Abstract
Immunotherapy with ex vivo generated dendritic cells (DCs) is reported to be of low toxicity and of diverse effectiveness in cancer treatment. The synthetic antigens are frequently used for immunotherapy especially for patients with stable disease after prior treatment. We described the effect of peptide-loaded DCs-based immunotherapy on patient with recurrent surgically resected adenocarcinoma with bronchoalveolar feature with co-existing of Takayasu arteritis and chronic hepatitis B. In January 2010, 61-year-old patient received subcutaneously four bi-weekly vaccinations of DCs loaded with MUC1 and MAGE-3 epitopes. Additionally, he received three bi-weekly booster vaccinations after 7 months from the first course of immunotherapy. Delayed-type hypersensitivity test was positive only for MAGE-3 antigen. The evidence expansion of MAGE-3-specific CD8+ cells after first vaccination and after third vaccination during boosters injections was observed (from 0.08% before vaccination to 0.5% after first vaccination; from 0.05% before booster vaccination to 0.24% after third injection). Computed tomography scans performed after first course and after booster course of vaccination until April 2011 did not shown any presence of lung tumour or metastases. Based on clinical factors (no completed wedge-resection and recurrent character of cancer) as well as on the presence of the tumour-antigen-specific immunological response, we could speculated that immunotherapy prolonged disease free-survival in our patient. Over 16 months from first vaccination, the patient remains without symptoms of cancer.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Dendritic cells (DCs) are effective antigen-presenting cells which strongly participate in the initiation and regulation of primary immune responses (Dubsky et al. 2005; Ueno et al. 2010). Therefore, their use for the active immunotherapy against cancers has been studied with extensive interest (Dubsky et al. 2005; Fujii et al. 2009; Ueno et al. 2010). The schema of ex vivo DCs generation consists of three steps: differentiation of DCs precursors (e.g. peripheral blood monocytes) into immature DCs, maturation and loading with tumour antigen using different kind of delivery (Nencioni et al. 2008; Rossi and Young 2005). The most important issue is to choose the appropriate tumour antigens. In the previous studies, lysates or apoptotic bodies of tumour cells were used for DCs stimulation (Dubsky et al. 2005; Nencioni et al. 2008). Recently, the synthetic oligopeptide (9–12 amino acids) with epitopes of well-defined tumour antigens are widely used in immunotherapy (Bleumer et al. 2007). In non-small cell lung cancer (NSCLC), MUC-1 and MAGE-A3 antigens are frequently used for both ex vivo DCs loading (adoptive immunotherapy) as well as for active immunotherapy (antigenic vaccine) (Bradbury and Shepherd 2008; Romero 2008). Moreover, the results of conducted clinical trials are encouraging (Raez et al. 2005; Wierecky et al. 2006).
The opportunity to application of highly specific maintenance immunotherapy, especially as an adjuvant therapy or after successful radio-chemotherapy of NSCLC, depends on continually access to tumour antigens during vaccine preparation (tumour tissue). Thus, synthetic tumour antigens are suitable for repeated vaccine administration. Moreover, for the efficacy of immunotherapy, the expression of tumour antigens in HLA-A context is required. Although, DCs immunotherapy is reported to be of very low toxicity (autologous vaccine), it could be also of low efficacy for the strategies of tumour immune evasion (Bradbury and Shepherd 2008; Raez et al. 2005; Romero 2008). In an advanced stages of lung cancer, the tumour mass is difficult to be accessed for immunological cells. Moreover, NSCLC patients received numerous immunosuppressive agents (Bradbury and Shepherd 2008; Raez et al. 2005; Romero 2008). However, the development of an autoimmune disorder as well as the stimulation of patients’ immune system by an immunomodulators could modify natural course of disease. In the case of NSCLC patient presented here, the lack of previous chemotherapy, surgical resection of lung tumour as well as the presence of autoimmune Takayasu arteritis (TA) and chronic hepatitis, may have a strong influence on immunotherapy effectiveness.
