Abstract
Chemokines proved able to induce release of enzymes relevant in cartilage damage. The present study addressed the levels of CXCL8 and CCL5 and the potential role of these chemokines in predicting the morphological changes in the course of osteoarthritis (OA). Synovial fluid (SF) and blood serum were obtained from 20 patients undergoing knee replacement surgery because of OA. For comparison, samples were also obtained from another 20 patients during diagnostic or therapeutic arthroscopy performed because of knee injury. The samples were analyzed for CXCL8 and CCL5 using enzyme-linked immunosorbent assay. SF from the group with OA showed significantly (p = 0.024) increased levels of CXCL8 when compared with the group after knee injury. We have not demonstrated any significant correlation between chemokine expression and clinical or radiological signs of OA. Mediators of inflammation are the potential predicting factors of OA, however, with respect to examined chemokines development of a diagnostic test can be limited by the low serum concentration and lack of correlation with clinical and radiological signs of the disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chemokines are a large family of mediators of inflammation able to induce chemotaxis in a variety of cells including neutrophils, monocytes, lymphocytes, eosinophils, and fibroblasts (Proudfoot 2002). They were originally described as having a primary role in directing lymphocytes to sites of inflammation, however, their physiological role is more complex than originally described, and new functions continue to be identified. Chemokines proved able to induce release of enzymes relevant in cartilage damage: alkaline phosphatase, N-acetyl-β-D-glucosamine and matrix metalloproteinases (MMP)-3 (Borzì et al. 2000; Lisignoli et al. 2003).
CXCL8 is one of the most potent chemoattractants for polymorphonuclear neutrophils (PMNs) stimulating their chemotaxis and generating reactive oxygen metabolites. This chemokine is synthesized by a variety of cells including monocytes/macrophages, chondrocytes, fibroblasts and osteoblasts (Lisignoli et al. 1999). CXCL8 can enhance the release of inflammatory cytokines (IL-1β, IL-6, and TNF-α) in mononuclear cells, which may further modulate the inflammatory reaction. CXCL8 also stimulates neutrophil degranulation and adherence to endothelial cells by macrophage antigen-1.
CCL5 can be produced by activated T cells and many other cell types, including fibroblasts, chondrocytes, and osteoblast (Lisignoli et al. 2002; Pulsatelli et al. 1999). It is a chemoattractant for monocytes, eosinophils, and T cells, and it activates both eosinophils and basophils to release granule content. It is proved that chondrocytes expressed the CCL5 receptors CCR1, CCR3, and CCR5 (Alaaeddine et al. 2001).
The primary aim of this study was to estimate the level of CXCL8 and CCL5 in synovial fluid (SF) and blood serum (BS) in patients with osteoarthritis (OA) of the knee. Secondary aim was to find the correlation between CXCL8 and CCL5 levels and clinical and radiological signs of OA.
Materials and Methods
The study was performed on patients with OA undergoing knee replacement surgery (OA group). SF and BS were obtained from 20 patients (mean age 67.7 years; range 59–78 years). Diagnosis of OA was based on clinical, radiological and laboratory parameters (Altman et al. 1986).
For comparison, SF and BS samples were obtained from 20 patients (mean age 34.9 years; range 21–47 years) during diagnostic or therapeutic arthroscopy performed because of knee injury (posttraumatic group). In all these cases the time from injury to arthroscopy exceeded 3 weeks.
Patients were not included in the study if they had presented signs of acute or chronic inflammatory knee disease or rheumatoid arthritis (RA) according to criteria of The American Rheumatism Association (Arnett et al. 1988). Individuals suffering from serious systemic or autoimmune diseases were also excluded from the study. In case of non steroidal anti-inflammatory drugs usage, these drugs were withdrawn at least 1 week prior to surgery.
The study was approved by the ethical committee of Silesian Medical University and informed consent was obtained from the patients.
For clinical assessment of patients with OA the WOMAC index was used (Bellamy et al. 1988). Radiological severity of OA was measured according to the Altman classification (Altman and Gold 2007), which is a four grade scale, based on marginal osteophytes formation and joint space narrowing (Table 1).
