulation of MDSCs. TGF-β1-deficient mice spontaneously develop an acute CD4+ T cell-mediated autoimmune liver disease (Rudner et al. 2003) that is dependent on IFN-γ (Gorham et al. 2001). MDSCs from Tgfb1 −/− livers readily suppressed CD4+ T cell proliferation via NO, IFN-γ, and cell–cell contact. Moreover, this inhibition was specifically associated with Mo-MDSCs, and IFN-γ had an important role in the development of the Mo-MDSC population (Cripps et al. 2010). The authors suggested that Th1 cells, via their release of IFN-γ, drive the accumulation of a regulatory myeloid cell population to the site of inflammation. Although the existence of MDSCs in AIH patients has yet to be demonstrated, CD11b+ cells are accumulated in liver biopsies of such patients (Senaldi et al. 1992).
MDSCs in Arthritis
Rheumatoid arthritis (RA) is a common systemic autoimmune disease that is mainly characterized by inflammation of the joints (Smolen and Aletaha 2015). Recent evidence supports a role for MDSCs in RA (Zhang et al. 2014). MDSCs block joint inflammation and histological damage in CIA and antigen-induced arthritis models. In these models, MDSCs significantly reduce the clinical score of arthritis and alleviate joint inflammation and histological damage compared with levels in PBS-treated control groups. The transfer of MDSCs down-regulated the levels of pro-inflammatory cytokines (tumor necrosis factor (TNF)-α, IL-6, IL-17, and IL-10) in serum and joints, accompanied by significant reductions in Th17 cells and macrophages in draining lymph nodes and joint tissues (Zhang et al. 2014). Fujii et al. (2013) clarified that MDSCs play crucial roles in the regulation of the pro-inflammatory immune response in the CIA mouse model. MDSCs accumulated in the spleen when the arthritis severity peaked and inhibited the proliferation of CD4+ T cells and their differentiation into Th17 cells in vitro. Moreover, intravenous transfer of spleen-derived MDSCs was followed by a decrease in the number of CD4+ T cells and a reduction in the arthritis severity in recipient mice. Conversely, in vivo depletion of MDSCs prevented the spontaneous resolution of joint inflammation (Fujii et al. 2013). While observations of MDSCs in human arthritis are lacking, Jiao et al. (2013) reported that an increased level of circulating MDSCs negatively correlated with the number of Th17 cells in patients with RA. Unfortunately, in that study, MDSC-like cells were only defined by surface marker expression, and the suppressor activity of these cells toward T cells was not tested.
Egelston et al. (2012) identified a MDSC-like population among synovial fluid (SF) cells from the joints of mice with proteoglycan-induced arthritis. SF cells from proteoglycan-induced arthritis mice significantly suppressed the maturation of DCs upon co-culture. These SF cells exhibited all the characteristics of MDSCs and exerted suppression primarily through the production of NO and ROS, which are usually produced by Gr-MDSCs. Importantly, SF cells also exhibited a profound suppressive effect on the DC- and antigen-dependent proliferation of proteoglycan-specific T cells, and this effect was maintained after the depletion of monocytic cells from the SF cell population. Gr-MDSCs-defined cells in the SF cell population have the potential to limit the expansion of autoreactive T cells, thereby breaking the vicious cycle of autoimmunity and inflammation (Egelston et al. 2012). A pilot study revealed that MDSCs are also present in the SF of RA patients (Kurko et al. 2014). Similar to SF cells collected from mice, SF cells from RA patients significantly suppressed anti-CD3/CD28 antibody-induced proliferation of autologous T cells. However, unlike mouse SF cells, human SF MDSCs can also significantly inhibit the vigorous proliferation of anti-CD3/CD28 antibody-stimulated T cells. These observations suggest that SF MDSCs are non-selective suppressors of T cell expansion and that they can limit the expansion of joint-infiltrating T cells in RA. Although further studies are needed to investigate the suppressive mechanism of SF cells in RA, the concentration of nitrite (formed from NO) is reportedly elevated in the SF of RA patients (Farrell et al. 1992). This strongly suggests that NO production is one mechanism via which SF MDSCs suppress T cell proliferation. SKG mice are BALB/c mice with a spontaneously arising point mutation in ZAP-70, leading to abnormal T cell selection and function (Sakaguchi et al. 2003). Charles et al. (2012) identified a Mo-MDSC-like bone marrow population with osteoclast precursor (OCP) activity in this mouse model. These cells diminished inflammatory arthritis in vivo and suppressed CD4+ and CD8+ T cell proliferation in vitro through the production of NO, similar to Mo-MDSCs (Charles et al. 2012). The authors discussed that the OCP population could suppress T cell proliferation in vitro, but these cells did not show osteoclastogenic potential. Thus, while the OCP population has a MDSC function, not all MDSCs are OCPs. These studies show that it is important to precisely characterize each MDSC subpopulation (Gr-MDSCs and Mo-MDSCs) and understand their immunosuppressive mechanism. Recently, the role of MDSC subpopulations in the pathogenesis of autoimmune arthritis was characterized in the CIA mouse model. During CIA, the population of mononuclear MDSCs increased in association with the severity of joint inflammation, while the population of PMN-MDSCs (identical to Gr-MDSCs according to the defined markers used in this study) decreased (Charles et al. 2012). Interestingly, PMN-MDSCs suppressed polyclonal T cell proliferation more potently than mononuclear MDSCs in vitro. Moreover, the adoptive transfer of PMN-MDSCs, but not of mononuclear MDSCs, decreased joint inflammation, accompanied by reduced serum cytokine secretion and reduced frequencies of Th1 and Th17 cells in draining lymph nodes. Based on their data, Wang et al. (2015) suggested that the shift between PMN-MDSCs and mononuclear MDSCs during the induction of CIA could influence the severity of joint inflammation. To apply MDSCs in clinical practice, the functional features of both subpopulations need to be elucidated, and the migration and plasticity of these cells need to be characterized.
Asthma
Asthma is caused by an imbalance of Th1/Th2 cells, and Th2 cells recruit other immune cells to the lungs and induce allergic airway inflammation (Hamid and Tulic 2009). Th2 cells induce reversible airway obstruction and airway hyper-responsiveness (Djukanovic et al. 1990), and MDSCs have a suppressive function in Th2-dominant allergic inflammation (Deshane et al. 2011). Th2 cytokine production by helper T lymphocytes plays important roles in the pathogenesis of asthma (Barnes 2001). MDSCs produce large amounts of IL-10 and thereby reduce the level of IL-12 during severe inflammation (Bunt et al. 2009). Zhang et al. (2013) examined the levels of MDSCs, IL-10, and IL-12 in children and mice, and reported that MDSC accumulation positively correlates with the level of IL-10 and negatively correlates with that of IL-12 during the onset and development of asthma. They suggested that the interaction between MDSCs and macrophages significantly increases the level of IL-10 and significantly decreases the level of IL-12, which promotes the development of chronic inflammation seen in asthma. However, the underlying mechanisms are unknown. TGF-β1 may be critical to understanding the mechanism via which MDSCs act in asthma. Song et al. (2014) reported that tumor-derived MDSCs shift the balance back to normal in a Th2-dominant asthmatic environment. In an ovalbumin (OVA)-induced asthma mouse model, injected tumor-derived MDSCs were recruited to the lungs and suppressed the infiltration of inflammatory cells, and levels of the Th2 cytokine IL-4 and OVA-specific IgE. However, the level of TGF-β1 increased, and anti-TGF-β1 antibody treatment abolished the inhibitory effects of MDSCs. This suggests that tumor-derived MDSCs inhibit the Th2 cell-mediated response to allergens in a TGF-β1-dependent manner (Song et al. 2014). TGF-β1 appears to suppress a wide variety of inflammatory responses in vivo (Christ et al. 1994; Shull et al. 1992).