TA is an autoimmune, idiopathic, large-vessel vasculitis that primarily concerns the aorta. It usually affects young people, but delayed diagnosis is common (Maksimowicz-McKinnon and Hoffman 2007; Nakajima et al. 2007; Noris 2001). The incidence of TA is estimated to be 1.3 in 1 million inhabitants in Europe. The cause of TA is unknown, however chronic or subaccute inflammatory disease plays an important role (chronic hepatitis B, Crohn disease, ulcerative colitis, chronic tonsillitis). Takayasu arteritis may be associated with abnormalities of the immune system, such as expanded populations of natural effector cells (Maksimowicz-McKinnon and Hoffman 2007; Nakajima et al. 2007; Noris 2001).
Here, we investigated the effect of peptide-loaded DCs-based immunotherapy on patient with lung acinar adenocarcinoma with bronchoalveolar feature with co-existing of TA and chronic hepatitis B.
Materials and Methods
Patient’s Characteristic
In September 2004, diagnosis of right lung adenocarcinoma was performed in heavy smoker (30-pack-year) 61-year-old male patient. Moreover, patient suffering for chronic hepatitis B and Takayasu disease. The patient was HBs-Ag-positive and anti-HBs-positive with normal function of liver (anti-HBs IgG level: 249.4 mIU/ml; bilirubine: 0.5 mg/dl; ALAT: 22 U/l; ASPAT: 23 U/l). Takayasu arteritis was diagnosed according to the American College of Rheumatology Criteria: abdominal aortic murmur, leg claudication, abdominal aortic and iliac artery stenosis in angiography. Moreover, the patient presented recurrent subfebrile status with constant elevated level of C-reactive protein (CRP).
In October 2004, patient underwent upper right lobectomy for stage IB tumour (T2aN0M0). Recurrence of adenocarcinoma was observed in 2006 and in January 2007 patient underwent right middle lobe resection with mediastinal lymph node dissection (T2aN1M0, stage IIA). The tumour was moderately differentiated and exhibits acinar and bronchoalveolar pattern.
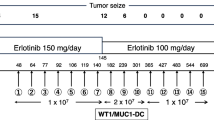
The patient definitively refused to receive adjuvant chemotherapy, which would have been the standard therapy for the disease. However, he was treated with erlotinib for 1 year until June 2008. Molecular analysis had shown positive epidermal growth factor receptor (EGFR) protein expression (IHC method), low copy number of EGFR gene (FISH method) and no mutation of EGFR in exon 19 and 21 (DNA fragments analysis method).
The patient had remained in good performance status (PS = 0). In July 2009, positron emission tomography-computed tomography (PET-CT) scan had shown a small tumour (14 × 10 mm) in the lower lobe of right lung, which was suggestive for a relapse of the disease. In November 2009, wedge-resection of sixth segment of the lower lobe of the right lung was performed. The tumour had a size of 3.5 × 2 cm. Histopathological diagnosis of moderately differentiated adenocarcinoma of lung with acinar and bronchoalveolar growth pattern was maintained. Moreover, lymphatic and blood vessels invasion was detected. However, the resected lymph nodes were negative for cancer cells.
Patient remained in good physical condition (PS = 1) with moderate forced dyspnea and anemia (Hb 9.4 g/dL). The serum lactate dehydrogenase (LDH) level was normal (170 U/L, LDH range: 100–190). During the clinical course of the disease, patient had refused any type of chemotherapy. Finally, he opted for immunotherapy, which had started in January 2010. The vaccination was approved by the Ethical Committee of the Medical University of Lublin (decision no. KE-0254/207/2008).