Before surgery venous blood samples of all patients were collected. Immediately prior to surgery SF samples were obtained by puncture of the knee joint. The samples were centrifuged at 2,500 rpm for 10 min, then the supernatant extracted and immediately frozen and preserved at −72°C. The samples were analyzed for CXCL8 and CCL5 using commercially available enzyme-linked immunosorbent assay (DuoSet ELISA Development System R&D). An ELISA is a five-step procedure: (1) coat the microtiter plate wells with antigen; (2) block all unbound sites to prevent false positive results; (3) add antibody to the wells; (4) add anti-mouse IgG conjugated to an enzyme; (5) reaction of a substrate with the enzyme to produce a colored product, thus indicating a positive reaction. The extinction was measured with ELx800 Bio-Tec Inst. Inc. reader. Analyses were performed twice for each sample and a mean value was taken as a result.
We used the unpaired Student’s t test for statistical analysis. A p value of <0.05 was considered statistically significant. To estimate the degree of correlation we used the Pearson’s correlation coefficient.
Results
OA, in contrary to RA, is a non-systemic disease. The inflammation in OA is limited to the joints and it is uncommon to find typical markers of inflammation in the BS of OA patients. That is the reason of high discrepancy between the SF values and the serum values of examined chemokines.
The average CXCL8 level of 18.4640 ± 17.0761 pg/ml was found in BS collected form OA patients. The average CXCL8 level of 10.1236 ± 19.1862 pg/ml was found in BS collected from posttraumatic (PT) patients. There was no significant difference between these two groups (p = 0.154).
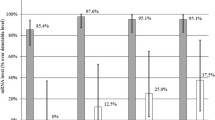
The average CXCL8 level of 188.8450 ± 324.6087 pg/ml was found in SF aspirated from OA knees. The average CXCL8 level of 18.1508 ± 14.0439 pg/ml was found in SF aspirated from PT knees. The synovial fluid from the group with OA showed significantly (p = 0.024) increased levels of CXCL8 when compared with the group after knee injury (Fig. 1).
The average concentrations of CCL5 in BS among OA and PT patients were 127.8453 ± 233.2532 and 89.8684 ± 78.2591 pg/ml, respectively. The difference was not significant (p = 0.494).
The average concentrations of CCL5 in synovial fluid collected from OA and PT knees were 690.5762 ± 163.4080 and 563.0747 ± 309.4597 pg/ml, respectively. The difference was not significant (p = 0.111).
There was no significant correlation between chemokines levels and WOMAC or Altman scale (Table 2).
Discussion
Deleuran et al. (1994) reported that the strongest expression of CXCL8, in both OA and RA patients, was detected in the blood vessels and lining cell layers of the synovial membrane. The presence of CXCL8 in the lining cell layers may result in delivery to the SF, and could explain the high amount of CXCL8 in this location. CXCL8 leads to lymphocyte activation and increased migration from vessels to the joint tissues. In consequence the accumulation of PMNs is observed. PMNs are the main source of MMPs (collagenases, gelatinases, stromelysins), which participate in the development of the clinical signs (swelling, pain) of inflammation (Kraan et al. 2001). Immunohistochemistry evaluation and in situ hybridization have proved significant correlation between level of CXCL8 and the clinical stage of disease among RA patients (Kraan et al. 2001). In our study, however, we have not found correlation between CXCL8 level and clinical scale of OA (WOMAC). These observations correspond with the study of Hamada et al. (2008) in which they also have not observed any relation between cytokines levels and clinical stage.
Bendre et al. (2003), in turn, have demonstrated that CXCL8 stimulates osteoclastogenesis and bone resorption. In osteoblasts CXCL8 potentiates the expression of osteoclast differentiation factor RANKL which is necessary for osteoclasts activation. Furthermore, CXCL8 can depress the activity of osteoblasts and their ability of osteogenesis (Rothe et al. 1998). This phenomenon can explain the bone loss observed in RA and OA. Yet in our study we have not demonstrated any correlation between CXCL8 level and bone remodelling expressed by Altman scale.
CCL5 was found to induce expression of MMP-1, MMP-3 and inducible nitric oxide synthase (Borzì et al. 2000; Nakamura et al. 2006). In cartilage it inhibits proteoglycan synthesis and increases the release of glycosaminoglycans. Alaaeddine et al. (2001) have demonstrated the role of CCL5 in the degradation of cartilage extracellular matrix by the induction of MMP-1 release from cultured chondrocytes. CCL5 was as effective as or even more effective than IL-1β in causing a loss of proteoglycans. CCL5 could have similar function in bone tissue (i.e. recruitment of osteoclasts, regulation of hematopoiesis, and modulation of bone remodeling) (Lisignoli et al. 2002). Joint cartilage degeneration in OA is associated with subchondral bone modification (Westacott et al. 1997). This modification is caused by a higher subchondral bone metabolism characterized by an increased collagen type I synthesis (Mansell and Bailey 1998) and abnormal production of cytokines/chemokines by stromal cells and osteoblasts (Lisignoli et al. 2004). SFs obtained from RA patients show high CCL5 level, and high serum concentration of CCL5 has been associated with a rapid progression of radiographic changes (Katschke et al. 2001). In addition, the study of Ellingsen et al. (2000) showed a correlation between CCL5 SF level and clinical signs of RA).