Similar to arthritis, a MDSC subpopulation can regulate inflammatory responses to asthma via known mechanisms. Shi et al. (2014) showed that the population of PMN-MDSCs dramatically reduces in aspirin-treated/COX-1-deficient mice and in patients with asthma, which correlates with the arginase activity in PMN-MDSCs. They further demonstrated that COX-1-derived PGE2 governs arginase-1 expression predominantly through an EP4/cAMP/PKA/CREB pathway in bone marrow-derived PMN-MDSCs (Shi et al. 2014), which are analogous to bone marrow-derived macrophages (Modolell et al. 1995). Lipopolysaccharide (LPS) was recently shown to induce the expansion of CD11b+Gr1intF4/80+ immature myeloid cells in patients with allergic airway inflammation (Arora et al. 2011). This is extremely interesting because LPS associates with protection from allergic diseases, such as asthma, in humans (Rodriguez et al. 2003). These cells alleviate asthma by suppressing the DC-mediated reaction of primed Th2 cells (Arora et al. 2010), suggesting a potential protective effect of MDSCs in the development of asthma.
MDSCs in IBD
IBD encompasses several diseases, including Crohn’s disease and ulcerative colitis, and many studies reported a relationship between MDSCs and IBD in humans and experimental mouse model systems (Ostanin and Bhattacharya 2013). IBD can be induced in transgenic mice harboring enterocyte-specific expression of hemagglutinin (HA) after a single adoptive transfer of HA-specific CD8+ T cells (CL4-TCR) (Fagundes et al. 2007). Haile et al. (2008) described the development of an MDSC population in this model. Interestingly, two transfers of CL4-TCR cells, separated by an interval of 12 days, rendered mice tolerant to enterocolitis at a third CL4-TCR cell adoptive transfer. Three CL4-TCR transfers caused a substantial induction of MDSCs in both the spleen and intestine, and these MDSCs suppressed CD8+ T cell proliferation ex vivo via NO production. Peripheral blood from IBD patients exhibited an increased frequency of cell populations with a surface phenotype (CD14+HLA-DR−/low) suggestive of a MDSC population (Haile et al. 2008). This research group recently showed that the adhesion protein CD49d is expressed specifically on Mo-MDSCs in the mouse enterocyte-HA model system and in murine cancer models (Haile et al. 2010). While Haile et al. (2008) suggested that Mo-MDSCs function as immune suppressors in IBD, Su et al. (2013) recently evaluated the therapeutic efficacy of Gr-MDSC subsets transplanted from normal mice into mice with colitis. After this transplantation, recipient mice showed an increased survival rate and decreased injury scores, myeloperoxidase activities, and IL-6 levels (Su et al. 2013).
Dextran sulfate sodium (DSS) is commonly used to chemically induce acute intestinal inflammation in rodent IBD models. DSS-induced colitis is characterized by weight loss, bloody diarrhea, epithelial cell damage, and immune cell infiltration. In this model, the population of spleen MDSCs is significantly increased, and this correlates with the severity of intestinal inflammation (Zhang et al. 2011a). Adoptive transfer of MDSCs in such models reduced inflammation and promoted efficient colonic mucosal healing (Zhang et al. 2011b). Trinitrobenzene sulfonic acid (TNBS) can also induce murine colitis as a common animal model of human Crohn’s disease. Guan et al. (2013) identified subsets of MDSCs and showed that adoptive transfer of MDSCs isolated from mice with colitis could significantly suppress TNBS-induced intestinal inflammation and down-regulate cytokine production (IL-17, TNF, and IFN-γ) in colon tissue.
As mentioned above, MDSCs are induced by strong oxidizing agents such as H2O2, NO, and peroxynitrite. Therefore, it is expected that antioxidants can control the expansion of MDSCs. Resveratrol is a natural phenol and exhibits strong anti-inflammatory properties in various autoimmune diseases (Birrell et al. 2005; Elmali et al. 2005; Singh et al. 2007, 2010). Singh et al. (2012) showed that resveratrol-induced immunosuppressive CD11b+Gr-1+ cells that express arginase-1, which correlated with reduced colitis severity, reduced the percentage of activated T cells in colitis.
MDSCs in Multiple Sclerosis
EAE is a commonly used mouse model of multiple sclerosis (MS), a T cell-mediated autoimmune inflammatory disease of the central nervous system (CNS) (Roder and Hickey 1996). Immunization of susceptible mice with myelin proteins can induce EAE. Myelin-specific T cells are activated in the periphery and migrate into the CNS, where they cause demyelination and CNS damage (Croxford et al. 2011). T cell inflammation in the CNS is accompanied by the recruitment of myeloid cells from the periphery, and recent studies reported a role for MDSCs in MS. Zhu et al. (2007) showed that CD11b+ cells accumulated in the blood, spleen, and CNS of mice following the induction of EAE, and Mo-MDSCs isolated from the spleen potently suppressed the proliferation of CD4+ T cells and CD8+ T cells ex vivo. Mo-MDSCs induced T cell apoptosis via NO production. Although the kinetics of Mo-MDSC accumulation positively correlated with the mouse clinical score/disease severity of EAE in this study, they did not directly address the specific role of Mo-MDSCs in vivo (Zhu et al. 2007). Indeed, other studies suggest that MDSCs contribute to CNS damage.
Mice deficient in CCR2 exhibit reduced accumulation of myeloid cells in inflamed tissue and develop less severe EAE (Gaupp et al. 2003). Mildner et al. (2009) demonstrated that CCR2 expression in myeloid cells is particularly important for maximum EAE pathology. In this study, decreased pathology was associated with a reduction in the accumulation of CD11b+Ly-6Chi monocytes, similar to Mo-MDSCs, in the CNS, suggesting that these cells are pathologic effectors of MS (Mildner et al. 2009). King et al. (2009) reported that CD11b+Ly-6Chi cells accumulate in blood and the CNS prior to and during the onset of flares of EAE in a model of remitting/relapsing murine EAE. An interesting finding of this study that should not be overlooked is that the transit of the MDSC subset from the blood into the CNS associated with a 1000-fold increase in the expression of iNOS mRNA (King et al. 2009). EAE can also be induced in susceptible mouse strains at 3 weeks after infection of Theiler’s murine encephalomyelitis virus (TMEV), a natural mouse pathogen that results in demyelinating disease (Olson et al. 2001). In this model system, MDSCs infiltrated the CNS at an early stage of MS and treatment with an anti-Gr1 or anti-Ly-6C monoclonal antibody reduced EAE pathology. Interestingly, the depletion of CD11b+Ly-6C+ cells increased expression of inflammatory cytokines in the CNS and significantly increased TMEV-specific T cell responses. These observations indicate that MDSCs have multiple roles as organ-specific effectors in EAE, performing both pro-inflammatory and anti-inflammatory functions.
Others
Interestingly, over 15 years ago, McIntosh and Drachman (1999) described a population of large suppressive macrophages (LSMs) in a mouse model of myasthenia gravis, a neuromuscular disorder in which antibodies specific for the acetylcholine receptor at neuromuscular junctions lead to weakness and fatigability of skeletal muscle. Ex vivo, LSMs induced apoptosis of activated T cells (McIntosh and Drachman 1999). Marhaba et al. (2007) reported that MDSCs can be induced in a mouse model of the autoimmune disease alopecia areata, in which inflammatory immune pathology leads to hair loss. These MDSCs inhibited T cell proliferation in vitro and, following their in vivo application, partially restored hair growth (Marhaba et al. 2007). MDSCs were also described in a mouse model of experimental autoimmune uveoretinitis (EAU), an autoimmune inflammatory disease of the eye induced in mice by the injection of eye-specific proteins along with adjuvant. These cells resembled Mo-MDSCs and accumulated in the eye as inflammation in the eye progressed. Cells derived from inflamed eyes inhibited the proliferation of activated T cells ex vivo (Kerr et al. 2008). Subsequent studies from this group showed that the suppressor function of MDSCs in EAU requires an intact TNF response axis (Raveney et al. 2009). A recent study in a mouse model of type 1 diabetes demonstrated that MDSCs isolated from tumor-bearing mice could abrogate CD4+ T cell-mediated pancreatic islet damage (Yin et al. 2010). Drujont et al. (2014) evaluated the potential of adoptive transfer of in vitro-derived MDSCs from bone marrow cells with GM-CSF and IL-6. These cells strongly inhibited CD8+ T cell proliferation in vitro. However, adoptive transfer of these cells did not alter antigen-specific CD8+ T cell proliferation and cytotoxicity in a stringent model of type 1 diabetes (Drujont et al. 2014). MRL-Fas lpr mice develop a multi-organ inflammatory disorder that resembles human systemic lupus erythematosus. A recent study identified CD11b+Gr1low cells in these mice. These cells increased in the kidney and blood during disease progression and suppressed CD4+ T cell proliferation ex vivo. Interestingly, inclusion of the arginase-specific inhibitor Nor-NOHA blocked this suppression, indicating that arginase, rather than iNOS, is the principal enzyme mediating suppression by MDSCs in this mouse model (Iwata et al. 2010). Although the mechanism of suppression was not specifically examined, MDSCs obviously play a critical role in various autoimmune diseases.