Patient’s Immunological Status Before Vaccination
The patient’s immunological status before vaccination results from coexisting of TA and chronic hepatitis B, was as follows: the levels of serum IgA and IgG were in the upper normal range (407 and 1,533 mg/dL, respectively), while the IgM level was in the lower normal range (85 mg/dL). The elevated level of CRP was observed (14.5 mg/L). The white blood cell count (WBC) was normal (6,470 cells/μL), including 48.4% of neutrophils, 37.5% of lymphocytes and 4.4% of monocytes. Immunophenotyping of peripheral blood lymphocyte (PBL) subpopulation by flow cytometry had shown: a low percentage of B lymphocytes (1.86%) and a high percentage of NK cells (33.3%) with simultaneously normal percentage of CD4+ and CD8+ T lymphocytes (46.6 and 27.5%, respectively, CD4:CD8 ratio: 1.7). The percentage of Th memory cells (CD45RO+) was 25.1% of total PBL. The percentage of T CD4+ cells with intracellular expression of interleukin (IL)-2 (defined as Th1 lymphocytes) was 23.4%, while the percentage of T CD8+ cells with intracellular expression of interferon (IFN)-γ (defined as cytotoxic T lymphocytes) was 14.2%. The percentage of T regulatory lymphocytes determined as CD4-positive cells with simultaneous expression of intracellular transcription factor FoxP3 and surface receptor for IL-2, CD25 antigen, was 3.91%. When the total lymphocyte populations were analyzed, the percentages of Th1, cytotoxic T lymphocytes (CTLs) and Treg were evaluated as follows: 10.87, 2.96 and 2.48%, respectively. In addition, PBLs expressed the HLA-A2 antigen. Moreover, tumour cells expressed MAGE-3 and MUC-1 in immunohistochemistry.
Preparation and Administration of DCs Vaccine
Blood samples were drawn into heparinised tubes and peripheral blood mononuclear cells (PBMC) were isolated using density gradient centrifugation. CD14-positive cells were isolated by immunomagnetic beads and cultured in DC CellGrow (CellGenix, Germany) medium supplemented with 1,000 IU/ml granulocyte–macrophage colony stimulating factor (GM-CSF) (Gentaur, Belgium) and 500 IU/ml IL-4 (Gentaur, Belgium) for 7 days. For the last 24 h, the immature DCs were stimulated with tumour necrosis factor α (50 ng/ml, Gentaur, Belgium). Thereafter, the mature DCs were pulsed for 2 h with the following tumour-specific peptides: MAGE-3 (FLWGPRALV, 20 μg/ml), MUC-1 (M1.1-STAPPVHNV, M1.2-LLLLTVLTV, 20 μg/ml; Glycotope, Germany). After incubation, the cells were washed and re-suspended in isotonic solution in total volume of 1 ml.
The patient received four bi-weekly vaccinations of total DC doses of 8.8, 7.2, 13.8 and 14.4 × 106 cells, respectively. After an interval of 7 months, the patient received three additional bi-weekly booster vaccinations of total DC doses of 8.3, 7.8 and 9.2 × 106 cells, respectively. The vaccine was administered subcutaneously in right arm region.
The patient was monitored for local and systemic toxicity according to the grading system of the National Cancer Institute-Common Toxicity Criteria (NCI-CTC). All vaccines were well tolerated. We did not observe any systemic adverse events, only subfebrile status was observed after first vaccination (NCI-CTC v.3.0 toxicity ≤ 1). Takayasu disease did not aggravate and during therapeutic process we did not observe any changes in the abdominal CT scans.
The phenotype of DCs after peptide stimulation was checked using a panel of the following specific monoclonal antibodies: CD14-PE, CD11c-PeCy5, CD83-PeCy5 and CD83-FITC, B7-H1-FITC (plays an important role in regulating T cell responses), CD86-PE, CD209-PerCP-Cy5.5 (DC-SIGN), B7-H4-PE (receptor on DCs which regulates immunotolerance activity by T cells), DCIR-AlexaFlour647 (DCs immunoreceptor, strongly expressed on DCs derived from PBMC in the presence of GM-CSF and IL-4) and HLA-DR-TC (Becton–Dickinson, USA). The vaccine consisted of at least 90% of CD11c+/CD14−/B7-H1+/B7-H4−/CD83+/CD209+/DCIR+/CD86+/HLA-DR+ cells, what indicates the maturity and immunocompetence of generated DCs (Fig. 1).