In OA patients, we have found increased levels of CCL5 in SF and BS in comparison to the concentration of this chemokine in SF and BS of PT patients, however, the difference was non significant. We have also not demonstrated any significant correlation between CCL5 levels and clinical or radiological signs of OA.
We should be mindful that in our study we have compared OA samples with those from not healthy but PT knees. We can certainly expect some inflammatory response in post-traumatic SF. Lotz et al. (1992) have shown that human articular chondrocytes express the CXCL8 gene and secrete bioactive CXCL8 during the response to cartilage injury. However, Irie et al. (2003) have proved that significantly high levels of proinflammatory cytokines persist just for few days after injury. With an eye to this, we have included in the control group only the samples which were obtained more then 3 weeks after knee injury. Still, we cannot treat this group as the control, but as another pathological condition in which chemokines are also expressed. In the recent paper Cuellar et al. (2009) have observed a greater concentration of inflammatory cytokines (IL-6, CCL2, CCL4, and IFN- γ) in SF from knees of patients with meniscal injury compared with SF from knees of asymptomatic control subjects in witch these cytokines were nearly absent.
As we know that injury evokes immune response (cytokine expression), the question arises—whether this effect will always lead to degenerative changes (OA)? Whether some factor which is predisposing to this process must also exist?
In conclusion, mediators of inflammation are the potential predicting factor of OA, however, with respect to examined chemokines development of a diagnostic test can be limited by the low serum concentration and lack of correlation with clinical and radiological signs of the disease. The problem also arises to distinguish OA from other pathological conditions (e.g. injury) in which chemokines are expressed.
Abbreviations
- BS:
-
Blood serum
- CCL2:
-
Monocyte chemoattractant protein 1
- CCL4:
-
Macrophage inhibiting protein 1β
- CCL5:
-
Chemokine RANTES
- RANTES:
-
Regulated upon activation, normal T-cell expressed, and secreted
- CXCL8:
-
Chemokine IL-8
- IL-1β:
-
Interleukin-1β
- MMPs:
-
Matrix metalloproteinases
- OA:
-
Osteoarthritis
- PMNs:
-
Polymorphonuclear neutrophils
- PT:
-
Posttraumatic
- SF:
-
Synovial fluid
- TNF-α:
-
Tumor necrosis factor α
- WOMAC:
-
Western Ontario and McMaster Universities index of osteoarthritis
References
Alaaeddine N, Olee T, Hashimoto S et al (2001) Production of the chemokine RANTES by articular chondrocytes and role in cartilage degradation. Arthritis Rheum 44:1633–1643
Altman RD, Gold GE (2007) Atlas of individual radiographic features in osteoarthritis, revised. Osteoarthritis Cartilage 15 (suppl A):A1–A56
Altman R, Asch E, Bloch D et al (1986) Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and Therapeutic Criteria Committee of the American Rheumatism Association. Arthritis Rheum 29:1039–1049
Arnett FC, Edworthy SM, Bloch DA et al (1988) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31:315–324
Bellamy N, Buchanan WW, Goldsmith CH et al (1988) Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol 15:1833–1840
Bendre MS, Montague DC, Peery T et al (2003) Interleukin-8 stimulation of osteoclastogenesis and bone resorption is a mechanism for the increased osteolysis of metastatic bone disease. Bone 33:28–37
Borzì RM, Mazzetti I, Cattini L et al (2000) Human chondrocyte express functional chemokine receptors and release matrix-degrading enzymes in response to C-X-C and C-C chemokines. Arthritis Rheum 43:1734–1741
Cuellar JM, Scuderi GJ, Cuellar VG et al (2009) Diagnostic utility of cytokine biomarkers in the evaluation of acute knee pain. J Bone Joint Surg Am 91:2313–2320
Deleuran B, Lemche P, Kristensen M et al (1994) Localisation of interleukin 8 in the synovial membrane, cartilage-pannus junction and chondrocytes in rheumatoid arthritis. Scand J Rheumatol 23:2–7