MDSCs in Infectious Diseases
Potent pro-inflammatory cytokines, such as TNF-α and IL-1β, are elevated in chronic viral infections and promote the survival and accumulation of MDSCs (Tu et al. 2008; Zhao et al. 2012). Oncogenic viruses, such as hepatitis B virus (Anthony et al. 2011; Chen et al. 2011; Kong et al. 2014) and human papillomavirus (Sunthamala et al. 2014), establish cancers in which levels of MDSCs are increased. Several reviews described the relationship between MDSCs and cancer-associated viruses (Khaled et al. 2013; Ostrand-Rosenberg and Sinha 2009). This review focuses on the latest studies of MDSCs in non-neoplastic infections. MDSCs were recently reported in a variety of non-tumor pathologies, including bacterial (Poe et al. 2013), parasitic (Van Ginderachter et al. 2010), fungal (Mencacci et al. 2002), and viral (Goh et al. 2013) infections. Viral infections utilize diverse pathways to induce the local and peripheral accumulation of MDSCs. More than one subset of MDSCs with different mechanisms of suppression can be found in the same type of viral infections (Chen et al. 2011; Qin et al. 2013).
The hepatitis C virus (HCV) core protein plays a critical role in the pathogenesis of hepatitis C because it can inhibit T cell activation and proliferation (Yao et al. 2003, 2004), IL-12 production by macrophages (Lee et al. 2001), and apoptosis of infected hepatocytes (Nguyen et al. 2006). This core protein can also activate the STAT3 pathway in APCs (Tacke et al. 2011), and STAT3 stimulates the generation of MDSCs (Cheng et al. 2008). STAT3 signaling up-regulates the myeloid-related protein S100A9, which not only prevents DC differentiation but also contributes to the accumulation of MDSCs. Furthermore, STAT3 enhances the immunosuppressive activity of MDSCs by up-regulating NADPH oxidase, leading to increased ROS production (Corzo et al. 2009). The addition of the HCV core protein to healthy human PBMCs led to the production of a distinct population of MDSCs, which effectively suppressed CD4+ and CD8+ T cell proliferation and IFN-γ production in a ROS-dependent manner (Tacke et al. 2011). The population of MDSCs in the blood of chronically infected HCV patients transiently decreases during antiviral therapy (Cai et al. 2013). IL-1β is another critical inflammatory mediator, and its level increases not only upon infection with HCV in vitro (Burdette et al. 2012) but also in the blood and livers of HCV-infected patients (Lapinski 2001), and it promotes the survival of MDSCs in the tumor microenvironment (Elkabets et al. 2010). Therefore, the effect of IL-1β on MDSC differentiation should be studied. However, Goh et al. (2013) discussed that, given that IL-1β is present in nearly every immune response, this effect is unlikely to be specific to HCV and may instead represent a more general mechanism of MDSC generation.
Human immunodeficiency virus (HIV) can generate MDSCs to facilitate its propagation (Macatangay et al. 2012). Specifically, the transcriptional transactivator protein of HIV contributes to the immunosuppressive environment by inhibiting MHC class II expression (Kanazawa et al. 2000), which is characteristically low on human MDSCs. Qin et al. (2013) showed that HIV-1 seropositive patients had increased numbers of Mo-MDSCs in their peripheral blood, which suppressed T cell proliferation via arginase-1. Treatment with highly active antiretroviral therapy (HAART) significantly decreased the level of MDSCs, which was matched by a steep drop in viral load. Interestingly, they did not find a notable difference in Gr-MDSCs with HIV infection (Qin et al. 2013). By contrast, Vollbrecht et al. (2012) reported that HIV patients who did not receive HAART had higher levels of Gr-MDSCs than healthy controls, which decreased upon treatment with HAART. In the SIV vaccine model, MDSC expansion inhibits protective cellular immunity (Sui et al. 2014). HIV gp120 is responsible for MDSC expansion, and IL-6 is the key molecule for MDSC-mediated immune suppression (Garg and Spector 2014).
A recent study reported an increase in splenic MDSCs in C57BL/6 mice infected with vesicular stomatitis virus (Liu et al. 2011). This is a negative-strand RNA virus that can infect a variety of animals, including humans, and typically causes an acute, mild, flu-like disease. Interestingly, MDSCs were only generated during a relatively prolonged infection of 5 days and were decreased when the infection was limited to 1 day, suggesting that MDSCs are recruited only during sustained immune responses. Adenoviruses can induce Gr-MDSCs to suppress NK cell proliferation and activation via H2O2 (Zhu et al. 2012). C57BL/6 mice intravenously infected with an adenovirus generated a substantial population of MDSCs, the depletion of which enhanced NK cell activity and viral clearance. Remarkably, infected mice displayed a dramatic increase in splenic Gr-MDSCs as early as 4 h post-infection, which gradually decreased to basal levels within 72 h. By contrast, the kinetics of Gr-MDSCs in bone was opposite to the influx of MDSCs into the spleen. The authors of this study concluded that these results are evidence of the migration of MDSCs from bone marrow into the spleen. If this is true, MDSCs not only elicit organ-specific immunosuppressive effects in accordance with their subtype, but their precursors can move to locations in which immune suppression is required and generate MDSC subtypes suitable for the environment. Although this is an extremely interesting result, many additional studies are needed to better understand this mechanism.
Greifenberg et al. (2009) reported that IFN-γ and LPS boost the development and activation of MDSCs while blocking the further differentiation of bone marrow precursors into DCs. They suggested that when pathogens bring LPS into the host immune system during chronic infection or sepsis, the adaptive immune response generates T cells that deliver IFN-γ into the microenvironment. MDSC activation, both in vivo and in vitro, requires signals provided by both LPS and IFN-γ, cannot be triggered by either component alone, and likely results in NO production by MDSCs (Greifenberg et al. 2009). Burn injuries in mouse models lead to massive myelopoiesis, including MDSC production, which suppresses lymphocyte proliferation (Noel et al. 2005, 2007). MDSCs at the burn site prevent keratinocyte production of murine defensins, which normally prevent infection, and thus augment susceptibility to infection with Pseudomonas aeruginosa (Kobayashi et al. 2008). Additionally, MDSCs infiltrate the burn wound itself, and these and other wound cells increase the levels of various cytokines, such as IL-6, IL-10, keratinocyte chemoattractant, and MCP-1 (Schwacha et al. 2010). Thus, although MDSCs and T cells likely expand to limit tissue damage as a result of increased inflammation following a burn injury, this may also lead to immunosuppression and increased susceptibility to infection.