Results
Monitoring of Immune Response
During vaccination, normalization of CRP level to 4.58 mg/L and haemoglobin level to 13 g/dL as well as continual reducing of LDH level to 153 U/L was observed. After booster course of vaccination, we observed reduction LDH level from 198 to 160 U/L, while CRP level was ranged between 5.5 and 6 mg/L. The WBC number was reduced below 6,000 cell per 1 μl with predominance of lymphocytes (range 40–45%). There are no important changes of lymphocytes subpopulation level.
Delayed-type hypersensitivity (DTH) skin test was performed intradermally with synthetic tumour antigens and isotonic salt solution as a control. Antigens were injected into the forearm before vaccination and after first and second vaccinations and the results were measured after 48 h of each injection. The positive skin reaction defined by >1.5 cm erythema with induration of the skin was observed only in case of DTH test with MAGE-3 antigen after first vaccination.
The presence of peripheral blood peptide-specific CTLs was assessed by tetramer staining and flow cytometry technique. Isolated PBMC were incubated with appropriate PE-conjugated tetramer (Glycotope, Germany) in staining buffer for 40 min at 4°C. Then, the cells were washed and stained with CD8-PerCP and CD4-FITC-conjugated monoclonal antibodies. After 30 min of incubation at 4°C, the cells were fixed with staining buffer and immediately analyzed with flow cytometry and presented as a percentage of total PBL (Fig. 2).
Of note, assays of pre and postvaccination PBMC samples provided evidence for the expansion of MAGE-3-specific CD8+ cells (from 0.08% before vaccination to 0.5% after first vaccination). Moreover, the mean fluorescence intensity (MFI) of MAGE-3-specific CD8-positive cells was increased and ranged between 1,533 value before vaccination to 3,863 MFI at the day of second vaccination. After the second vaccination, the percentage of MAGE-3-specific CTLs decreased to 0.03% and reached plateau until termination of vaccination (Fig. 3). A significant alteration of immunological response was not observed for MUC-1 peptides (Fig. 3). At the same time, the percentage of CD8 and CD95L double-positive cells was increased from 0.14% before to 0.79% after first vaccination. We conclude these cells are competent to potentially destroy cancer cells through Fas-FasL interaction.
During the booster vaccinations, the percentage of MAGE-3-specific CD8+ cells was increased from 0.05% before booster vaccination to 0.24% after third injection (Fig. 3).
Interestingly, we detected twofold increase of percentage of CTLs expressed T cell receptor against cytomegalovirus (CMV) antigen (the measurement of these cells was used as control in our experiment). In addition, during booster vaccination, the percentage of CTLs-CMV-specific had increased from 0.13 to 0.20% and finally was established at 0.06% after third injection.
The anti-CMV IgM was below detection level, but the level of anti-CMV IgG was high (321 U/mL) and increased to 366 U/mL after vaccination finishing.
The percentage of CTLs and Th1 cells produced IFN-γ and IL-2 was detected by staining for intracellular cytokines. CD14-negative cells were seeded in completed medium in 6-well plates for 24 h. Then, phorbol myristate-acetate (PMA, 2.5 ng/mL), ionomycin (2 ng/mL) and brefeldin (2 ng/mL; Sigma, Germany) were added directly to the culture wells. Within 5 h, culture cells were collected and washed in PBS without Ca2+ and Mg2+. In order to determine the expression of CD4 and CD8 antigens, FITC-conjugated anti-CD4 and PerCP-conjugated anti-CD8 monoclonal antibodies were used (Becton, Dickinson, USA). For fixation of antibodies binding and permeabilisation of cell membrane, IntraPrep kit was used (Beckman, Coulter, USA). The expression of intracellular cytokines was detected by means of the PE-conjugated anti-IL-2, anti-IFN-γ monoclonal antibodies (BD Biosciences, USA).