Ellingsen T, Buus A, Moller BK et al (2000) In vitro migration of mononuclear cells towards synovial fluid and plasma from rheumatoid arthritis patients correlates to RANTES synovial fluid levels and to clinical pain parameters. Scand J Rheumatol 29:216–221
Hamada Y, Holmlund AB, Kondoh T et al (2008) Severity of arthroscopically observed pathology and levels of inflammatory cytokines in the synovial fluid before and after visually guided temporomandibular joint irrigation correlated with the clinical outcome in patients with chronic closed lock. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 106:343–349
Irie K, Uchiyama E, Iwaso H (2003) Intraarticular inflammatory cytokines in acute anterior cruciate ligament injured knee. Knee 10:93–96
Katschke KJ Jr, Rottman JB, Ruth JH et al (2001) Differential expression of chemokine receptors on peripheral blood, synovial fluid, and synovial tissue monocytes/macrophages in rheumatoid arthritis. Arthritis Rheum 44:1022–1032
Kraan MC, Patel DD, Haringman JJ et al (2001) The development of clinical signs of rheumatoid synovial inflammation is associated with increased synthesis of the chemokine CXCL8 (interleukin-8). Arthritis Res 3:65–71
Lisignoli G, Toneguzzi S, Pozzi C et al (1999) Proinflammatory cytokines and chemokine production and expression by human osteoblasts isolated from patients with rheumatoid arthritis and osteoarthritis. J Rheumatol 26:791–799
Lisignoli G, Toneguzzi S, Grassi F et al (2002) Different chemokines are expressed in human arthritic bone biopsies: IFN-gamma and IL-6 differently modulate IL-8, MCP-1 and rantes production by arthritic osteoblasts. Cytokine 20:231–238
Lisignoli G, Toneguzzi S, Piacentini A et al (2003) Human osteoblasts express functional CXC chemokine receptors 3 and 5: activation by their ligands, CXCL10 and CXCL13, significantly induces alkaline phosphatase and beta-N-acetylhexosaminidase release. J Cell Physiol 194:71–79
Lisignoli G, Toneguzzi S, Piacentini A et al (2004) Recruitment and proliferation of T lymphocytes is supported by IFNgamma- and TNFalpha-activated human osteoblasts: involvement of CD54 (ICAM-1) and CD106 (VCAM-1) adhesion molecules and CXCR3 chemokine receptor. J Cell Physiol 198:388–398
Lotz M, Terkeltaub R, Villiger PM (1992) Cartilage and joint inflammation. Regulation of IL-8 expression by human articular chondrocytes. J Immunol 148:466–473
Mansell JP, Bailey AJ (1998) Abnormal cancellous bone collagen metabolism in osteoarthritis. J Clin Invest 101:1596–1603
Nakamura H, Tanaka M, Masuko-Hongo K et al (2006) Enhanced production of MMP-1, MMP-3, MMP-13, and RANTES by interaction of chondrocytes with autologous T cells. Rheumatol Int 26:984–990
Proudfoot AE (2002) Chemokine receptors: multifaceted therapeutic targets. Nat Rev Immunol 2:106–115
Pulsatelli L, Dolzani P, Piacentini A et al (1999) Chemokine production by human chondrocytes. J Rheumatol 26:1992–2001
Rothe L, Collin-Osdoby P, Chen Y et al (1998) Human osteoclasts and osteoclast-like cells synthesize and release high basal and inflammatory stimulated levels of the potent chemokine interleukin-8. Endocrinology 139:4353–4363
Westacott CI, Webb GR, Warnock MG et al (1997) Alteration of cartilage metabolism by cells from osteoarthritic bone. Arthritis Rheum 40:1282–1291
Acknowledgments
We thank the patients for contributing to this study and the clinical staff for help with collection of samples. We also thank Ewa Sozańska for technical help with ELISA measurements.
Conflict of interest
The authors declare that they have no competing interests.
Author information
Authors and Affiliations
Corresponding author
About this article
Cite this article
Pierzchala, A.W., Kusz, D.J. & Hajduk, G. CXCL8 and CCL5 Expression in Synovial Fluid and Blood Serum in Patients with Osteoarthritis of the Knee. Arch. Immunol. Ther. Exp. 59, 151–155 (2011). https://doi.org/10.1007/s00005-011-0115-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00005-011-0115-4