MDSCs in Transplantation
The induction of immune tolerance continues to be an important goal of clinical organ and tissue transplantation. MDSCs are suggested to have clinical therapeutic applications for tolerance induction (Wu et al. 2014). In transplantation, a complex immune reaction between donor- and recipient-immune cells occurs, which can lead to donor tissue rejection and/or graft-versus-host disease (GVHD), leading to attack of the recipient’s tissues by immune cells of the donor (Bhushan and Collins 2003; Goker et al. 2001). It is thought that these immune reactions can involve a direct pathway of activating allogeneic-reactive T cells, which recognize foreign MHC molecules, and an indirect pathway, in which APCs take up and present foreign peptides to their syngeneic T cells (Lechler et al. 2001). A protective role of MDSCs was also described in murine allogenic transplantation models (De Wilde et al. 2009; Dilek et al. 2012; Dugast et al. 2008; Zhang et al. 2008). In humans, a recent report showed the accumulation of MDSCs and T cells in patients who had received a kidney transplant (Luan et al. 2013).
MDSCs accumulated in a rat model of kidney transplant tolerance. These MDSCs suppressed T cell proliferation in a contact- and iNOS-dependent manner, and selectively suppressed activated effector T cells, whereas natural Treg cells were largely resistant to this effect (Haspot et al. 2005). These data illustrate a novel immunoregulatory mechanism associated with transplant tolerance (Dugast et al. 2008). In addition to kidney transplantation, iNOS is also involved in MDSC-mediated islet allograft survival (Arakawa et al. 2014), and Smad3 is suggested to regulate NO production in the skin and a heart graft mouse model (Wu et al. 2012). Complement component 3 is involved in MDSC induction in a mouse liver transplantation model (Hsieh et al. 2013). Dilek et al. (2012) used DNA microarrays to analyze gene expression in blood-derived MDSCs from rat recipients of kidney allografts and found that CCL5 (Rantes), a chemotactic C–C motif 5 chemokine, was strongly down-regulated after treatment with a tolerizing regimen. Given that CCL5 is expressed in the kidney and participates in the recruitment of leukocytes and particularly T cells, MDSCs may contribute to the establishment of a graft-to-periphery CCL5 gradient in tolerant kidney allograft recipients, which controls the recruitment of T cells to the graft, where they likely help to maintain tolerance (Dilek et al. 2012).
HLA-G (a human immunosuppressive non-classical MHC molecule) protects the fetus from attack by NK cells, macrophages, DCs, monocytes, and T cells by interacting with immune inhibitory receptors, such as immunoglobulin-like transcript (ILT) 2 and ILT4, and modifying cell functions (LeMaoult et al. 2004, 2005; Liang et al. 2008; Rajagopalan and Long 2005; Ristich et al. 2005). MDSCs can be induced by ILT2 inhibitory receptor/HLA-G ligand and may prevent rejection of highly immunogenic organs/tissues in clinical transplantation (Zhang et al. 2008). LPS was repeatedly injected to elicit the emergence of MDSCs using a protocol that induces endotoxin tolerance (Vaknin et al. 2008). These LPS-induced MDSCs suppressed allogeneic and polyclonal T cell responses in vitro and had the potential to control skin allograft rejection. These MDSCs, generated by endotoxin tolerance, produced large amounts of IL-10 and expressed heme oxygenase-1 (HO-1) (De Wilde et al. 2009). HO-1 is a stress-responsive enzyme with immunoregulatory and cytoprotective properties, and its expression may be an additional mechanism by which MDSCs regulate alloreactive T cells (De Wilde et al. 2009). Indeed, HO-1 expression in allografts and xenografts associates with improved graft survival and protection against ischemia reperfusion injury, arteriosclerosis, and chronic rejection, and HO-1 overexpression by gene transfer in heart transplants facilitates tolerance induction (Braudeau et al. 2004; Chauveau et al. 2002; Soares et al. 1998; Tsuchihashi et al. 2007; Yamashita et al. 2006). Recently, Zhu et al. (2013) showed that exogenous administration of granulocyte colony-stimulation-factor (G-CSF) to donor mice caused the accumulation of MDSCs during stem cell mobilization and could lead to the apoptosis of T lymphocytes. They suggested that these data offer a new strategy to prevent and treat GVHD. The effect of G-SCF on GVHD was also investigated in human patients (Lv et al. 2015; Vendramin et al. 2014). Both studies suggested that G-CSF can modulate MDSCs and thereby prevent GVHD after allogeneic transplantation. MDSC subtypes appear to have different functions in transplant models, as in disease models. Luan et al. (2013) demonstrated that while levels of Mo-MDSCs and Gr-MDSCs were increased and the number of circulating Treg cells was elevated in kidney allograft recipients, Mo-MDSCs and a subset of Gr-MDSCs could inhibit CD4+ T cell proliferation.
By contrast, Wang et al. (2013) reported that MDSCs accumulated in the blood and spleens of allogeneic BMT recipient mice, and gradually reduced to physiological levels if GVHD did not occur. MDSC accumulation positively correlated with the severity of GVHD and further increased upon tumor release in BMT. Le Blanc et al. (2013) highlighted that MDSCs can be a double-edged sword in allogeneic hematopoietic stem cell transplantation. Thus, further studies of MDSCs in humans are required to use these cells in transplantation.
Conclusions and Future Perspectives
This review focused on the latest findings regarding MDSCs in non-neoplastic inflammatory diseases, rather than in cancer-related inflammation. MDSCs have a strong impact on almost all immune-related diseases. In particular, the strong immunosuppressive function of MDSCs may make these cells a promising therapy for immune disorders induced by autoimmune responses and some infections, and for transplantation. However, there are substantial gaps in the literature and regarding the clinical utility of these cells. The markers of human MDSCs have not been clearly determined, although human Mo-MDSCs and Gr-MDSCs are currently defined as CD33+CD11b+HLA-DRlow/−CD14±CD15low/− and CD33+CD11b+HLA-DRlow/−CD14−CD15+CD66b+, respectively (Gantt et al. 2014). By contrast, murine MDSCs have well-defined markers, thereby ensuring consistency among studies and research groups.
Adoptive transfer of self-MDSCs is an extremely promising therapy with minimal side effects to cure hypersensitivity immune disorders against foreign antigens. However, a serious problem limits the clinical application of MDSCs, namely, adoptive transfer of MDSCs may promote the immune response in some cases, and it is not clear whether this is due to the transferred MDSCs or the further differentiation of MDSCs. Moreover, immune responses appear to differ depending on the type of disease and the individual. Thus, it must be elucidated whether MDSCs, or a specific subset thereof, suppress or promote the immune response in various conditions. This may help to determine the optimal timing of the administration of a specific subset of MDSCs to suppress T cells in each patient. In addition, MDSC populations need to be characterized in more detail with specific markers because they comprise heterogeneous cells.
MDSCs not only vary in terms of their total number according to disease progress, but also in terms of the distribution of subpopulations (Gr-MDSCs and Mo-MDSCs) and their immunosuppressive function. As mentioned above, several reports showed that the number of MDSCs increases with the progress of autoimmune diseases. Therefore, the introduction of MDSCs may have therapeutic effects during early inflammation when MDSCs have not fully developed; however, it is unlikely that this would have such benefits in late inflammation when the number of MDSCs has greatly increased. The distribution of MDSC subpopulations varies according to disease progress, and this appears to influence the immune response of MDSCs. In particular, this review describes studies of the distributions of MDSC subpopulations because they may provide new evidence to address the current paradoxical analysis of MDSC functions in vivo. Investigation of MDSC chemotaxis may also unravel this problem. Recently, Katoh et al. (2013) showed that loss of CXCR2 in a colitis mouse model dramatically suppresses chronic colonic inflammation and tumorigenesis via inhibiting MDSCs in colonic mucosa and tumors. This proves that MDSC chemotaxis plays an important role in inflammation and cancer development. MDSCs can also be subject to epigenetic regulation, and this can vary depending on the state of inflammation and the microenvironment. In the future, the number of patients with immune disorders will steadily increase due to environmental factors, meaning an effective immune therapy must be developed. To develop such a therapy using MDSCs, although mechanistic studies are important, the genetic etiology must be determined using bioinformatics approaches such as microarrays, next-generation sequencing, and mass spectroscopy.