The percentages of CD4+ and CD8+ cells with intracellular expression of IFN-γ were stable during the course of vaccination (Table 1). However, the percentage of CD4+ and CD8+ cells produced IL-2 was elevated more than threefold after the second vaccination (Table 1). The percentage of cells with intracellular expression of IL-2 among CD4+ lymphocyte subpopulation (Th1 cells) was 23.4% before vaccination and 75.3% after second vaccination (when total PBL was analyzed, the percentages were as follow: 10.9 and 40.6%, respectively). Percentage of cells with intracellular expression of IL-2 among CD8+ lymphocyte subpopulation was 31.9% before vaccination and 62.3% after second vaccination (when total PBL was analyzed, the percentages were as follow: 2.87 and 9.03%, respectively).
The percentage of regulatory T cell before and during vaccination was performed using Human FoxP3 Staining Buffers and set of monoclonal antibodies according to manufacturer’s instruction (BD Pharmingen, USA). The percentage of CD4-positive, CD25 highly positive and Fox-P3-positive T regulatory lymphocytes was estimated from CD4-positive cells and from total PBL (Fig. 4). The percentage of Treg cells was rather stable during the course of vaccination, but the lowest percentage of Treg cells was noticed after first vaccination. Before the booster vaccination, the percentage of Treg cells was slightly higher when compared with the percentage during first course of vaccination. It was decreased during booster vaccination (Table 1).
The flow cytometry analysis of regulatory T lymphocytes: CD4-positive cells were gated; the cells with high expression of CD25 antigen and expression of FoxP3 transcription factor were defined as regulatory T cells. R1 included only CD4-positive cells, R2 included triple-positive cells CD4+/CD25+/FoxP3+
In addition, as a control group, the peripheral blood from 10 healthy subjects (median age: 57) was taken and the percentages of CD4+ and CD8+ cells with intracellular expression of IFN-γ and IL-2, as well as the percentage of T regulatory cells, were estimated as described above. The results are presented as median value in Table 1.
Clinical Monitoring
In January 2010, before first vaccination, patient underwent computed tomography (CT) scanning which shown 13–14 mm diameter enlargement of three lymph nodes: mediastinal, paratracheal and aortic-pulmonary window. Moreover, a hypodense area in the zone of the surgical resection scar and pleural thickening with a small amount of effusion on the right were also shown. The wall thickening and parietal thrombus in the aorta before aortic bifurcation and in iliac arteries due to Takayasu disease were visualized in abdominal CT scans (Fig. 5).
Control CT scans were performed in March 2010 after cessation of immunotherapy. Enlargement of lymph nodes was limited only to a mediastinal lymph node (13 mm), but cluster of nodules of 3–13 mm in diameter in inferior lobe of right lung were appeared (Fig. 6a). Nodules were encircled by grand glass opacities and expressed “the tree-in-bud” sign. The obtained results of CT scans might suggest recurrence of lung adenocarcinoma, however, bronchopneumonia occurs secondary to mucus plugging in deformed bronchi is also possible. Nevertheless, in PET-CT examination performed on May 30th 2010, the alteration mimicking cancer recurrence was not observed (Fig. 6b). Until October 2010, the patient remains in good condition without clinical symptoms of cancer progression. Due to the lack of symptoms of cancer recurrence in CT examination, the booster course of vaccination was started in November 2010. After cessation of booster vaccination, the symptoms of cancer recurrence were not observed until April 2011 (18 months from surgical resection).
The cluster of nodules of 3–13 mm in diameter in inferior lobe of right lung mimicking recurrence of lung cancer after first course of vaccinations (a). After following examination, it was ascertained that these changes were of inflammation character (b control CT scans performed before booster vaccinations)
Discussion
Immunological analysis of the patient’s immune status before vaccination had indicated the moderate activation of the immune system with the predominant role of Th1 lymphocytes, cytotoxic T lymphocytes and NK cells. Together with the histopathological diagnosis of lung adenocarcinoma with acinar and bronchoalveolar growth pattern, this might explain the slow development of lung cancer in the case of recurrent cancer and partial success of surgical resection. We concluded that activation of patient’s immunological system might control tumour growth. However, this type of lung cancer has minimally invasive character with low metastatic activity (Dacic 2009; Noris 2001). Innate immune response and co-existing of Takayasu disease might influence both the effect of DC-based immunotherapy and the clinical course of lung adenocarcinoma.
MUC1 is a glycoprotein expressed on the cell surface of many normal epithelial tissues and overexpressed or aberrantly glycosylated on many carcinoma cells, including NSCLC. Phase I and II of clinical trials had demonstrated the potential early activity of L-BLP25 liposomal vaccine, which included the MUC-1 peptide, in maintenance therapy of advanced NSCLC patients (Butts et al. 2005; Powell and Chow 2008). The vaccine consists of MUC-1-(sequences: M1.1-STAPPVHNV and M1.2-LLLLTVLTV)-pulsed DCs were used by Wierecky et al. (2006) in metastatic renal cancer. MUC-1 peptide-specific T cell responses were detected in PBMC of 75% of patients, while clinical responses were observed in 25% of patients (Wierecky et al. 2006). Kontani et al. (2003) analyzed the clinical response in 5 NSCLC patients with pleural effusion after MUC-1-loaded DCs vaccination using oligopeptides containing 30 amino acids whose sequence corresponds to MUC1 tandem repeats (TRPAPGSTAPPAHGVTSAPDTRPAPGSTAP) but differing from those MUC-1 sequence used in our and others reports. Moreover, Kontani et al. (2003) had observed one stable disease, one disappearance of a pleural effusion and two partial responses. Early disease progression was observed in one patient (Kontani et al. 2003). Based on these results, one might speculate that DC vaccines targeting MUC-1 are useful for immunotherapy of cancer.
The human MAGE-3 gene was originally discovered in melanoma cells that encode tumour antigens, and has been reported to be expressed in various types of tumours, including lung cancer, but not in normal tissue other than testis or placenta (Marchand et al. 2003). It was previously reported that vaccine consists of DNA recombinant MAGE-3 protein with a lipidated protein D from Haemophilus influenzae at its N-terminus as well as sequence of several histidine residues at the C-terminus (Prot.D MAGE-3/His) is highly effective in lung cancer treatment (Marchand et al. 2003). Moreover, Atanackovic et al. (2004) looked for the presence of MAGE-3 specific T cells in patients that had been vaccinated by Prot.D MAGE-3/His by performing tetramer analysis and ELISPOT analysis. Of nine HLA-A2-positive patients, one showed a significant increase in tetramer-positive MAGE-3-specific CD8+ T cells (Atanackovic et al. 2004). The sequence of MAGE-3 epitope used in our report was matching with the sequence listed by Atanackovic et al. (2004). Hersey et al. (2004) had conducted clinical trial using DCs pulsed with peptides from melanoma antigens MAGE-3.A2 (sequence: FLWGPRALV), tyrosine, gp100 and MART-1 or with tumour cell lysates in metastatic melanoma patients. Authors did not show clinical responses in the group treated with DCs pulsed with melanoma antigens. However, three immune responses against MAGE-3.A2 epitope (increased IFN-γ production by CTLs) were observed (Hersey et al. 2004). A very interesting results are achieved by immunotherapy with a recombinant MAGE-A3 fusion protein together with immune adjuvant AS02B, that was injected in the group of 182 patients with resected stage IB/II, MAGE-A3-positive NSCLC. Investigators observed a delayed time to recurrence and reduction in relative risk of cancer relapse. The 44-month follow-up analysis of this randomized phase II study confirms the positive activity and immunogenicity of the MAGE-A3 treatment in adjuvant NSCLC. The encouraging results from the phase II study have led to a phase III (MAGRIT) trial of MAGE-A3 positive NSCLC patients in stage IB, II or IIIA (Vansteenkiste et al. 2008a, b). Based on the literature review, our report is the first study with DCs pulsed with the MAGE-3 epitope conducted in NSCLC patient. Moreover, we observed an increased immune response during vaccination.