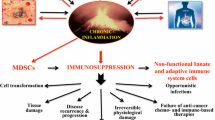
Through clinical and experimental results, it is clear that MDSCs are involved in almost every pathway from T cell proliferation to immune cell migration and infiltration, and the immune evasion of cancer cells. Lack of understanding of these pathways has hindered the cure of immune-related diseases including cancer. Thus, if MDSCs can effectively control such diseases, this would be a landmark point in immune therapy. This review closes by emphasizing that the distribution of MDSC subpopulations and how this changes in immune disorders must be understood to treat such diseases with cell therapies (Fig. 1).
The role of myeloid-derived suppressor cells (MDSCs) in inflammatory diseases. The strong immunosuppressive function of MDSCs may make these cells a promising therapy for immune disorders induced by autoimmune responses and some infections, and for transplantation. MDSCs not only vary in terms of their total number according to disease progress, but also in terms of the distribution of subpopulations [granulocytic MDSCs (Gr-MDSCs) and monocytic MDSCs (Mo-MDSCs)] and their immunosuppressive function. MDSCs inhibit immunity by blocking T cells, natural killer (NK) cells, and dendritic cells (DCs) via mediators such as nitric oxide (NO), reactive oxygen species (ROS), arginase-1 (Arg-1), and cytokines
References
Anthony DD et al (2011) Lower peripheral blood CD14+ monocyte frequency and higher CD34+ progenitor cell frequency are associated with HBV vaccine induced response in HIV infected individuals. Vaccine 29:3558–3563
Arakawa Y et al (2014) Cotransplantation with myeloid-derived suppressor cells protects cell transplants: a crucial role of inducible nitric oxide synthase. Transplantation 97:740–747
Arora M et al (2010) TLR4/MyD88-induced CD11b+Gr-1 int F4/80+ non-migratory myeloid cells suppress Th2 effector function in the lung. Mucosal Immunol 3:578–593
Arora M et al (2011) LPS-induced CD11b+Gr1(int)F4/80+ regulatory myeloid cells suppress allergen-induced airway inflammation. Int Immunopharmacol 11:827–832
Baniyash M (2004) TCR zeta-chain downregulation: curtailing an excessive inflammatory immune response. Nat Rev Immunol 4:675–687
Barnes PJ (2001) Th2 cytokines and asthma: an introduction. Respir Res 2:64–65
Bhushan V, Collins RH Jr (2003) Chronic graft-vs-host disease. JAMA 290:2599–2603
Bingisser RM et al (1998) Macrophage-derived nitric oxide regulates T cell activation via reversible disruption of the Jak3/STAT5 signaling pathway. J Immunol 160:5729–5734
Birrell MA et al (2005) Resveratrol, an extract of red wine, inhibits lipopolysaccharide induced airway neutrophilia and inflammatory mediators through an NF-kappaB-independent mechanism. FASEB J 19:840–841
Bowen JL, Olson JK (2009) Innate immune CD11b+Gr-1+ cells, suppressor cells, affect the immune response during Theiler’s virus-induced demyelinating disease. J Immunol 183:6971–6980
Braudeau C et al (2004) Induction of long-term cardiac allograft survival by heme oxygenase-1 gene transfer. Gene Ther 11:701–710
Brito C et al (1999) Peroxynitrite inhibits T lymphocyte activation and proliferation by promoting impairment of tyrosine phosphorylation and peroxynitrite-driven apoptotic death. J Immunol 162:3356–3366
Bunt SK et al (2009) Inflammation enhances myeloid-derived suppressor cell cross-talk by signaling through Toll-like receptor 4. J Leukoc Biol 85:996–1004
Burdette D et al (2012) Hepatitis C virus activates interleukin-1beta via caspase-1-inflammasome complex. J Gen Virol 93(Pt 2):235–246
Cai W et al (2013) Clinical significance and functional studies of myeloid-derived suppressor cells in chronic hepatitis C patients. J Clin Immunol 33:798–808
Charles JF et al (2012) Inflammatory arthritis increases mouse osteoclast precursors with myeloid suppressor function. J Clin Invest 122:4592–4605
Chauveau C et al (2002) Gene transfer of heme oxygenase-1 and carbon monoxide delivery inhibit chronic rejection. Am J Transplant 2:581–592
Chen S et al (2011) Immunosuppressive functions of hepatic myeloid-derived suppressor cells of normal mice and in a murine model of chronic hepatitis B virus. Clin Exp Immunol 166:134–142
Cheng P et al (2008) Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J Exp Med 205:2235–2249
Christ M et al (1994) Immune dysregulation in TGF-beta 1-deficient mice. J Immunol 153:1936–1946
Corzo CA et al (2009) Mechanism regulating reactive oxygen species in tumor-induced myeloid-derived suppressor cells. J Immunol 182:5693–5701
Cripps JG, Gorham JD (2011) MDSC in autoimmunity. Int Immunopharmacol 11:789–793
Cripps JG et al (2010) Type 1 T helper cells induce the accumulation of myeloid-derived suppressor cells in the inflamed Tgfb1 knockout mouse liver. Hepatology 52:1350–1359
Croxford AL et al (2011) Mouse models for multiple sclerosis: historical facts and future implications. Biochim Biophys Acta 1812:177–183
De Santo C et al (2008) Invariant NKT cells reduce the immunosuppressive activity of influenza A virus-induced myeloid-derived suppressor cells in mice and humans. J Clin Invest 118:4036–4048
De Wilde V et al (2009) Endotoxin-induced myeloid-derived suppressor cells inhibit alloimmune responses via heme oxygenase-1. Am J Transplant 9:2034–2047
Delano MJ et al (2007) MyD88-dependent expansion of an immature GR-1(+)CD11b(+) population induces T cell suppression and Th2 polarization in sepsis. J Exp Med 204:1463–1474
Deshane J et al (2011) Free radical-producing myeloid-derived regulatory cells: potent activators and suppressors of lung inflammation and airway hyperresponsiveness. Mucosal Immunol 4:503–518
Dietlin TA et al (2007) Mycobacteria-induced Gr-1+ subsets from distinct myeloid lineages have opposite effects on T cell expansion. J Leukoc Biol 81:1205–1212
Dilek N et al (2012) Control of transplant tolerance and intragraft regulatory T cell localization by myeloid-derived suppressor cells and CCL5. J Immunol 188:4209–4216
Djukanovic R et al (1990) Mucosal inflammation in asthma. Am Rev Respir Dis 142:434–457
Dong C (2008) TH17 cells in development: an updated view of their molecular identity and genetic programming. Nat Rev Immunol 8:337–348
Drujont L et al (2014) Evaluation of the therapeutic potential of bone marrow-derived myeloid suppressor cell (MDSC) adoptive transfer in mouse models of autoimmunity and allograft rejection. PLoS One 9:e100013
Dugast AS et al (2008) Myeloid-derived suppressor cells accumulate in kidney allograft tolerance and specifically suppress effector T cell expansion. J Immunol 180:7898–7906
Egelston C et al (2012) Suppression of dendritic cell maturation and T cell proliferation by synovial fluid myeloid cells from mice with autoimmune arthritis. Arthritis Rheum 64:3179–3188
Ekmekcioglu S et al (2000) Inducible nitric oxide synthase and nitrotyrosine in human metastatic melanoma tumors correlate with poor survival. Clin Cancer Res 6:4768–4775
Elkabets M et al (2010) IL-1beta regulates a novel myeloid-derived suppressor cell subset that impairs NK cell development and function. Eur J Immunol 40:3347–3357
Elmali N et al (2005) Effect of resveratrol in experimental osteoarthritis in rabbits. Inflamm Res 54:158–162
Enioutina EY et al (2011) A role for immature myeloid cells in immune senescence. J Immunol 186:697–707