Our methods of monitoring of the immune response to anticancer vaccines are reliable and consistent with state-of-the art on immune-monitoring in patients with malignancies (Keilholz et al. 2002; Nagorsen et al. 2004; Whiteside et al. 2003). We had shown the increase of the percentage of CTLs specific to MAGE-3 with simultaneously positive DTH test. Our assays revealed also the increasing of T lymphocytes with intracellular expression of IL-2 and slightly decreasing of Treg lymphocytes. One might speculate that the stimulation of immunological responses to anticancer vaccination with DCs pulsed with MAGE-3 epitope was even clinically successful. A very important observation regarding the application of the future vaccinations’ schedules is the dynamics in the activation of tumour-specific immunological responses visible as a peak in the frequency of MAGE-3 specific CD8+ T cells. The increase in the percentage of MAGE-3 specific T cells was observed after second injection in both first and booster course of vaccinations. Taking into consideration the time of antigen stimulation after which antigen-specific CTLs are activated, as well as the recurrent character of the injections, we might believe that specific immunological responses had expanded after second vaccination. We suggest that continuous monitoring of the immune responses during vaccinations is necessary.
Vaccines for the therapy of cancer differ from classical prophylactic vaccines. Therapeutic vaccines aim at inducing strong antigen-specific T-cell responses (Bradbury and Shepherd 2008; Raez et al. 2005). Vaccine therapy is feasible and safe. It is probable that vaccines could be beneficial only for certain subgroups of patients (Bradbury and Shepherd 2008; Raez et al. 2005). The immunotherapy as a consolidation therapy after surgical resection or radiochemotherapy might be more efficient than immunotherapy in advanced malignancies. Nevertheless, clinical monitoring of such immunotherapy is very difficult (Keilholz et al. 2002; Nagorsen et al. 2004; Whiteside et al. 2003). In the cases lacking evident clinical disease symptoms and measurable tumours, only monitoring of immune response can be performed. However, disease-free survival can be evaluated. Despite the appearance of poor prognostic factors (no completed wedge-resection and tumour vessels infiltration by cancer cells), our patient remains without clinical symptoms of disease over 18 months from the tumour resection. In the light of the previous assumption, we speculate that immunotherapy prolonged the disease-free survival in our patient.
References
Atanackovic D, Altorki NK, Stockert E et al (2004) Vaccine-induced CD4+ T cell response to MAGE-3 protein in lung cancer patients. J Immunol 172:3289–3296
Bleumer I, Tiemessen DM, Oosterwijk-Wakka JC (2007) Preliminary analysis of patients with progressive renal cell carcinoma vaccinated with CA9-peptide-pulsed mature dendritic cells. J Immunother 30:116–122
Bradbury PA, Shepherd FA (2008) Immunotherapy for lung cancer. J Thorac Oncol 3(6 suppl 2):S164–S170
Butts C, Murray N, Maksymiuk A et al (2005) Randomized phase IIB trial of BLB25 liposome vaccine in stage IIIB and IV non-small cell lung cancer. J Clin Oncol 23:6674–6681
Dacic S (2009) Minimally invasive adenocarcinomas of the lung. Adv Anat Pathol 16:166–171
Dubsky P, Ueno H, Piqueras B et al (2005) Human dendritic cell subsets for vaccination. J Clin Immunol 25:551–572
Fujii S, Takayama T, Asakura M et al (2009) Dendritic cell-based cancer immunotherapies. Arch Immunol Ther Exp 57:189–198
Hersey P, Menzies SW, Halliday GM et al (2004) Phase I/II study of treatment with dendritic cell vaccines in patients with disseminated melanoma. Cancer Immunol Immunother 53:125–134
Keilholz U, Weber J, Finke JH et al (2002) Immunologic monitoring of cancer vaccine therapy: results of a workshop sponsored by the Society for Biological Therapy. J Immunother 25:97–138
Kontani K, Taguchi O, Ozaki Y et al (2003) Dendritic cell vaccine immunotherapy of cancer targeting MUC1 mucin. Int J Mol Med 12:493–502
Maksimowicz-McKinnon K, Hoffman GS (2007) Takayasu arteritis: what is the long-term prognosis? Rheum Dis Clin North Am 33:777–786
Marchand M, Punt CJ, Aamdal S et al (2003) Immunisation of metastatic cancer patients with MAGE-3 protein combined with adjuvant SBAS-2: a clinical report. Eur J Cancer 39:70–77
Nagorsen D, Scheibenbogen C, Thiel E et al (2004) Immunological monitoring of cancer vaccine therapy. Expert Opin Biol Ther 4:1677–1684
Nakajima N, Masuda M, Imamaki M et al (2007) A case of pulmonary artery bypass surgery for a patient with isolated Takayasu pulmonary arteritis and a review of the literature. Ann Thorac Cardiovasc Surg 13:267–271
Nencioni A, Grünebach F, Schmidt SM et al (2008) The use of dendritic cells in cancer immunotherapy. Crit Rev Oncol Hematol 65:191–199
Noris M (2001) Pathogenesis of Takayasu’s arteritis. J Nephrol 14:506–513
Powell E, Chow LQ (2008) BLP-25 liposomal vaccine: a promising potential therapy in non-small-cell lung cancer. Expert Rev Respir Med 2:37–45
Raez LE, Fein S, Podack ER (2005) Lung cancer immunotherapy. Clin Med Res 3:221–228
Romero P (2008) Current state of vaccine therapies in non-small cell lung cancer. Clin Lung Cancer 9(suppl 1):S28–S36
Rossi M, Young JW (2005) Human dendritic cells: potent antigen-presenting cells at the crossroads of innate and adaptive immunity. J Immunol 175:1373–1381
Ueno H, Schmitt N, Klechevsky E et al (2010) Harnessing human dendritic cell subsets for medicine. Immunol Rev 234:199–212
Vansteenkiste J, Zielinski M, Linder A et al (2008a) Association of gene expression signature and clinical efficacy of MAGE-A3 antigen-specific cancer immunotherapeutic (ASCI) as adjuvant therapy in resected stage IB/II non-small cell lung cancer (NSCLC). J Clin Oncol 26(suppl):9045
Vansteenkiste J, Zielinski M, Linder A et al (2008b) Final results of a multi-center, double-blind, randomized, placebo-controlled phase II study to assess the efficacy of MAGE-A3 immunotherapeutic as adjuvant therapy in stage IB/II non-small cell lung cancer (NSCLC). J Clin Oncol 25(suppl):7554
Whiteside TL, Zhao Y, Tsukishiro T et al (2003) Enzyme-linked immunospot, cytokine flow cytometry and tetramers in the detection of T-cell responses to a dendritic cell-based multipeptide vaccine in patients with melanoma. Clin Cancer Res 9:641–649
Wierecky J, Müller MR, Wirths S et al (2006) Immunologic and clinical response after vaccinations with peptide-pulsed dendritic cells in metastatic renal cancer patients. Cancer Res 66:5910–5918
Acknowledgments
This work was supported by the Ministry of Science and Higher Education (Poland) grant no. NN 401028937.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Wojas-Krawczyk, K., Krawczyk, P., Buczkowski, J. et al. Immunotherapy of Lung Adenocarcinoma Patient with Peptide-Pulsed Dendritic Cells: a Case Report. Arch. Immunol. Ther. Exp. 60, 69–77 (2012). https://doi.org/10.1007/s00005-011-0157-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00005-011-0157-7