Fagundes CT et al (2007) ST2, an IL-1R family member, attenuates inflammation and lethality after intestinal ischemia and reperfusion. J Leukoc Biol 81:492–499
Farrell AJ et al (1992) Increased concentrations of nitrite in synovial fluid and serum samples suggest increased nitric oxide synthesis in rheumatic diseases. Ann Rheum Dis 51:1219–1222
Fu B et al (2014) Subsets of human natural killer cells and their regulatory effects. Immunology 141:483–489
Fujii W et al (2013) Myeloid-derived suppressor cells play crucial roles in the regulation of mouse collagen-induced arthritis. J Immunol 191:1073–1081
Gabrilovich DI, Nagaraj S (2009) Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 9:162–174
Gabrilovich DI et al (2001) Mechanism of immune dysfunction in cancer mediated by immature Gr-1 + myeloid cells. J Immunol 166:5398–5406
Gantt S et al (2014) The role of myeloid-derived suppressor cells in immune ontogeny. Front Immunol 5:387
Garg A, Spector SA (2014) HIV type 1 gp120-induced expansion of myeloid derived suppressor cells is dependent on interleukin 6 and suppresses immunity. J Infect Dis 209:441–451
Gaupp S et al (2003) Experimental autoimmune encephalomyelitis (EAE) in CCR2(−/−) mice: susceptibility in multiple strains. Am J Pathol 162:139–150
Gervassi A et al (2014) Myeloid derived suppressor cells are present at high frequency in neonates and suppress in vitro T cell responses. PLoS One 9:e107816
Goh C et al (2013) Myeloid-derived suppressor cells: the dark knight or the joker in viral infections? Immunol Rev 255:210–221
Goker H et al (2001) Acute graft-vs-host disease: pathobiology and management. Exp Hematol 29:259–277
Gorham JD et al (2001) Genetic regulation of autoimmune disease: bALB/c background TGF-beta 1-deficient mice develop necroinflammatory IFN-gamma-dependent hepatitis. J Immunol 166:6413–6422
Greifenberg V et al (2009) Myeloid-derived suppressor cell activation by combined LPS and IFN-gamma treatment impairs DC development. Eur J Immunol 39:2865–2876
Guan Q et al (2013) The role and potential therapeutic application of myeloid-derived suppressor cells in TNBS-induced colitis. J Leukoc Biol 94:803–811
Haile LA et al (2008) Myeloid-derived suppressor cells in inflammatory bowel disease: a new immunoregulatory pathway. Gastroenterology 135:871–881 (881 e871–875)
Haile LA et al (2010) CD49d is a new marker for distinct myeloid-derived suppressor cell subpopulations in mice. J Immunol 185:203–210
Hamid Q, Tulic M (2009) Immunobiology of asthma. Ann Rev Physiol 71:489–507
Harari O, Liao JK (2004) Inhibition of MHC II gene transcription by nitric oxide and antioxidants. Curr Pharm Des 10:893–898
Haspot F et al (2005) Anti-CD28 antibody-induced kidney allograft tolerance related to tryptophan degradation and TCR class II B7 regulatory cells. Am J Transplant 5:2339–2348
Hoechst B et al (2009) Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology 50:799–807
Hsieh CC et al (2013) The role of complement component 3 (C3) in differentiation of myeloid-derived suppressor cells. Blood 121:1760–1768
Huang B et al (2006) Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res 66:1123–1131
Iwata Y et al (2010) Involvement of CD11b+ GR-1 low cells in autoimmune disorder in MRL-Fas lpr mouse. Clin Exp Nephrol 14:411–417
Jiang J et al (2014) Phenotypes, accumulation, and functions of myeloid-derived suppressor cells and associated treatment strategies in cancer patients. Hum Immunol 75:1128–1137
Jiao Z et al (2013) Increased circulating myeloid-derived suppressor cells correlated negatively with Th17 cells in patients with rheumatoid arthritis. Scand J Rheumatol 42:85–90
Kanazawa S et al (2000) Tat competes with CIITA for the binding to P-TEFb and blocks the expression of MHC class II genes in HIV infection. Immunity 12:61–70
Katoh H et al (2013) CXCR2-expressing myeloid-derived suppressor cells are essential to promote colitis-associated tumorigenesis. Cancer Cell 24:631–644
Kerr EC et al (2008) Analysis of retinal cellular infiltrate in experimental autoimmune uveoretinitis reveals multiple regulatory cell populations. J Autoimmun 31:354–361
Khaled YS et al (2013) Myeloid-derived suppressor cells in cancer: recent progress and prospects. Immunol Cell Biol 91:493–502
Kim YJ et al (2011) Phagocytosis, a potential mechanism for myeloid-derived suppressor cell regulation of CD8+ T cell function mediated through programmed cell death-1 and programmed cell death-1 ligand interaction. J Immunol 187:2291–2301
King IL et al (2009) Circulating Ly-6C+ myeloid precursors migrate to the CNS and play a pathogenic role during autoimmune demyelinating disease. Blood 113:3190–3197
Kinnula VL et al (2004) Ultrastructural and chromosomal studies on manganese superoxide dismutase in malignant mesothelioma. Am J Respir Cell Mol Biol 31:147–153
Ko HJ et al (2009) Immunosuppressive myeloid-derived suppressor cells can be converted into immunogenic APCs with the help of activated NKT cells: an alternative cell-based antitumor vaccine. J Immunol 182:1818–1828
Kobayashi M et al (2008) Gr-1(+)CD11b(+) cells as an accelerator of sepsis stemming from Pseudomonas aeruginosa wound infection in thermally injured mice. J Leukoc Biol 83:1354–1362
Kong X et al (2014) gammadeltaT cells drive myeloid-derived suppressor cell-mediated CD8+ T cell exhaustion in hepatitis B virus-induced immunotolerance. J Immunol 193:1645–1653
Krawitt EL (2006) Autoimmune hepatitis. N Engl J Med 354:54–66
Kropf P et al (2007) Arginase activity mediates reversible T cell hyporesponsiveness in human pregnancy. Eur J Immunol 37:935–945
Kurko J et al (2014) Identification of myeloid-derived suppressor cells in the synovial fluid of patients with rheumatoid arthritis: a pilot study. BMC Musculoskelet Disord 15:281
Kusmartsev S, Gabrilovich DI (2003) Inhibition of myeloid cell differentiation in cancer: the role of reactive oxygen species. J Leukoc Biol 74:186–196
Kusmartsev S et al (2005) Tumor-associated CD8+ T cell tolerance induced by bone marrow-derived immature myeloid cells. J Immunol 175:4583–4592
Lapinski TW (2001) The levels of IL-1beta, IL-4 and IL-6 in the serum and the liver tissue of chronic HCV-infected patients. Arch Immunol Ther Exp 49:311–316
Le Blanc K et al (2013) Myeloid-derived suppressor cells in allogeneic hematopoietic stem cell transplantation: a double-edged sword? Oncoimmunology 2:e25009
Lechler R et al (2001) Dendritic cells in transplantation—friend or foe? Immunity 14:357–368
Lee CH et al (2001) Hepatitis C virus core protein inhibits interleukin 12 and nitric oxide production from activated macrophages. Virology 279:271–279
LeMaoult J et al (2004) HLA-G1-expressing antigen-presenting cells induce immunosuppressive CD4+ T cells. Proc Natl Acad Sci USA 101:7064–7069
LeMaoult J et al (2005) HLA-G up-regulates ILT2, ILT3, ILT4, and KIR2DL4 in antigen presenting cells, NK cells, and T cells. FASEB J 19:662–664
Li H et al (2009) Cancer-expanded myeloid-derived suppressor cells induce anergy of NK cells through membrane-bound TGF-beta 1. J Immunol 182:240–249
Liang S et al (2008) Modulation of dendritic cell differentiation by HLA-G and ILT4 requires the IL-6–STAT3 signaling pathway. Proc Natl Acad Sci USA 105:8357–8362
Liu C et al (2007) Expansion of spleen myeloid suppressor cells represses NK cell cytotoxicity in tumor-bearing host. Blood 109:4336–4342
Liu C et al (2011) Poly(I:C) induce bone marrow precursor cells into myeloid-derived suppressor cells. Mol Cell Biochem 358:317–323
Lu T et al (2011) Tumor-infiltrating myeloid cells induce tumor cell resistance to cytotoxic T cells in mice. J Clin Invest 121:4015–4029
Luan Y et al (2013) Monocytic myeloid-derived suppressor cells accumulate in renal transplant patients and mediate CD4(+) Foxp3(+) Treg expansion. Am J Transplant 13:3123–3131
Lv M et al (2015) Monocytic and promyelocytic myeloid-derived suppressor cells may contribute to G-CSF-induced immune tolerance in haplo-identical allogeneic hematopoietic stem cell transplantation. Am J Hematol 90:E9–E16
Macatangay BJ et al (2012) MDSC: a new player in HIV immunopathogenesis. AIDS 26:1567–1569
Marhaba R et al (2007) The importance of myeloid-derived suppressor cells in the regulation of autoimmune effector cells by a chronic contact eczema. J Immunol 179:5071–5081
Markowitz J et al (2013) Myeloid-derived suppressor cells in breast cancer. Breast Cancer Res Treat 140:13–21
McIntosh KR, Drachman DB (1999) Induction of apoptosis in activated T cell blasts by suppressive macrophages: a possible immunotherapeutic approach for treatment of autoimmune disease. Cell Immunol 193:24–35
Mencacci A et al (2002) CD80+Gr-1+ myeloid cells inhibit development of antifungal Th1 immunity in mice with candidiasis. J Immunology 169:3180–3190
Mildner A et al (2009) CCR2+ Ly-6Chi monocytes are crucial for the effector phase of autoimmunity in the central nervous system. Brain 132:2487–2500
Modolell M et al (1995) Reciprocal regulation of the nitric oxide synthase/arginase balance in mouse bone marrow-derived macrophages by TH1 and TH2 cytokines. Eur J Immunol 25:1101–1104
Movahedi K et al (2008) Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T cell-suppressive activity. Blood 111:4233–4244
Nagaraj S et al (2007) Altered recognition of antigen is a mechanism of CD8+ T cell tolerance in cancer. Nat Med 13:828–835
Nagaraj S et al (2010) Mechanism of T cell tolerance induced by myeloid-derived suppressor cells. J Immunol 184:3106–3116
Nakamura Y et al (2006) Nitric oxide in breast cancer: induction of vascular endothelial growth factor-C and correlation with metastasis and poor prognosis. Clin Cancer Res 12:1201–1207
Nausch N et al (2008) Mononuclear myeloid-derived “suppressor” cells express RAE-1 and activate natural killer cells. Blood 112:4080–4089
Nguyen H et al (2006) Hepatitis C virus core protein induces expression of genes regulating immune evasion and anti-apoptosis in hepatocytes. Virology 354:58–68
Noel JG et al (2005) Effect of thermal injury on splenic myelopoiesis. Shock 23:115–122
Noel JG et al (2007) Thermal injury elevates the inflammatory monocyte subpopulation in multiple compartments. Shock 28:684–693
Obermajer N et al (2011) Positive feedback between PGE2 and COX2 redirects the differentiation of human dendritic cells toward stable myeloid-derived suppressor cells. Blood 118:5498–5505
Olson JK et al (2001) Direct activation of innate and antigen-presenting functions of microglia following infection with Theiler’s virus. J Virol 75:9780–9789
Ostanin DV, Bhattacharya D (2013) Myeloid-derived suppressor cells in the inflammatory bowel diseases. Inflamm Bowel Dis 19:2468–2477
Ostrand-Rosenberg S (2010) Myeloid-derived suppressor cells: more mechanisms for inhibiting antitumor immunity. Cancer Immunol Immunother 59:1593–1600
Ostrand-Rosenberg S, Sinha P (2009) Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol 182:4499–4506
Ostrand-Rosenberg S et al (2012) Cross-talk between myeloid-derived suppressor cells (MDSC), macrophages, and dendritic cells enhances tumor-induced immune suppression. Semin Cancer Biol 22:275–281
Pak AS et al (1995) Mechanisms of immune suppression in patients with head and neck cancer: presence of CD34(+) cells which suppress immune functions within cancers that secrete granulocyte-macrophage colony-stimulating factor. Clin Cancer Res 1:95–103
Poe SL et al (2013) STAT1-regulated lung MDSC-like cells produce IL-10 and efferocytose apoptotic neutrophils with relevance in resolution of bacterial pneumonia. Mucosal Immunol 6:189–199
Popovic PJ et al (2007) Arginine and immunity. J Nutr 137:1681S–1686S
Pulendran B et al (2010) Programming dendritic cells to induce T(H)2 and tolerogenic responses. Nat Immunol 11:647–655
Qin A et al (2013) Expansion of monocytic myeloid-derived suppressor cells dampens T cell function in HIV-1-seropositive individuals. J Virol 87:1477–1490
Rajagopalan S, Long EO (2005) Viral evasion of NK-cell activation. Trends Immunol 26:403–405
Raveney BJ et al (2009) TNFR1-dependent regulation of myeloid cell function in experimental autoimmune uveoretinitis. J Immunol 183:2321–2329
Rieber N et al (2013) Neutrophilic myeloid-derived suppressor cells in cord blood modulate innate and adaptive immune responses. Clin Exp Immunol 174:45–52
Ristich V et al (2005) Tolerization of dendritic cells by HLA-G. Eur J Immunol 35:1133–1142
Roder J, Hickey WF (1996) Mouse models, immunology, multiple sclerosis and myelination. Nat Genet 12:6–8
Rodriguez D et al (2003) Bacterial lipopolysaccharide signaling through Toll-like receptor 4 suppresses asthma-like responses via nitric oxide synthase 2 activity. J Immunol 171:1001–1008
Rodriguez PC et al (2004) Arginase I production in the tumor microenvironment by mature myeloid cells inhibits T-cell receptor expression and antigen-specific T-cell responses. Cancer Res 64:5839–5849
Rodriguez PC et al (2007) L-arginine availability regulates T-lymphocyte cell-cycle progression. Blood 109:1568–1573
Rudner LA et al (2003) Necroinflammatory liver disease in BALB/c background, TGF-beta 1-deficient mice requires CD4+ T cells. J Immunol 170:4785–4792
Sakaguchi N et al (2003) Altered thymic T-cell selection due to a mutation of the ZAP-70 gene causes autoimmune arthritis in mice. Nature 426:454–460
Schwacha MG et al (2010) Impact of thermal injury on wound infiltration and the dermal inflammatory response. J Surg Res 158:112–120
Senaldi G et al (1992) Immunohistochemical features of the portal tract mononuclear cell infiltrate in chronic aggressive hepatitis. Arch Dis Child 67:1447–1453
Serafini P et al (2008) Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res 68:5439–5449
Shi M et al (2014) Myeloid-derived suppressor cell function is diminished in aspirin-triggered allergic airway hyperresponsiveness in mice. J Allergy Clin Immunol 134(1163–1174):e16
Shull MM et al (1992) Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature 359:693–699
Singh NP et al (2007) Resveratrol (trans-3,5,4′-trihydroxystilbene) ameliorates experimental allergic encephalomyelitis, primarily via induction of apoptosis in T cells involving activation of aryl hydrocarbon receptor and estrogen receptor. Mol Pharmacol 72:1508–1521
Singh UP et al (2010) Resveratrol (trans-3,5,4′-trihydroxystilbene) induces silent mating type information regulation-1 and down-regulates nuclear transcription factor-kappaB activation to abrogate dextran sulfate sodium-induced colitis. J Pharmacol Exp Ther 332:829–839
Singh UP et al (2012) Role of resveratrol-induced CD11b(+) Gr-1(+) myeloid derived suppressor cells (MDSCs) in the reduction of CXCR3(+) T cells and amelioration of chronic colitis in IL-10(−/−) mice. Brain Behav Immun 26:72–82
Sinha P et al (2007) Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol 179:977–983
Smolen JS, Aletaha D (2015) Rheumatoid arthritis therapy reappraisal: strategies, opportunities and challenges. Nat Rev Rheumatol 11:276–289
Soares MP et al (1998) Expression of heme oxygenase-1 can determine cardiac xenograft survival. Nat Med 4:1073–1077
Song C et al (2014) Passive transfer of tumour-derived MDSCs inhibits asthma-related airway inflammation. Scand J Immunol 79:98–104
Su H et al (2013) Transplantation of granulocytic myeloid-derived suppressor cells (G-MDSCs) could reduce colitis in experimental murine models. J Digestive Dis 14:251–258
Sui Y et al (2014) Vaccine-induced myeloid cell population dampens protective immunity to SIV. J Clin Invest 124:2538–2549
Sunthamala N et al (2014) HPV16 E2 protein promotes innate immunity by modulating immunosuppressive status. Biochem Biophys Res Commun 446:977–982
Suzuki E et al (2005) Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin Cancer Res 11:6713–6721
Tacke RS et al (2011) Extracellular hepatitis C virus core protein activates STAT3 in human monocytes/macrophages/dendritic cells via an IL-6 autocrine pathway. J Biol Chem 286:10847–10855
Torres-Aguilar H et al (2010) Tolerogenic dendritic cells in autoimmune diseases: crucial players in induction and prevention of autoimmunity. Autoimmun Rev 10:8–17
Tsuchihashi S et al (2007) Heme oxygenase-1 mediated cytoprotection against liver ischemia and reperfusion injury: inhibition of type-1 interferon signaling. Transplantation 83:1628–1634
Tu S et al (2008) Overexpression of interleukin-1beta induces gastric inflammation and cancer and mobilizes myeloid-derived suppressor cells in mice. Cancer Cell 14:408–419
Turnquist HR et al (2011) IL-33 expands suppressive CD11b+Gr-1(int) and regulatory T cells, including ST2L+ Foxp3+ cells, and mediates regulatory T cell-dependent promotion of cardiac allograft survival. J Immunol 187:4598–4610
Vaknin I et al (2008) A common pathway mediated through Toll-like receptors leads to T- and natural killer-cell immunosuppression. Blood 111:1437–1447
Van Ginderachter JA et al (2010) Myeloid-derived suppressor cells in parasitic infections. Eur J Immunol 40:2976–2985
Vendramin A et al (2014) Graft monocytic myeloid-derived suppressor cell content predicts the risk of acute graft-versus-host disease after allogeneic transplantation of granulocyte colony-stimulating factor-mobilized peripheral blood stem cells. Biol Blood Marrow Transplant 20:2049–2055
Vickers SM et al (1999) Association of increased immunostaining for inducible nitric oxide synthase and nitrotyrosine with fibroblast growth factor transformation in pancreatic cancer. Arch Surg 134:245–251
Vollbrecht T et al (2012) Chronic progressive HIV-1 infection is associated with elevated levels of myeloid-derived suppressor cells. AIDS 26:F31–F37
Wang Y et al (2012) Myeloid-derived suppressor cells participate in preventing graft rejection. Clin Dev Immunol 2012:731486
Wang D et al (2013) Dynamic change and impact of myeloid-derived suppressor cells in allogeneic bone marrow transplantation in mice. Biol Blood Marrow Transplant 19:692–702
Wang W et al (2015) Functional characterization of myeloid-derived suppressor cell subpopulations during the development of experimental arthritis. Eur J Immunol 45:464–473
Westendorf AM et al (2006) Autoimmune-mediated intestinal inflammation-impact and regulation of antigen-specific CD8 + T cells. Gastroenterology 131:510–524
Wu T et al (2012) Smad3-deficient CD11b(+)Gr1(+) myeloid-derived suppressor cells prevent allograft rejection via the nitric oxide pathway. J Immunol 189:4989–5000
Wu T et al (2014) The roles of myeloid-derived suppressor cells in transplantation. Expert Rev Clin Immunol 10:1385–1394
Yamashita K et al (2006) Heme oxygenase-1 is essential for and promotes tolerance to transplanted organs. FASEB J 20:776–778
Yang R et al (2006) CD80 in immune suppression by mouse ovarian carcinoma-associated Gr-1+CD11b1+ myeloid cells. Cancer Res 66:6807–6815
Yao ZQ et al (2003) HCV core/gC1qR interaction arrests T cell cycle progression through stabilization of the cell cycle inhibitor p27Kip1. Virology 314:271–282
Yao ZQ et al (2004) Direct binding of hepatitis C virus core to gC1qR on CD4+ and CD8+ T cells leads to impaired activation of Lck and Akt. J Virol 78:6409–6419
Yi H et al (2012) Mouse CD11b+Gr-1+ myeloid cells can promote Th17 cell differentiation and experimental autoimmune encephalomyelitis. J Immunol 189:4295–4304
Yin B et al (2010) Myeloid-derived suppressor cells prevent type 1 diabetes in murine models. J Immunol 185:5828–5834
Youn JI, Gabrilovich DI (2010) The biology of myeloid-derived suppressor cells: the blessing and the curse of morphological and functional heterogeneity. Eur J Immunol 40:2969–2975
Young MR, Newby M, Wepsic HT (1987) Hematopoiesis and suppressor bone marrow cells in mice bearing large metastatic Lewis lung carcinoma tumors. Cancer Res 47:100–105
Zhang W et al (2008) Human inhibitory receptor immunoglobulin-like transcript 2 amplifies CD11b+Gr1+ myeloid-derived suppressor cells that promote long-term survival of allografts. Transplantation 86:1125–1134
Zhang R et al (2011a) Up-regulation of Gr1+CD11b+ population in spleen of dextran sulfate sodium administered mice works to repair colitis. Inflamm Allergy Drug Targets 10:39–46
Zhang R et al (2011b) Dextran sulphate sodium increases splenic Gr1(+)CD11b(+) cells which accelerate recovery from colitis following intravenous transplantation. Clin Exp Immunol 164:417–427
Zhang YL et al (2013) Peripheral blood MDSCs, IL-10 and IL-12 in children with asthma and their importance in asthma development. PLoS One 8:e63775
Zhang L et al (2014) Myeloid-derived suppressor cells protect mouse models from autoimmune arthritis via controlling inflammatory response. Inflammation 37:670–677
Zhao X et al (2012) TNF signaling drives myeloid-derived suppressor cell accumulation. J Clin Invest 122:4094–4104
Zhong H et al (2014) Origin and pharmacological modulation of tumor-associated regulatory dendritic cells. J Int Cancer 134:2633–2645
Zhu B et al (2007) CD11b+Ly-6C(hi) suppressive monocytes in experimental autoimmune encephalomyelitis. J Immunol 179:5228–5237
Zhu J et al (2012) Myeloid-derived suppressor cells regulate natural killer cell response to adenovirus-mediated gene transfer. J Virol 86:13689–13696
Zhu XJ et al (2013) Amplification of functional myeloid-derived suppressor cells during stem cell mobilization induced by granulocyte colony-stimulation-factor. J Huazhong Univ Sci Technolog Med Sci 33:817–821
Acknowledgments
This work was supported by grants from the Korean Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health and Welfare, Republic of Korea (Grant numbers: HI11C1791 and HI14C1466).
Author information
Authors and Affiliations
Corresponding author
Additional information
Y. Kwak and H.-E. Kim contributed equally to this work.
About this article
Cite this article
Kwak, Y., Kim, HE. & Park, S.G. Insights into Myeloid-Derived Suppressor Cells in Inflammatory Diseases. Arch. Immunol. Ther. Exp. 63, 269–285 (2015). https://doi.org/10.1007/s00005-015-0342-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00005-015-0342-1